Effectiveness of Azvudine and Nirmatrelvir-ritonavir in Kidney Transplant Recipients With COVID-19: A Retrospective Cohort Study
et al., Infections in the immunosuppressed and immunocompromised host, doi:10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A2917, Apr 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
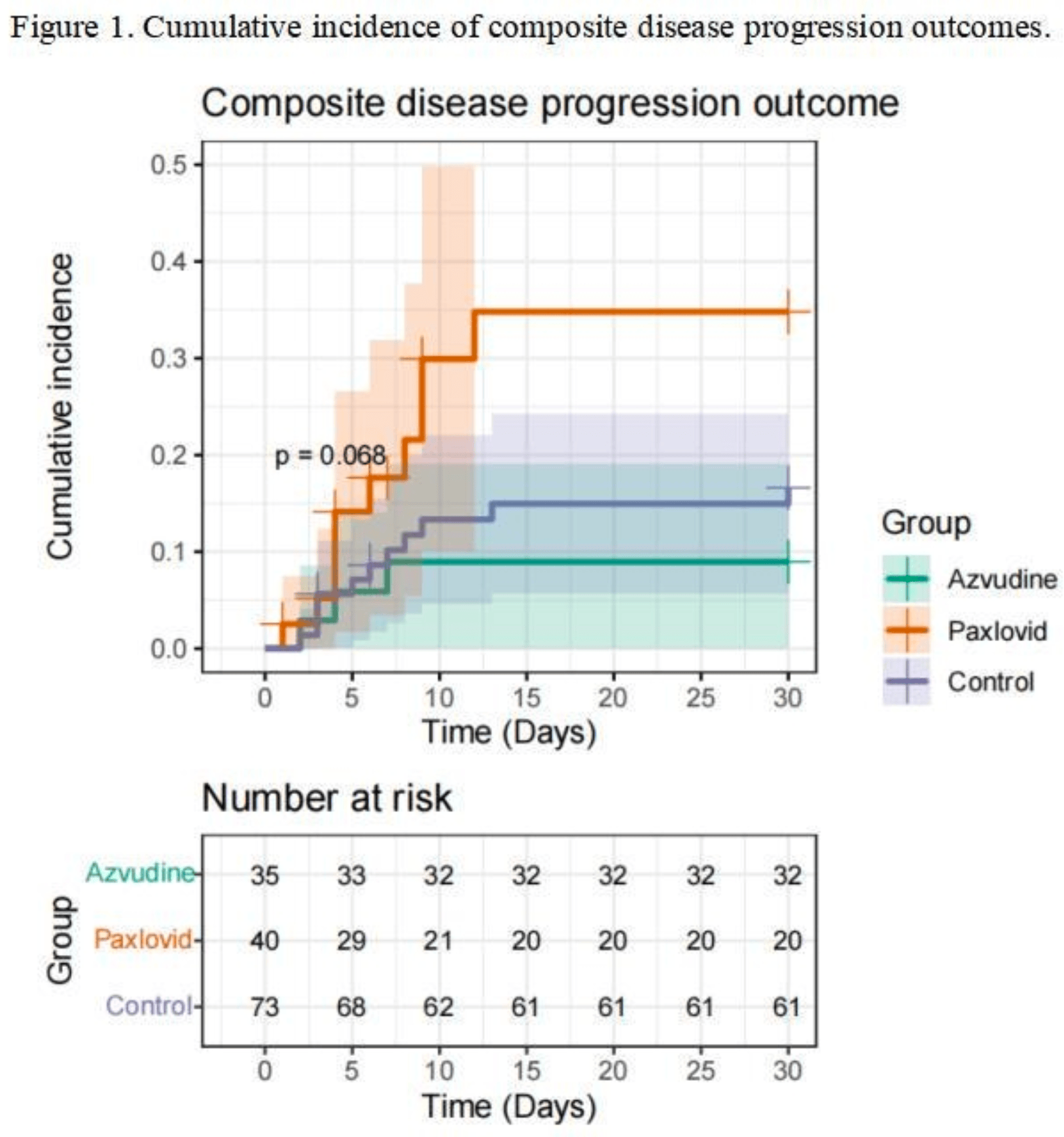
Retrospective 148 hospitalized kidney transplant patients with COVID-19 in China showing lower risk of disease progression with azvudine treatment compared, and higher risk with paxlovid treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
Study covers azvudine and paxlovid.
|
risk of progression, 45.1% lower, HR 0.55, p = 0.36, inverted to make HR<1 favor treatment, propensity score matching, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Liu et al., 30 Apr 2024, retrospective, China, peer-reviewed, 3 authors, study period 1 December, 2022 - 19 January, 2023.
Contact: liuyi200402@163.com.
Effectiveness of Azvudine and Nirmatrelvir-ritonavir in Kidney Transplant Recipients With COVID-19: A Retrospective Cohort Study
Rationale: The effectiveness of azvudine and nirmatrelvir-ritonavir in treating COVID-19 patients has been demonstrated. However, evidence regarding their real-world effectiveness in kidney transplant recipients remains limited.Methods: We conducted a single-centre retrospective cohort study, focusing on hospitalised kidney transplant patients with COVID-19 between December 1st, 2022 and anuary 19th, 2023 during the omicron BA.5.2 variant-dominant period. Patients were eligible for inclusion if they tested positive for SARS-CoV-2 within 3 days before or after hospital admission and were diagnosed with both COVID-19 and having undergone kidney transplant. Exclusion criteria encompassed the administration of other antiviral treatments within 10 days of a positive SARS-CoV-2 test, breastfeeding, pregnancy, or known allergies to nirmatrelvir-ritonavir or azvudine. The primary outcome was a composite outcome of all-cause mortality, disease progression to critical COVID-19, Intensive care unit admission or upgrade of respiratory support, as well as their separate events. Hazard ratios (HRs) was estimated using Cox regression models for each event outcome between the groups.Results: We identified 1761 hospitalised patients with COVID-19 during the study period. After exclusions and propensity score matching, a total of 148 kidney transplant patients were included, with 40 receiving azvudine, 73 receiving nirmatrelvirritonavir, and 35 not receiving antiviral treatment. Azvudine was associated with a lower risk of composite disease progression outcome than those with no antiviral treatment (HR, 1.82; 95% CI, 0.51 to 6.53), whereas risk of composite disease progression outcome was similar in nirmatrelvirritonavir groups (HR, 3.72; 95% CI, 1.02 to 13.55). There was no evidence indicating a reduction in all-cause death, disease progression or incidence of the Intensive care unit admission. Conclusions: During the Omicron BA.5.2 variant wave, azvudine improved the prognosis of hospitalised kidney transplant recipients with COVID-19. However, the findings are constrained by the small sample size and a short follow-up period.
DOI record:
{
"DOI": "10.1164/ajrccm-conference.2024.209.1_meetingabstracts.a2917",
"URL": "http://dx.doi.org/10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A2917",
"alternative-id": [
"10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A2917",
"10.1164/ajrccm-conference.2024.A107"
],
"author": [
{
"affiliation": [
{
"name": "Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Liu",
"given": "Y.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Zhang",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Liu",
"given": "D.",
"sequence": "additional"
}
],
"container-title": "A107. INFECTIONS IN THE IMMUNOSUPPRESSED AND IMMUNOCOMPROMISED HOST",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
4,
30
]
],
"date-time": "2024-04-30T20:10:21Z",
"timestamp": 1714507821000
},
"deposited": {
"date-parts": [
[
2024,
4,
30
]
],
"date-time": "2024-04-30T20:10:28Z",
"timestamp": 1714507828000
},
"event": "American Thoracic Society 2024 International Conference, May 17-22, 2024 - San Diego, CA",
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:34:05Z",
"timestamp": 1714523645357
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
4,
30
]
]
},
"member": "19",
"original-title": [],
"prefix": "10.1164",
"published": {
"date-parts": [
[
2024,
4,
30
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
30
]
]
},
"published-print": {
"date-parts": [
[
2024,
5
]
]
},
"publisher": "American Thoracic Society",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.atsjournals.org/doi/10.1164/ajrccm-conference.2024.209.1_MeetingAbstracts.A2917"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effectiveness of Azvudine and Nirmatrelvir-ritonavir in Kidney Transplant Recipients With COVID-19: A Retrospective Cohort Study",
"type": "proceedings-article"
}
