Effects of remdesivir on SARS-CoV-2 viral dynamics and mortality in viraemic patients hospitalized for COVID-19
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkad295, Sep 2023
Retrospective 318 hospitalized COVID-19 patients in Sweden, showing improvements in viral clearance but no improvement for mortality with remdesivir treatment.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
|
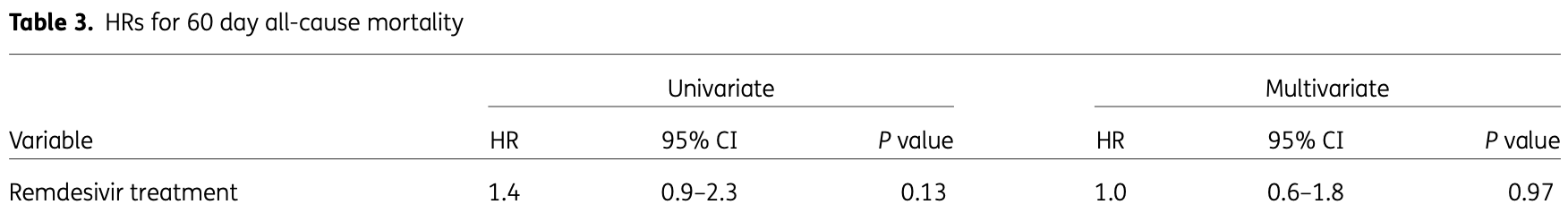
risk of death, no change, HR 1.00, p = 0.97, treatment 105, control 213, adjusted per study, multivariable, day 60.
|
|
risk of death, no change, HR 1.00, p = 0.99, treatment 105, control 213, adjusted per study, multivariable, day 28.
|
|
risk of death, 20.0% lower, HR 0.80, p = 0.74, treatment 105, control 213, adjusted per study, multivariable, day 7.
|
|
risk of progression, 40.0% higher, OR 1.40, p = 0.31, treatment 105, control 213, adjusted per study, multivariable, Table S7, RR approximated with OR.
|
|
risk of no viral clearance, 28.6% lower, HR 0.71, p = 0.11, treatment 105, control 213, adjusted per study, inverted to make HR<1 favor treatment, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Hagman et al., 26 Sep 2023, retrospective, Sweden, peer-reviewed, 9 authors, average treatment delay 6.0 days.
Effects of remdesivir on SARS-CoV-2 viral dynamics and mortality in viraemic patients hospitalized for COVID-19
doi:10.1093/jac/dkad295/7283037
Background: Studies on the antiviral effects of remdesivir have shown conflicting results. SARS-CoV-2 viraemia could identify patients in whom antiviral treatment may be particularly beneficial.
Objectives: To investigate antiviral effects and clinical outcomes of remdesivir treatment in viraemic patients. Methods: Viraemic patients hospitalized for COVID-19 with ratio of arterial oxygen partial pressure to fractional inspired oxygen of ≤300, symptom duration ≤10 days, and estimated glomerular filtration rate ≥30 mL/min were included in a cohort. The rate of serum viral clearance and serum viral load decline, 60 day mortality and in-hospital outcomes were estimated. A subgroup analysis including patients with symptom duration ≤7 days was performed. Results: A total of 318 viraemic patients were included. Thirty-three percent (105/318) received remdesivir. The rate of serum viral clearance [subhazard risk ratio (SHR) 1.4 (95% CI 0.9-2.0), P = 0.11] and serum viral load decline (P = 0.11) were not significantly different between remdesivir-treated patients and controls. However, the rate of serum viral clearance was non-significantly higher [SHR 1.6 (95% CI 1.0-2.7), P = 0.051] and the viral load decline was faster (P = 0.03) in remdesivir-treated patients with symptom duration ≤7 days at admission. The 60 day mortality [HR 1.0 (95% CI 0.6-1.8), P = 0.97] and adverse in-hospital outcomes [OR 1.4 (95% CI 0.8-2.4), P = 0.31] were not significantly different between remdesivir-treated patients and controls. Conclusions: Remdesivir treatment did not significantly change the duration of SARS-CoV-2 viraemia, decline of serum viral load, 60 day mortality or in-hospital adverse outcomes in patients with ≤10 days of symptoms at admission. Remdesivir appeared to reduce the duration of viraemia in a subgroup of patients with ≤7 days of symptoms at admission.
Ethics The Swedish Ethical Review Authority approved of the study (registration numbers 2020-01770 and 2020-06337). Informed consent was waived.
Author contributions Conceptualization: K.H., M.H., P.G.-J., J.U. Methodology: K.H., M.H., J.J., J.U. Investigation: K.H., J.W., M.H., E.A., B.H., L.G., J.U. Data curation: K.H., B.H., L.G., J.U. Formal analysis: K.H., J.U. Writing-original draft: K.H., J.U. Writing-review and editing: K.H., J.W., M.H., E.A., B.H., L.G., J.J., P.G.-J., J.U. Visualization: K.H., J.U.
Supplementary data Figure S1 and Tables S1 to S8 are available as Supplementary data at JAC Online.
References
Ader, Bouscambert-Duchamp, Hites, Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00485-0
Agarwal, Mukherjee, Kumar, Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial), BMJ, doi:10.1136/bmj.m3939
Barratt-Due, Olsen, Nezvalova-Henriksen, Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19 : a randomized trial, Ann Intern Med, doi:10.7326/M21-0653
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Biancofiore, Mirijello, Puteo, Remdesivir significantly reduces SARS-CoV-2 viral load on nasopharyngeal swabs in hospitalized patients with COVID-19: a retrospective case-control study, J Med Virol, doi:10.1002/jmv.27598
Consortium, Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO solidarity randomised trial and updated meta-analyses, Lancet, doi:10.1016/S0140-6736(22)00519-0
Dillner, Ursing, Convalescent plasma for treatment of COVID-19: study protocol for an open randomised controlled trial in Sweden, BMJ Open, doi:10.1136/bmjopen-2020-048337
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Folgueira, Luczkowiak, Lasala, Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.02.014
Garcia-Vidal, Alonso, Camon, Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19, J Antimicrob Chemother, doi:10.1093/jac/dkab321
Goldberg, Zvi, A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.02.029
Gordon, Tchesnokov, Woolner, Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, J Biol Chem, doi:10.1074/jbc.RA120.013679
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Group, Horby, Lim, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Hagman, Hedenstierna, Gille-Johnson, Severe acute respiratory syndrome coronavirus 2 RNA in serum as predictor of severe outcome in coronavirus disease 2019: a retrospective cohort study, Clin Infect Dis, doi:10.1093/cid/ciaa1285
Hagman, Hedenstierna, Rudling, Duration of SARS-CoV-2 viremia and its correlation to mortality and inflammatory parameters in patients hospitalized for COVID-19: a cohort study, Diagn Microbiol Infect Dis, doi:10.1016/j.diagmicrobio.2021.115595
Hagman, Hedenstierna, Widaeus, Correlation of SARS-CoV-2 nasopharyngeal CT values with viremia and mortality in adults hospitalized with COVID-19, Open Forum Infect Dis, doi:10.1093/ofid/ofac463
Jacobs, Bain, Naqvi, SARS-CoV-2 viremia is associated with COVID-19 severity and predicts clinical outcomes, Clin Infect Dis, doi:10.1093/cid/ciab686
Jacobs, Mellors, Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in blood of patients with coronavirus disease 2019 (COVID-19): what does it mean?, Clin Infect Dis, doi:10.1093/cid/ciaa1316
Jacobs, Naqvi, Shah, Plasma SARS-CoV-2 RNA levels as a biomarker of lower respiratory tract SARS-CoV-2 infection in critically ill patients with COVID-19, J Infect Dis, doi:10.1093/infdis/jiac157
Jones, Biele, Muhlemann, Estimating infectiousness throughout SARS-CoV-2 infection course, Science, doi:10.1126/science.abi5273
Joo, Ko, Kim, Clinical and virologic effectiveness of remdesivir treatment for severe coronavirus disease 2019 (COVID-19) in Korea: a nationwide multicenter retrospective cohort study, J Korean Med Sci, doi:10.3346/jkms.2021.36.e83
Li, Schneider, Mehta, SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes, J Clin Invest, doi:10.1172/JCI148635
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.10044
Lingas, Neant, Gaymard, Effect of remdesivir on viral dynamics in COVID-19 hospitalized patients: a modelling analysis of the randomized, controlled, open-label DisCoVeRy trial, J Antimicrob Chemother, doi:10.1093/jac/dkac048
Manzini, Ciccone, Rosa, Convalescent or standard plasma versus standard of care in the treatment of COVID-19 patients with respiratory impairment: short and long-term effects. A three-arm randomized controlled clinical trial, BMC Infect Dis, doi:10.1186/s12879-022-07716-5
Padilla, Polotskaya, Fernandez, Survival benefit of remdesivir in hospitalized COVID-19 patients with high SARS-CoV-2 viral loads and Effects of remdesivir on SARS-CoV-2 viraemia low-grade systemic inflammation, J Antimicrob Chemother, doi:10.1093/jac/dkac144
Rojas, Rodriguez, Hernandez, Safety and efficacy of convalescent plasma for severe COVID-19: a randomized, single blinded, parallel, controlled clinical study, BMC Infect Dis, doi:10.1186/s12879-022-07560-7
Rovito, Bono, Augello, Association between SARS-CoV-2 RNAemia and dysregulated immune response in acutely ill hospitalized COVID-19 patients, Sci Rep, doi:10.1038/s41598-022-23923-1
Van Cleemput, Van Snippenberg, Lambrechts, Organ-specific genome diversity of replication-competent SARS-CoV-2, Nat Commun, doi:10.1038/s41467-021-26884-7
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Williamson, Feldmann, Schwarz, Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2, Nature, doi:10.1038/s41586-020-2423-5
Yoon, Yoo, Lee, Viable SARS-CoV-2 shedding under remdesivir and dexamethasone treatment, J Infect, doi:10.1016/j.jinf.2022.03.022
DOI record:
{
"DOI": "10.1093/jac/dkad295",
"ISSN": [
"0305-7453",
"1460-2091"
],
"URL": "http://dx.doi.org/10.1093/jac/dkad295",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Studies on the antiviral effects of remdesivir have shown conflicting results. SARS-CoV-2 viraemia could identify patients in whom antiviral treatment may be particularly beneficial.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Objectives</jats:title>\n <jats:p>To investigate antiviral effects and clinical outcomes of remdesivir treatment in viraemic patients.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Viraemic patients hospitalized for COVID-19 with ratio of arterial oxygen partial pressure to fractional inspired oxygen of ≤300, symptom duration ≤10 days, and estimated glomerular filtration rate ≥30 mL/min were included in a cohort. The rate of serum viral clearance and serum viral load decline, 60 day mortality and in-hospital outcomes were estimated. A subgroup analysis including patients with symptom duration ≤7 days was performed.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 318 viraemic patients were included. Thirty-three percent (105/318) received remdesivir. The rate of serum viral clearance [subhazard risk ratio (SHR) 1.4 (95% CI 0.9–2.0), P = 0.11] and serum viral load decline (P = 0.11) were not significantly different between remdesivir-treated patients and controls. However, the rate of serum viral clearance was non-significantly higher [SHR 1.6 (95% CI 1.0–2.7), P = 0.051] and the viral load decline was faster (P = 0.03) in remdesivir-treated patients with symptom duration ≤7 days at admission. The 60 day mortality [HR 1.0 (95% CI 0.6–1.8), P = 0.97] and adverse in-hospital outcomes [OR 1.4 (95% CI 0.8–2.4), P = 0.31] were not significantly different between remdesivir-treated patients and controls.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Remdesivir treatment did not significantly change the duration of SARS-CoV-2 viraemia, decline of serum viral load, 60 day mortality or in-hospital adverse outcomes in patients with ≤10 days of symptoms at admission. Remdesivir appeared to reduce the duration of viraemia in a subgroup of patients with ≤7 days of symptoms at admission.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5965-6130",
"affiliation": [
{
"name": "Department of Infectious Diseases, Sahlgrenska University Hospital , Diagnosvagen 21, 416 50 Gothenburg , Sweden"
},
{
"name": "Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet , Stockholm , Sweden"
}
],
"authenticated-orcid": false,
"family": "Hagman",
"given": "Karl",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-8539-9820",
"affiliation": [
{
"name": "Department of Infectious Diseases, Danderyd Hospital , Stockholm , Sweden"
}
],
"authenticated-orcid": false,
"family": "Hedenstierna",
"given": "Magnus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Danderyd Hospital , Stockholm , Sweden"
}
],
"family": "Widaeus",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Danderyd Hospital , Stockholm , Sweden"
}
],
"family": "Arvidsson",
"given": "Emelie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Microbiology, Karolinska University Hospital , Stockholm Sweden"
}
],
"family": "Hammas",
"given": "Berit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Microbiology, Karolinska University Hospital , Stockholm Sweden"
}
],
"family": "Grillner",
"given": "Lena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5829-1803",
"affiliation": [
{
"name": "Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet , Stockholm , Sweden"
},
{
"name": "Department of Anaesthesia and Intensive Care, Danderyd Hospital , Stockholm , Sweden"
}
],
"authenticated-orcid": false,
"family": "Jakobsson",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Danderyd Hospital , Stockholm , Sweden"
}
],
"family": "Gille-Johnson",
"given": "Patrik",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5508-9327",
"affiliation": [
{
"name": "Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet , Stockholm , Sweden"
},
{
"name": "Department of Infectious Diseases, Danderyd Hospital , Stockholm , Sweden"
}
],
"authenticated-orcid": false,
"family": "Ursing",
"given": "Johan",
"sequence": "additional"
}
],
"container-title": "Journal of Antimicrobial Chemotherapy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
9,
18
]
],
"date-time": "2023-09-18T21:07:12Z",
"timestamp": 1695071232000
},
"deposited": {
"date-parts": [
[
2023,
9,
27
]
],
"date-time": "2023-09-27T21:35:05Z",
"timestamp": 1695850505000
},
"funder": [
{
"DOI": "10.13039/501100004722",
"award": [
"FO2017-0482"
],
"doi-asserted-by": "publisher",
"name": "Lars Hierta Memorial Foundation"
},
{
"DOI": "10.13039/501100005689",
"award": [
"GLS-972295"
],
"doi-asserted-by": "publisher",
"name": "Gothenburg Society of Medicine"
}
],
"indexed": {
"date-parts": [
[
2023,
9,
28
]
],
"date-time": "2023-09-28T12:45:24Z",
"timestamp": 1695905124891
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
9,
26
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
26
]
],
"date-time": "2023-09-26T00:00:00Z",
"timestamp": 1695686400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkad295/51771535/dkad295.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkad295/51771535/dkad295.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
9,
26
]
]
},
"published-online": {
"date-parts": [
[
2023,
9,
26
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1074/jbc.RA120.013679",
"article-title": "Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "6785",
"journal-title": "J Biol Chem",
"key": "2023092721025247600_dkad295-B1",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(22)00519-0",
"article-title": "Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO solidarity randomised trial and updated meta-analyses",
"author": "Consortium WHOST",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "Lancet",
"key": "2023092721025247600_dkad295-B2",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 - final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "2023092721025247600_dkad295-B3",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "2023092721025247600_dkad295-B4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkac144",
"article-title": "Survival benefit of remdesivir in hospitalized COVID-19 patients with high SARS-CoV-2 viral loads and low-grade systemic inflammation",
"author": "Padilla",
"doi-asserted-by": "crossref",
"first-page": "2257",
"journal-title": "J Antimicrob Chemother",
"key": "2023092721025247600_dkad295-B5",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkab321",
"article-title": "Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19",
"author": "Garcia-Vidal",
"doi-asserted-by": "crossref",
"first-page": "3296",
"journal-title": "J Antimicrob Chemother",
"key": "2023092721025247600_dkad295-B6",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "2023092721025247600_dkad295-B7",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2021.02.029",
"article-title": "A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel",
"author": "Goldberg",
"doi-asserted-by": "crossref",
"first-page": "917.e1",
"journal-title": "Clin Microbiol Infect",
"key": "2023092721025247600_dkad295-B8",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(21)00485-0",
"article-title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial",
"author": "Ader",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Lancet Infect Dis",
"key": "2023092721025247600_dkad295-B9",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.7326/M21-0653",
"article-title": "Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19 : a randomized trial",
"author": "Barratt-Due",
"doi-asserted-by": "crossref",
"first-page": "1261",
"journal-title": "Ann Intern Med",
"key": "2023092721025247600_dkad295-B10",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27598",
"article-title": "Remdesivir significantly reduces SARS-CoV-2 viral load on nasopharyngeal swabs in hospitalized patients with COVID-19: a retrospective case-control study",
"author": "Biancofiore",
"doi-asserted-by": "crossref",
"first-page": "2284",
"journal-title": "J Med Virol",
"key": "2023092721025247600_dkad295-B11",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.3346/jkms.2021.36.e83",
"article-title": "Clinical and virologic effectiveness of remdesivir treatment for severe coronavirus disease 2019 (COVID-19) in Korea: a nationwide multicenter retrospective cohort study",
"author": "Joo",
"doi-asserted-by": "crossref",
"first-page": "e83",
"journal-title": "J Korean Med Sci",
"key": "2023092721025247600_dkad295-B12",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkac048",
"article-title": "Effect of remdesivir on viral dynamics in COVID-19 hospitalized patients: a modelling analysis of the randomized, controlled, open-label DisCoVeRy trial",
"author": "Lingas",
"doi-asserted-by": "crossref",
"first-page": "1404",
"journal-title": "J Antimicrob Chemother",
"key": "2023092721025247600_dkad295-B13",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2423-5",
"article-title": "Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "Nature",
"key": "2023092721025247600_dkad295-B14",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2022.03.022",
"article-title": "Viable SARS-CoV-2 shedding under remdesivir and dexamethasone treatment",
"author": "Yoon",
"doi-asserted-by": "crossref",
"first-page": "834",
"journal-title": "J Infect",
"key": "2023092721025247600_dkad295-B15",
"volume": "84",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofac463",
"article-title": "Correlation of SARS-CoV-2 nasopharyngeal CT values with viremia and mortality in adults hospitalized with COVID-19",
"author": "Hagman",
"doi-asserted-by": "crossref",
"first-page": "ofac463",
"journal-title": "Open Forum Infect Dis",
"key": "2023092721025247600_dkad295-B16",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-23923-1",
"article-title": "Association between SARS-CoV-2 RNAemia and dysregulated immune response in acutely ill hospitalized COVID-19 patients",
"author": "Rovito",
"doi-asserted-by": "crossref",
"first-page": "19658",
"journal-title": "Sci Rep",
"key": "2023092721025247600_dkad295-B17",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiac157",
"article-title": "Plasma SARS-CoV-2 RNA levels as a biomarker of lower respiratory tract SARS-CoV-2 infection in critically ill patients with COVID-19",
"author": "Jacobs",
"doi-asserted-by": "crossref",
"first-page": "2089",
"journal-title": "J Infect Dis",
"key": "2023092721025247600_dkad295-B18",
"volume": "226",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciaa1285",
"article-title": "Severe acute respiratory syndrome coronavirus 2 RNA in serum as predictor of severe outcome in coronavirus disease 2019: a retrospective cohort study",
"author": "Hagman",
"doi-asserted-by": "crossref",
"first-page": "e2995",
"journal-title": "Clin Infect Dis",
"key": "2023092721025247600_dkad295-B19",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/j.diagmicrobio.2021.115595",
"article-title": "Duration of SARS-CoV-2 viremia and its correlation to mortality and inflammatory parameters in patients hospitalized for COVID-19: a cohort study",
"author": "Hagman",
"doi-asserted-by": "crossref",
"first-page": "115595",
"journal-title": "Diagn Microbiol Infect Dis",
"key": "2023092721025247600_dkad295-B20",
"volume": "102",
"year": "2022"
},
{
"DOI": "10.1172/JCI148635",
"article-title": "SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "e148635",
"journal-title": "J Clin Invest",
"key": "2023092721025247600_dkad295-B21",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "2023092721025247600_dkad295-B22",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1126/science.abi5273",
"article-title": "Estimating infectiousness throughout SARS-CoV-2 infection course",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "eabi5273",
"journal-title": "Science",
"key": "2023092721025247600_dkad295-B23",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab686",
"article-title": "SARS-CoV-2 viremia is associated with COVID-19 severity and predicts clinical outcomes",
"author": "Jacobs",
"doi-asserted-by": "crossref",
"first-page": "1525",
"journal-title": "Clin Infect Dis",
"key": "2023092721025247600_dkad295-B24",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-26884-7",
"article-title": "Organ-specific genome diversity of replication-competent SARS-CoV-2",
"author": "Van Cleemput",
"doi-asserted-by": "crossref",
"first-page": "6612",
"journal-title": "Nat Commun",
"key": "2023092721025247600_dkad295-B25",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2021.02.014",
"article-title": "Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19",
"author": "Folgueira",
"doi-asserted-by": "crossref",
"first-page": "886",
"journal-title": "Clin Microbiol Infect",
"key": "2023092721025247600_dkad295-B26",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1316",
"article-title": "Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in blood of patients with coronavirus disease 2019 (COVID-19): what does it mean?",
"author": "Jacobs",
"doi-asserted-by": "crossref",
"first-page": "e2898",
"journal-title": "Clin Infect Dis",
"key": "2023092721025247600_dkad295-B27",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.10044",
"article-title": "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "460",
"journal-title": "JAMA",
"key": "2023092721025247600_dkad295-B28",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m3939",
"article-title": "Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial)",
"author": "Agarwal",
"doi-asserted-by": "crossref",
"first-page": "m3939",
"journal-title": "BMJ",
"key": "2023092721025247600_dkad295-B29",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "2023092721025247600_dkad295-B30",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "2023092721025247600_dkad295-B31",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1186/s12879-022-07560-7",
"article-title": "Safety and efficacy of convalescent plasma for severe COVID-19: a randomized, single blinded, parallel, controlled clinical study",
"author": "Rojas",
"doi-asserted-by": "crossref",
"first-page": "575",
"journal-title": "BMC Infect Dis",
"key": "2023092721025247600_dkad295-B32",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1186/s12879-022-07716-5",
"article-title": "Convalescent or standard plasma versus standard of care in the treatment of COVID-19 patients with respiratory impairment: short and long-term effects. A three-arm randomized controlled clinical trial",
"author": "Manzini",
"doi-asserted-by": "crossref",
"first-page": "879",
"journal-title": "BMC Infect Dis",
"key": "2023092721025247600_dkad295-B33",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1136/bmjopen-2020-048337",
"article-title": "Convalescent plasma for treatment of COVID-19: study protocol for an open randomised controlled trial in Sweden",
"author": "Dillner",
"doi-asserted-by": "crossref",
"first-page": "e048337",
"journal-title": "BMJ Open",
"key": "2023092721025247600_dkad295-B34",
"volume": "11",
"year": "2021"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jac/advance-article/doi/10.1093/jac/dkad295/7283037"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Effects of remdesivir on SARS-CoV-2 viral dynamics and mortality in viraemic patients hospitalized for COVID-19",
"type": "journal-article"
}