Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial)
et al., BMJ, doi:10.1136/bmj.m3939, PLACID, CTRI/2020/04/024775, Oct 2020
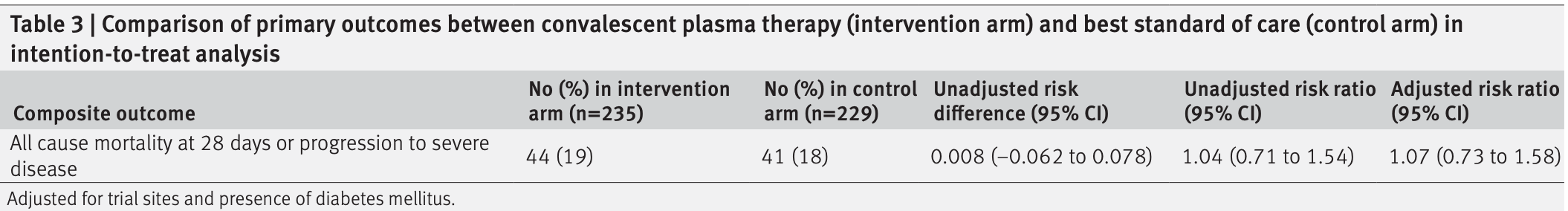
RCT 464 hospitalized patients in India, 235 treated with convalescent plasma, showing no improvement in combined death at 28 days or progression to severe disease.
|
risk of death, 7.0% higher, RR 1.07, p = 0.74, treatment 34 of 235 (14.5%), control 31 of 229 (13.5%).
|
|
combined death at 28 days or progression to severe disease, 7.0% higher, RR 1.07, p = 0.74, treatment 44 of 235 (18.7%), control 41 of 229 (17.9%).
|
|
risk of mechanical ventilation, 1.0% lower, RR 0.99, p = 0.98, treatment 19 of 227 (8.4%), control 19 of 224 (8.5%), NNT 892.
|
|
risk of no viral clearance, 28.0% lower, RR 0.72, p = 0.02, treatment 56 of 173 (32.4%), control 76 of 169 (45.0%), NNT 7.9, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Agarwal et al., 22 Oct 2020, Randomized Controlled Trial, India, peer-reviewed, 6 authors, study period 22 April, 2020 - 14 July, 2020, average treatment delay 8.0 days, trial CTRI/2020/04/024775 (PLACID).
Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial)
BMJ, doi:10.1136/bmj.m3939
OBJECTIVE To investigate the effectiveness of using convalescent plasma to treat moderate coronavirus disease 2019 (covid-19) in adults in India.
DESIGN Open label, parallel arm, phase II, multicentre, randomised controlled trial. SETTING 39 public and private hospitals across India. PARTICIPANTS 464 adults (≥18 years) admitted to hospital (screened 22 April to 14 July 2020) with confirmed moderate covid-19 (partial pressure of oxygen in arterial blood/ fraction of inspired oxygen (PaO 2 /FiO 2 ) ratio between 200 mm Hg and 300 mm Hg or a respiratory rate of more than 24/min with oxygen saturation 93% or less on room air): 235 were assigned to convalescent plasma with best standard of care (intervention arm) and 229 to best standard of care only (control arm).
INTERVENTIONS Participants in the intervention arm received two doses of 200 mL convalescent plasma, transfused 24 hours apart. The presence and levels of neutralising antibodies were not measured a priori; stored samples were assayed at the end of the study.
MAIN OUTCOME MEASURE Composite of progression to severe disease (PaO 2 / FiO 2 <100 mm Hg) or all cause mortality at 28 days post-enrolment.
RESULTS Progression to severe disease or all cause mortality at 28 days after enrolment occurred in 44 (19%) participants in the intervention arm and 41 (18%) in the control arm (risk difference 0.008 (95% confidence interval −0.062 to 0.078); risk ratio 1.04, 95% confidence interval 0.71 to 1.54).
CONCLUSION Convalescent plasma was not associated with a reduction in progression to severe covid-19 or all cause mortality. This trial has high generalisability and approximates convalescent plasma use in real life settings with limited laboratory capacity. A priori measurement of neutralising antibody titres in donors and participants might further clarify the role of convalescent plasma in the management of covid-19.
Supplementary information: Additional tables and figures Supplementary information: Detailed list of PLACID Trial collaborators
References
Abolghasemi, Eshghi, Cheraghali, Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study, Transfus Apher Sci, doi:10.1016/j.transci.2020.102875
Bl, Samk, Vr, As, Pb et al., Patient enrolment, conduct of study, clinical care and data collection: Madras Medical College
Casadevall, Joyner, Pirofski, SARS-CoV-2 viral load and antibody responses: the case for convalescent plasma therapy, J Clin Invest, doi:10.1172/JCI139760
Cheng, Wong, Soo, Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-004-1271-9
Devasenapathy, Ye, Loeb, Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and metaanalysis, CMAJ, doi:10.1503/cmaj.200642
Edwards, Aronson, Adverse drug reactions: definitions, diagnosis, and management, Lancet, doi:10.1016/S0140-6736(00)02799-9
Epstein, Burnouf, Points to consider in the preparation and transfusion of COVID-19 convalescent plasma, Vox Sang, doi:10.1111/vox.12939
Ford, Pragmatic Trials, N Engl J Med, doi:10.1056/NEJMra1510059
Gharbharan, Jordans, Kessel, Convalescent Plasma for COVID-19. A randomized clinical trial, medRxiv
Hegerova, Gooley, Sweerus, Use of convalescent plasma in hospitalized patients with COVID-19: case series, Blood, doi:10.1182/blood.2020006964
Joyner, Bruno, Klassen, Safety Update: COVID-19 Convalescent Plasma in 20 000 Hospitalized Patients, Mayo Clin Proc
Joyner, Senefeld, Klassen, Effect of convalescent plasma on mortality among hospitalized patients with covid-19: initial three-month experience, doi:10.1101/2020.08.12.20169359v1.full.pdf
Khan, Hizbullah, Jain, Coronavirus pandemic fuels blackmarket for plasma of recovered patients, India Today
Li, Zhang, Hu, Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.10044
Long, Tang, Shi, Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections, Nat Med, doi:10.1038/s41591-020-0965-6
Luke, Casadevall, Watowich, Hoffman, Beigel et al., Hark back: passive immunotherapy for influenza and other serious infections, Crit Care Med, doi:10.1097/CCM.0b013e3181d44c1e
Mm ;, Gk, Pc, Tb, Sd et al., Data Management Team: KK, RS. Generation of randomisation sequence: VSK. Central Implementation Team: AA, AM, GK, AT. Laboratory Analysis Team
Norton, Miller, Kleinman, Computing adjusted risk ratios and risk differences in Stata, Stata J, doi:10.1177/1536867X1301300304
Piechotta, Chai, Valk, Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review, Cochrane Database Syst Rev
Robbiani, Gaebler, Muecksch, Convergent antibody responses to SARS-CoV-2 in convalescent individuals, Nature, doi:10.1038/s41586-020-2456-9
Rojas, Rodríguez, Monsalve, Convalescent plasma in Covid-19: Possible mechanisms of action, Autoimmun Rev, doi:10.1016/j.autrev.2020.102554
Sarkale, Patil, Yadav, First isolation of SARS-CoV-2 from clinical samples in India, Indian J Med Res
Shen, Wang, Zhao, Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma, JAMA, doi:10.1001/jama.2020.4783
Van Griensven, Edwards, De Lamballerie, Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea, N Engl J Med, doi:10.1056/NEJMoa1511812
Wu, Wang, Liu, Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications, medRxiv
Xia, Li, Wu, Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion, Blood, doi:10.1182/blood.2020007079
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1136/bmj.m3939",
"ISSN": [
"1756-1833"
],
"URL": "http://dx.doi.org/10.1136/bmj.m3939",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Objective</jats:title><jats:p>To investigate the effectiveness of using convalescent plasma to treat moderate coronavirus disease 2019 (covid-19) in adults in India.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>Open label, parallel arm, phase II, multicentre, randomised controlled trial.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>39 public and private hospitals across India.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>464 adults (≥18 years) admitted to hospital (screened 22 April to 14 July 2020) with confirmed moderate covid-19 (partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO<jats:sub>2</jats:sub>/FiO<jats:sub>2</jats:sub>) ratio between 200 mm Hg and 300 mm Hg or a respiratory rate of more than 24/min with oxygen saturation 93% or less on room air): 235 were assigned to convalescent plasma with best standard of care (intervention arm) and 229 to best standard of care only (control arm).</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Participants in the intervention arm received two doses of 200 mL convalescent plasma, transfused 24 hours apart. The presence and levels of neutralising antibodies were not measured a priori; stored samples were assayed at the end of the study.</jats:p></jats:sec><jats:sec><jats:title>Main outcome measure</jats:title><jats:p>Composite of progression to severe disease (PaO<jats:sub>2</jats:sub>/FiO<jats:sub>2</jats:sub><100 mm Hg) or all cause mortality at 28 days post-enrolment.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Progression to severe disease or all cause mortality at 28 days after enrolment occurred in 44 (19%) participants in the intervention arm and 41 (18%) in the control arm (risk difference 0.008 (95% confidence interval −0.062 to 0.078); risk ratio 1.04, 95% confidence interval 0.71 to 1.54).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Convalescent plasma was not associated with a reduction in progression to severe covid-19 or all cause mortality. This trial has high generalisability and approximates convalescent plasma use in real life settings with limited laboratory capacity. A priori measurement of neutralising antibody titres in donors and participants might further clarify the role of convalescent plasma in the management of covid-19.</jats:p></jats:sec><jats:sec><jats:title>Trial registration</jats:title><jats:p>Clinical Trial Registry of India CTRI/2020/04/024775.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmj.m3939"
],
"author": [
{
"affiliation": [],
"family": "Agarwal",
"given": "Anup",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7298-5097",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mukherjee",
"given": "Aparna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Gunjan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chatterjee",
"given": "Pranab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhatnagar",
"given": "Tarun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malhotra",
"given": "Pankaj",
"sequence": "additional"
}
],
"container-title": "BMJ",
"container-title-short": "BMJ",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2020,
10,
23
]
],
"date-time": "2020-10-23T04:30:11Z",
"timestamp": 1603427411000
},
"deposited": {
"date-parts": [
[
2022,
11,
24
]
],
"date-time": "2022-11-24T10:11:45Z",
"timestamp": 1669284705000
},
"indexed": {
"date-parts": [
[
2024,
4,
1
]
],
"date-time": "2024-04-01T05:07:45Z",
"timestamp": 1711948065682
},
"is-referenced-by-count": 464,
"issued": {
"date-parts": [
[
2020,
10,
22
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
22
]
],
"date-time": "2020-10-22T00:00:00Z",
"timestamp": 1603324800000
}
}
],
"link": [
{
"URL": "http://data.bmj.org/tdm/10.1136/bmj.m3939",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmj.m3939",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "m3939",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2020,
10,
22
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
22
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1172/JCI139760",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.1"
},
{
"DOI": "10.1016/j.autrev.2020.102554",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.2"
},
{
"DOI": "10.1097/CCM.0b013e3181d44c1e",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.3"
},
{
"DOI": "10.1056/NEJMoa1511812",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.4"
},
{
"DOI": "10.1007/s10096-004-1271-9",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.5"
},
{
"DOI": "10.1503/cmaj.200642",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.6"
},
{
"DOI": "10.1038/s41586-020-2456-9",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.7"
},
{
"DOI": "10.1001/jama.2020.4783",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.8"
},
{
"DOI": "10.1016/j.transci.2020.102875",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.9"
},
{
"DOI": "10.1182/blood.2020006964",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.10"
},
{
"DOI": "10.1182/blood.2020007079",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.11"
},
{
"key": "2020102221301436000_371.oct22_8.m3939.12",
"unstructured": "Joyner MJ, Bruno KA, Klassen SA, et al. Safety Update: COVID-19 Convalescent Plasma in 20 000 Hospitalized Patients. Mayo Clin Proc 2020 Jul 19; www.ncbi.nlm.nih.gov/pmc/articles/PMC7368917/ Accessed on 25 Aug 2020."
},
{
"DOI": "10.1001/jama.2020.10044",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.13"
},
{
"key": "2020102221301436000_371.oct22_8.m3939.14",
"unstructured": "Gharbharan A Jordans CCE Geurtsvan Kessel C . Convalescent Plasma for COVID-19. A randomized clinical trial. medRxiv 2020; 2020.07.01.20139857."
},
{
"DOI": "10.1002/14651858.cd013600.pub2",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.15"
},
{
"key": "2020102221301436000_371.oct22_8.m3939.16",
"unstructured": "An EU programme of COVID-19convalescent plasma collection and transfusion. https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf Accessed on 17 Aug 2020."
},
{
"key": "2020102221301436000_371.oct22_8.m3939.17",
"unstructured": "Guidelines on Clinical Management of COVID-19 v.3. Ministry of Health and Family Welfare; Government of India. 2020. www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf Accessed on 25 Aug 2020."
},
{
"key": "2020102221301436000_371.oct22_8.m3939.18",
"unstructured": "Department of Health and Family Welfare, Ministry of Health and Family Welfare. Government of India. Gazette of India. https://cdsco.gov.in/opencms/opencms/en/Notifications/Gazette-Notifications/ Accessed on 25 Aug 2020."
},
{
"DOI": "10.1111/vox.12939",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.19"
},
{
"DOI": "10.1101/2020.03.30.20047365",
"doi-asserted-by": "crossref",
"key": "2020102221301436000_371.oct22_8.m3939.20",
"unstructured": "Wu F Wang A Liu M . Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020; 2020.03.30.20047365."
},
{
"DOI": "10.4103/ijmr.IJMR_1029_20",
"article-title": "First isolation of SARS-CoV-2 from clinical samples in India",
"author": "Sarkale",
"doi-asserted-by": "crossref",
"first-page": "244",
"journal-title": "Indian J Med Res",
"key": "2020102221301436000_371.oct22_8.m3939.21",
"volume": "151",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.22"
},
{
"DOI": "10.1016/S0140-6736(00)02799-9",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.23"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.24"
},
{
"DOI": "10.1177/1536867X1301300304",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.25"
},
{
"key": "2020102221301436000_371.oct22_8.m3939.26",
"unstructured": "Joyner MJ Senefeld JW Klassen SA . Effect of convalescent plasma on mortality among hospitalized patients with covid-19: initial three-month experience. MedRxiv. https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1.full.pdf"
},
{
"DOI": "10.1038/s41591-020-0965-6",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.27"
},
{
"DOI": "10.1056/NEJMra1510059",
"doi-asserted-by": "publisher",
"key": "2020102221301436000_371.oct22_8.m3939.28"
},
{
"key": "2020102221301436000_371.oct22_8.m3939.29",
"unstructured": "Central Drugs Standard Control Notice . https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NjA0Mw== Accessed on 25Aug 2020."
},
{
"key": "2020102221301436000_371.oct22_8.m3939.30",
"unstructured": "Khan J, Hizbullah M, Jain N. Coronavirus pandemic fuels black-market for plasma of recovered patients. India Today July 22, 2020. www.indiatoday.in/india/story/exclusive-coronavirus-pandemic-fuels-black-market-for-plasma-of-recovered-patients-1703332-2020-07-22 Accessed on 25 Aug 2020."
}
],
"reference-count": 30,
"references-count": 30,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.738880283.793579811",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.3410/f.738880283.793579632",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.bmj.com/lookup/doi/10.1136/bmj.m3939"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Industrial and Manufacturing Engineering",
"Metals and Alloys",
"Strategy and Management",
"Mechanical Engineering"
],
"subtitle": [],
"title": "Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy"
}