Safety and efficacy of convalescent plasma for severe COVID-19: a randomized, single blinded, parallel, controlled clinical study
et al., BMC Infectious Diseases, doi:10.1186/s12879-022-07560-7, CP-COVID-19, NCT04332835, Jun 2022
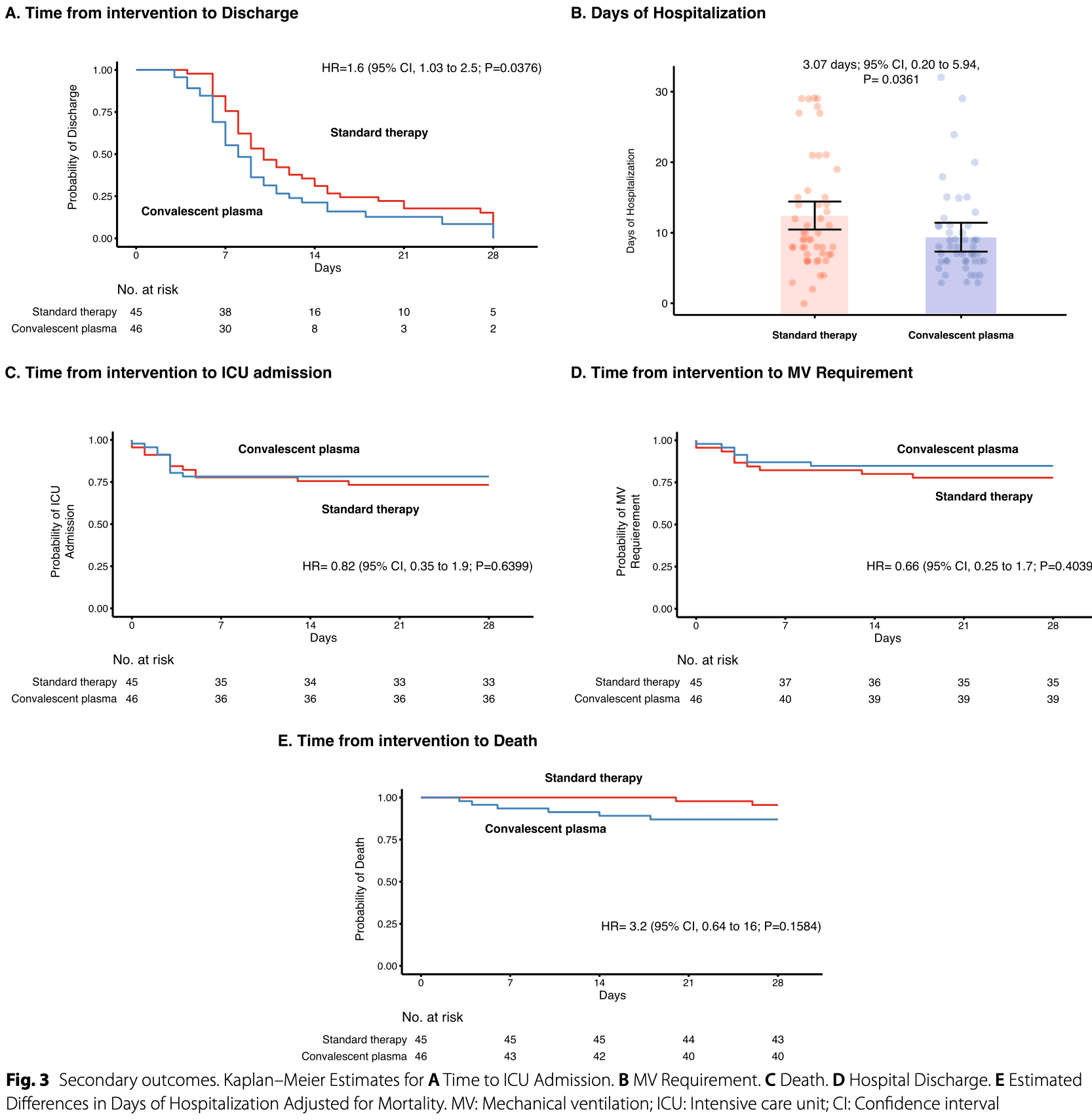
RCT 91 hospitalized patients in Colombia showing shorter time to discharge with convalescent plasma, but higher mortality (without statistical significance).
|
risk of death, 220.0% higher, HR 3.20, p = 0.16, treatment 46, control 45, Cox proportional hazards.
|
|
risk of no hospital discharge, 37.5% lower, HR 0.62, p = 0.04, treatment 46, control 45, inverted to make HR<1 favor treatment, Cox proportional hazards.
|
|
risk of no viral clearance, 25.0% higher, OR 1.25, p = 0.72, treatment 46, control 45, adjusted per study, mid-recovery, day 4, RR approximated with OR.
|
|
risk of no viral clearance, 16.0% higher, OR 1.16, p = 0.82, treatment 46, control 45, adjusted per study, day 7, RR approximated with OR.
|
|
risk of no viral clearance, 51.0% higher, OR 1.51, p = 0.60, treatment 46, control 45, adjusted per study, day 14, RR approximated with OR.
|
|
risk of no viral clearance, 12.0% higher, OR 1.12, p = 0.91, treatment 46, control 45, adjusted per study, day 28, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rojas et al., 27 Jun 2022, Single Blind Randomized Controlled Trial, Colombia, peer-reviewed, 45 authors, study period 8 August, 2020 - 13 November, 2020, average treatment delay 11.0 days, trial NCT04332835 (history) (CP-COVID-19).
Contact: anayajm@gmail.com (corresponding author).
Safety and efficacy of convalescent plasma for severe COVID-19: a randomized, single blinded, parallel, controlled clinical study
BMC Infectious Diseases, doi:10.1186/s12879-022-07560-7
Background: Convalescent plasma (CP) has been widely used to treat COVID-19 and is under study. However, the variability in the current clinical trials has averted its wide use in the current pandemic. We aimed to evaluate the safety and efficacy of CP in severe coronavirus disease 2019 (COVID-19) in the early stages of the disease.
Methods: A randomized controlled clinical study was conducted on 101 patients admitted to the hospital with confirmed severe COVID-19. Most participants had less than 14 days from symptoms onset and less than seven days from hospitalization. Fifty patients were assigned to receive CP plus standard therapy (ST), and 51 were assigned to receive ST alone. Participants in the CP arm received two doses of 250 mL each, transfused 24 h apart. All transfused plasma was obtained from "super donors" that fulfilled the following criteria: titers of anti-SARS-CoV-2 S1 IgG ≥ 1:3200 and IgA ≥ 1:800 antibodies. The effect of transfused anti-IFN antibodies and the SARS-CoV-2 variants at the entry of the study on the overall CP efficacy was evaluated. The primary outcomes were the reduction in viral load and the increase in IgG and IgA antibodies at 28 days of follow-up. The per-protocol analysis included 91 patients. Results: An early but transient increase in IgG anti-S1-SARS-CoV-2 antibody levels at day 4 post-transfusion was observed (Estimated difference [ED], − 1.36; 95% CI, − 2.33 to − 0.39; P = 0.04). However, CP was not associated with viral load reduction in any of the points evaluated. Analysis of secondary outcomes revealed that those patients in the CP arm disclosed a shorter time to discharge (ED adjusted for mortality, 3.1 days; 95% CI, 0.20 to 5.94; P = 0.0361) or a reduction of 2 points on the WHO scale when compared with the ST group (HR adjusted for mortality, 1.6; 95% CI, 1.03
Abbreviations
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12879-022-07560-7. Additional file 1. Lineages and mutations of interest in the studied population.
Author contributions
Declarations Ethics approval and consent to participate The institutional review board of the Universidad del Rosario approved the study design (Act. 421 CEI-UR). Written informed consent was obtained from all participants, and the trial was conducted following the principles stated in the Declaration of Helsinki and Good Clinical Practice guidelines.
Consent for publication All participants provided consent to publish.
Competing interests None.
Author contributions Conceptualization: JMA, JEG, RM; Acquisition of data: YR, JCH, JCD, JAD, VPV, JPM, IA, JTY, OBR, JMPO, DMM, YAA, CRS, PGG JSG, CRP, GSL, NM, LM, RRD, JEO, CRPM, AAB, ARV, CEP; Methodology: JMA, ARV, RM, GSL, JEG, BC; Statistical Analysis: MR, RM, JMA; Funding acquisition: JMA, BC, RM; Procurement of convalescent plasma: PGG, LAL, LDC, JSG, CRP, GSL, BC; Center Coordination: CF; Monitoring: IEO; Variants analysis: NB, LHP, SC, MM, JDR; Anti-IFN antibodies: PB, AG, LB, J-LC; Writing and editing: MR, JMA. All authors read and approved the final manuscript. • fast, convenient online submission • thorough peer review by experienced researchers in your field • rapid publication on acceptance • support for research data, including large and complex data..
References
Acosta-Ampudia, Monsalve, Rojas, Rodríguez, Gallo et al., COVID-19 convalescent plasma composition and immunological effects in severe patients, J Autoimmun
Acosta-Ampudia, Rojas, Monsalve, Rodríguez, Ramírez-Santana et al., Comment on: nature and dimensions of the systemic hyper-inflammation and its attenuation by convalescent plasma in severe COVID-19, J Infect Dis
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ, doi:10.1136/bmj.m4232
Alqahtani, Abdulrahman, Almadani, Alali, Zamrooni et al., Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease, Sci Rep, doi:10.1038/s41598-021-89444-5
Anaya, Monsalve, Rojas, Rodríguez, Montoya-García et al., Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19, J Transl Autoimmun
Avendaño-Solá, Ramos-Martínez, Muñez-Rubio, Ruiz-Antorán, Molina et al., A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia, J Clin Invest, doi:10.1172/JCI152740
Axfors, Janiaud, Schmitt, Hooft, Smith et al., Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials, BMC Infect Dis
Bajpai, Kumar, Maheshwari, Chhabra, Gupta, Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients: A Pilot Randomized Controlled Trial, medRxiv
Bajpai, Maheshwari, Dogra, Kumar, Gupta et al., Efficacy of convalescent plasma therapy in the patient with COVID-19: a randomised control trial (COPLA-II trial), BMJ Open, doi:10.1136/bmjopen-2021-055189
Balcells, Rojas, Corre, Martínez-Valdebenito, Ceballos et al., Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: A randomized phase II clinical trial, PLoS Med, doi:10.1371/journal.pmed.1003415
Bandopadhyay, 'rozario, Lahiri, Sarif, Ray et al., Nature and dimensions of systemic hyperinflammation and its attenuation by convalescent plasma in severe COVID-19, J Infect Dis
Bastard, Gervais, Voyer, Rosain, Philippot et al., Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths, Sci Immunol
Casadevall, Dragotakes, Johnson, Senefeld, Klassen et al., Convalescent plasma use in the USA was inversely correlated with COVID-19 mortality, Elife, doi:10.7554/eLife.69866
Castañeda, Patiño, Muñoz, Ballesteros, Guerrero-Araya et al., Evolution and epidemic spread of SARS-CoV-2 in Colombia: a year into the pandemic, Vaccines
Cepeda-Cuervo, Beta regression models: Joint mean and variance modeling, J Stat Theory Pract
Chang, Feng, Meng, Apostolidis, Mack et al., Newonset IgG autoantibodies in hospitalized patients with COVID-19, Nat Commun
Cárdenas-Turanzas, Ensor, Wakefield, Zhang, Wallace et al., Cross-validation of a Sequential Organ Failure Assessment scorebased model to predict mortality in patients with cancer admitted to the intensive care unit, J Crit Care
Diggle, Liang, Zeger, Longitudinal data analysis
Duan, Liu, Li, Zhang, Yu et al., Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci
Esmaeili, Esmaeili, Pourpak, Immunological effects of convalescent plasma therapy for coronavirus: a scoping review, BMC Infect Dis
Fitzmaurice, Davidian, Verbeke, Molenberghs, Longitudinal data analysis
Fitzmaurice, Laird, Ware, Applied longitudinal analysis
Foix, López, Díez-Fuertes, Mcconnell, Aj, Predicted impact of the viral mutational landscape on the cytotoxic response against SARS-CoV-2, PLoS Comput Biol
Fung, Nambiar, Pandey, Aldrich, Teraoka et al., Treatment of immunocompromised COVID-19 patients with convalescent plasma, Transpl Infect Dis, doi:10.1111/tid.13477
Gharbharan, Jordans, Geurtsvankessel, Hollander, Karim et al., Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat Commun, doi:10.1038/s41467-021-23469-2
Goldstein, Multilevel mixed linear model analysis using iterative generalized least squares, Biometrika
Harvey, Carabelli, Jackson, Gupta, Thomson et al., SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol
Joyner, Carter, Senefeld, Klassen, Mills et al., Convalescent plasma antibody levels and the risk of death from Covid-19
Li, Wu, Nie, Zhang, Hao et al., The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity, Cell
Li, Zhang, Hu, Tong, Zheng et al., Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA
Libster, Marc, Wappner, Coviello, Bianchi et al., Early high-titer plasma therapy to prevent severe covid-19 in older adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Naveca, Nascimento, De Souza, Corado, Nascimento et al., COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P1 emergence, Nat Med
O'toole, Hill, Pybus, Watts, Bogoch et al., Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2 with grinch, Wellcome Open Res, doi:10.12688/wellcomeopenres.16661.2
Okba, Müller, Wang, Geurtsvankessel, Corman, Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease patients, Emerg Infect Dis
Park, Tarpey, Liu, Goldfeld, Wu et al., Development and validation of a treatment benefit index to identify hospitalized patients with COVID-19 who may benefit from convalescent plasma, JAMA Netw open
Raadsen, Gharbharan, Jordans, Mykytyn, Lamers et al., Interferon-α2 auto-antibodies in convalescent plasma therapy for COVID-19, J Clin Immunol
Rambaut, Holmes, 'toole, Hill, Mccrone et al., A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology, Nat Microbiol
Rao, Estimation of variance and covariance components-MINQUE theory, J Multivar Anal
Ray, Paul, Bandopadhyay, 'rozario, Sarif et al., A phase 2 single center open label randomised control trial for convalescent plasma therapy in patients with severe COVID-19, Nat Commun, doi:10.1038/s41467-022-28064-7
Rodionov, Biener, Spieth, Achleitner, Hölig et al., Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID-19, The Lancet Microbe
Rojas, Anaya, Why will it never be known if convalescent plasma is effective for COVID-19, J Transl Autoimmun
Rojas, Rodríguez, Monsalve, Acosta-Ampudia, Camacho et al., Convalescent plasma in Covid-19: Possible mechanisms of action, Autoimmun Rev
Rs, RStudio: Integrated Development for R. RStudio
Savvateeva, Filippova, Valuev-Elliston, Nuralieva, Yukina et al., Microarray-based detection of antibodies against SARS-CoV-2 proteins, common respiratory viruses and type i interferons, Viruses
Shen, Wang, Zhao, Yang, Li et al., Treatment of 5 critically ill patients with COVID-19 with convalescent plasma, JAMA
Simonovich, Pratx, Scibona, Beruto, Vallone et al., A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia, N Engl J Med
Sullivan, Gebo, Shoham, Bloch, Lau et al., Early outpatient treatment for covid-19 with convalescent plasma, N Engl J Med, doi:10.1056/NEJMoa2119657
Theel, Harring, Hilgart, Granger, Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2, J Clin Microbiol
Tonn, Corman, Johnsen, Richter, Rodionov et al., Stability and neutralising capacity of SARS-CoV-2-specific antibodies in convalescent plasma, Lancet Microbe
Trujillo, Consenso colombiano de atención, diagnóstico y manejo de la infección por SARS-COV-2/COVID 19 en establecimientos de atención de la salud Recomendaciones basadas en consenso de expertos e informadas en la evidencia, Infect
Verbeke, Linear mixed models for longitudinal data
Weidner, Gänsdorfer, Unterweger, Weseslindtner, Drexler et al., Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays, J Clin Virol Off Publ Pan Am Soc Clin Virol
DOI record:
{
"DOI": "10.1186/s12879-022-07560-7",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-022-07560-7",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Convalescent plasma (CP) has been widely used to treat COVID-19 and is under study. However, the variability in the current clinical trials has averted its wide use in the current pandemic. We aimed to evaluate the safety and efficacy of CP in severe coronavirus disease 2019 (COVID-19) in the early stages of the disease.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A randomized controlled clinical study was conducted on 101 patients admitted to the hospital with confirmed severe COVID-19. Most participants had less than 14 days from symptoms onset and less than seven days from hospitalization. Fifty patients were assigned to receive CP plus standard therapy (ST), and 51 were assigned to receive ST alone. Participants in the CP arm received two doses of 250 mL each, transfused 24 h apart. All transfused plasma was obtained from \"super donors\" that fulfilled the following criteria: titers of anti-SARS-CoV-2 S1 IgG ≥ 1:3200 and IgA ≥ 1:800 antibodies. The effect of transfused anti-IFN antibodies and the SARS-CoV-2 variants at the entry of the study on the overall CP efficacy was evaluated. The primary outcomes were the reduction in viral load and the increase in IgG and IgA antibodies at 28 days of follow-up. The per-protocol analysis included 91 patients.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>An early but transient increase in IgG anti-S1-SARS-CoV-2 antibody levels at day 4 post-transfusion was observed (Estimated difference [ED], − 1.36; 95% CI, − 2.33 to − 0.39; P = 0.04). However, CP was not associated with viral load reduction in any of the points evaluated. Analysis of secondary outcomes revealed that those patients in the CP arm disclosed a shorter time to discharge (ED adjusted for mortality, 3.1 days; 95% CI, 0.20 to 5.94; P = 0.0361) or a reduction of 2 points on the WHO scale when compared with the ST group (HR adjusted for mortality, 1.6; 95% CI, 1.03 to 2.5; P = 0.0376). There were no benefits from CP on the rates of intensive care unit admission (HR, 0.82; 95% CI, 0.35 to 1.9; P = 0.6399), mechanical ventilation (HR, 0.66; 95% CI, 0.25 to 1.7; P = 0.4039), or mortality (HR, 3.2; 95% CI, 0.64 to 16; P = 0.1584). Anti-IFN antibodies and SARS-CoV-2 variants did not influence these results.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>CP was not associated with viral load reduction, despite the early increase in IgG anti-SARS-CoV-2 antibodies. However, CP is safe and could be a therapeutic option to reduce the hospital length of stay.</jats:p>\n <jats:p><jats:italic>Trial registration</jats:italic> NCT04332835\n</jats:p>\n </jats:sec>",
"alternative-id": [
"7560"
],
"article-number": "575",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "6 March 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "25 May 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "27 June 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The institutional review board of the Universidad del Rosario approved the study design (Act. 421 CEI-UR). Written informed consent was obtained from all participants, and the trial was conducted following the principles stated in the Declaration of Helsinki and Good Clinical Practice guidelines."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "All participants provided consent to publish."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "None."
},
{
"group": {
"label": "Author contributions",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "Conceptualization: JMA, JEG, RM; Acquisition of data: YR, JCH, JCD, JAD, VPV, JPM, IA, JTY, OBR, JMPO, DMM, YAA, CRS, PGG JSG, CRP, GSL, NM, LM, RRD, JEO, CRPM, AAB, ARV, CEP; Methodology: JMA, ARV, RM, GSL, JEG, BC; Statistical Analysis: MR, RM, JMA; Funding acquisition: JMA, BC, RM; Procurement of convalescent plasma: PGG, LAL, LDC, JSG, CRP, GSL, BC; Center Coordination: CF; Monitoring: IEO; Variants analysis: NB, LHP, SC, MM, JDR; Anti-IFN antibodies: PB, AG, LB, J-LC; Writing and editing: MR, JMA. All authors read and approved the final manuscript."
}
],
"author": [
{
"affiliation": [],
"family": "Rojas",
"given": "Manuel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rodríguez",
"given": "Yhojan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández",
"given": "Juan Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Díaz-Coronado",
"given": "Juan C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vergara",
"given": "José Alejandro Daza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vélez",
"given": "Verónica Posada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mancilla",
"given": "Jessica Porras",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Araujo",
"given": "Iván",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yepes",
"given": "Jairo Torres",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ricaurte",
"given": "Oscar Briceño",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pardo-Oviedo",
"given": "Juan Mauricio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monsalve",
"given": "Diana M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Acosta-Ampudia",
"given": "Yeny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramírez-Santana",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García",
"given": "Paula Gaviria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landinez",
"given": "Lina Acevedo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Correales",
"given": "Luisa Duarte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grass",
"given": "Jeser Santiago",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez",
"given": "Cristian Ricaurte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López",
"given": "Gustavo Salguero",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mateus",
"given": "Nataly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mancera",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devia",
"given": "Ronald Rengifo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orjuela",
"given": "Juan Esteban",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parra-Moreno",
"given": "Christian R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buitrago",
"given": "Andrés Alfonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ordoñez",
"given": "Inés Elvira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osorio",
"given": "Claudia Fabra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ballesteros",
"given": "Nathalia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patiño",
"given": "Luz H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castañeda",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muñoz",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramírez",
"given": "Juan David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bastard",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gervais",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bizien",
"given": "Lucy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casanova",
"given": "Jean-Laurent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camacho",
"given": "Bernardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallo",
"given": "Juan Esteban",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rojas-Villarraga",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez",
"given": "Carlos E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manrique",
"given": "Rubén",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mantilla",
"given": "Rubén D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anaya",
"given": "Juan-Manuel",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
6,
27
]
],
"date-time": "2022-06-27T06:03:13Z",
"timestamp": 1656309793000
},
"deposited": {
"date-parts": [
[
2022,
6,
27
]
],
"date-time": "2022-06-27T06:03:25Z",
"timestamp": 1656309805000
},
"funder": [
{
"DOI": "10.13039/501100008793",
"award": [
"ABN011",
"ABN011",
"ABN011",
"ABN011",
"ABN011",
"ABN011"
],
"doi-asserted-by": "publisher",
"name": "Universidad del Rosario"
},
{
"name": "ISA group"
},
{
"name": "Suramericana"
},
{
"name": "IDCBIS"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
21
]
],
"date-time": "2022-12-21T02:35:29Z",
"timestamp": 1671590129820
},
"is-referenced-by-count": 1,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
6,
27
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
27
]
],
"date-time": "2022-06-27T00:00:00Z",
"timestamp": 1656288000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
27
]
],
"date-time": "2022-06-27T00:00:00Z",
"timestamp": 1656288000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07560-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-022-07560-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07560-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
6,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.autrev.2020.102554",
"author": "M Rojas",
"doi-asserted-by": "publisher",
"journal-title": "Autoimmun Rev",
"key": "7560_CR1",
"unstructured": "Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev. 2020;19: 102554.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.jaut.2021.102598",
"author": "Y Acosta-Ampudia",
"doi-asserted-by": "publisher",
"journal-title": "J Autoimmun",
"key": "7560_CR2",
"unstructured": "Acosta-Ampudia Y, Monsalve DM, Rojas M, Rodríguez Y, Gallo JE, Salazar-Uribe JC, et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J Autoimmun. 2021;118: 102598.",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiab137",
"author": "Y Acosta-Ampudia",
"doi-asserted-by": "publisher",
"first-page": "1833",
"journal-title": "J Infect Dis",
"key": "7560_CR3",
"unstructured": "Acosta-Ampudia Y, Rojas M, Monsalve DM, Rodríguez Y, Ramírez-Santana C, Anaya J-M. Comment on: nature and dimensions of the systemic hyper-inflammation and its attenuation by convalescent plasma in severe COVID-19. J Infect Dis. 2021;223:1833–4.",
"volume": "223",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiab010",
"author": "P Bandopadhyay",
"doi-asserted-by": "publisher",
"first-page": "565",
"journal-title": "J Infect Dis",
"key": "7560_CR4",
"unstructured": "Bandopadhyay P, D’Rozario R, Lahiri A, Sarif J, Ray Y, Paul SR, et al. Nature and dimensions of systemic hyperinflammation and its attenuation by convalescent plasma in severe COVID-19. J Infect Dis. 2021;224:565–74.",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06981-0",
"author": "B Esmaeili",
"doi-asserted-by": "publisher",
"first-page": "1278",
"journal-title": "BMC Infect Dis",
"key": "7560_CR5",
"unstructured": "Esmaeili B, Esmaeili S, Pourpak Z. Immunological effects of convalescent plasma therapy for coronavirus: a scoping review. BMC Infect Dis. 2021;21:1278.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06829-7",
"author": "C Axfors",
"doi-asserted-by": "publisher",
"first-page": "1170",
"journal-title": "BMC Infect Dis",
"key": "7560_CR6",
"unstructured": "Axfors C, Janiaud P, Schmitt AM, Hooft J, Smith ER, Haber NA, et al. Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect Dis. 2021;21:1170.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.jtauto.2020.100069",
"author": "M Rojas",
"doi-asserted-by": "publisher",
"journal-title": "J Transl Autoimmun",
"key": "7560_CR7",
"unstructured": "Rojas M, Anaya J-M. Why will it never be known if convalescent plasma is effective for COVID-19. J Transl Autoimmun. 2020;3: 100069.",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.7554/eLife.69866",
"author": "A Casadevall",
"doi-asserted-by": "publisher",
"journal-title": "Elife",
"key": "7560_CR8",
"unstructured": "Casadevall A, Dragotakes Q, Johnson PW, Senefeld JW, Klassen SA, Wright RS, et al. Convalescent plasma use in the USA was inversely correlated with COVID-19 mortality. Elife. 2021. https://doi.org/10.7554/eLife.69866.",
"year": "2021"
},
{
"DOI": "10.1126/sciimmunol.abl4340",
"author": "P Bastard",
"doi-asserted-by": "publisher",
"first-page": "45",
"journal-title": "Sci Immunol.",
"key": "7560_CR9",
"unstructured": "Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol. 2021;6:45.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.jcrc.2012.04.018",
"author": "M Cárdenas-Turanzas",
"doi-asserted-by": "publisher",
"first-page": "673",
"journal-title": "J Crit Care",
"key": "7560_CR10",
"unstructured": "Cárdenas-Turanzas M, Ensor J, Wakefield C, Zhang K, Wallace SK, Price KJ, et al. Cross-validation of a Sequential Organ Failure Assessment score-based model to predict mortality in patients with cancer admitted to the intensive care unit. J Crit Care. 2012;27:673–80.",
"volume": "27",
"year": "2012"
},
{
"DOI": "10.1097/CM9.0000000000000819",
"doi-asserted-by": "publisher",
"key": "7560_CR11",
"unstructured": "Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin Med J (Engl). 2020;133:1087–95. http://doi.org/https://doi.org/10.1097/CM9.0000000000000819."
},
{
"DOI": "10.1016/S2666-5247(20)30037-9",
"author": "T Tonn",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Microbe",
"key": "7560_CR12",
"unstructured": "Tonn T, Corman VM, Johnsen M, Richter A, Rodionov RN, Drosten C, et al. Stability and neutralising capacity of SARS-CoV-2-specific antibodies in convalescent plasma. Lancet Microbe. 2020;1: e63.",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.22354/in.v24i3.851",
"author": "CH Saavedra Trujillo",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Infect",
"key": "7560_CR13",
"unstructured": "Saavedra Trujillo CH. Consenso colombiano de atención, diagnóstico y manejo de la infección por SARS-COV-2/COVID 19 en establecimientos de atención de la salud Recomendaciones basadas en consenso de expertos e informadas en la evidencia. Infect. 2020;24:1.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"author": "World Health Organisation",
"doi-asserted-by": "publisher",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "7560_CR14",
"unstructured": "World Health Organisation. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192.",
"volume": "20",
"year": "2020"
},
{
"author": "ES Theel",
"journal-title": "J Clin Microbiol",
"key": "7560_CR15",
"unstructured": "Theel ES, Harring J, Hilgart H, Granger D. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58: e01243.",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2020.104540",
"author": "L Weidner",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Virol Off Publ Pan Am Soc Clin Virol",
"key": "7560_CR16",
"unstructured": "Weidner L, Gänsdorfer S, Unterweger S, Weseslindtner L, Drexler C, Farcet M, et al. Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2020;129: 104540.",
"volume": "129",
"year": "2020"
},
{
"DOI": "10.3201/eid2607.200841",
"author": "NMA Okba",
"doi-asserted-by": "publisher",
"first-page": "1478",
"journal-title": "Emerg Infect Dis",
"key": "7560_CR17",
"unstructured": "Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–88.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-0770-5",
"author": "A Rambaut",
"doi-asserted-by": "publisher",
"first-page": "1403",
"journal-title": "Nat Microbiol",
"key": "7560_CR18",
"unstructured": "Rambaut A, Holmes EC, O’Toole Á, Hill V, McCrone JT, Ruis C, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–7.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.3390/vaccines9080837",
"author": "S Castañeda",
"doi-asserted-by": "publisher",
"first-page": "56",
"journal-title": "Vaccines",
"key": "7560_CR19",
"unstructured": "Castañeda S, Patiño LH, Muñoz M, Ballesteros N, Guerrero-Araya E, Paredes-Sabja D, et al. Evolution and epidemic spread of SARS-CoV-2 in Colombia: a year into the pandemic. Vaccines. 2021;9:56.",
"volume": "9",
"year": "2021"
},
{
"key": "7560_CR20",
"unstructured": "Global COVID-19 Clinical Platform: Rapid core case report form (CRF). WHO. 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Clinical_CRF-2020.4."
},
{
"DOI": "10.1007/978-1-4612-2294-1_3",
"doi-asserted-by": "crossref",
"key": "7560_CR21",
"unstructured": "Verbeke G. Linear mixed models for longitudinal data. In: Linear mixed models in practice. Springer; 1997. p. 63–153."
},
{
"DOI": "10.1016/0047-259X(71)90001-7",
"author": "CR Rao",
"doi-asserted-by": "publisher",
"first-page": "257",
"journal-title": "J Multivar Anal",
"key": "7560_CR22",
"unstructured": "Rao CR. Estimation of variance and covariance components—MINQUE theory. J Multivar Anal. 1971;1:257–75.",
"volume": "1",
"year": "1971"
},
{
"key": "7560_CR23",
"unstructured": "Team Rs. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, 2020."
},
{
"DOI": "10.1093/biomet/73.1.43",
"author": "H Goldstein",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "Biometrika",
"key": "7560_CR24",
"unstructured": "Goldstein H. Multilevel mixed linear model analysis using iterative generalized least squares. Biometrika. 1986;73:43–56.",
"volume": "73",
"year": "1986"
},
{
"author": "GM Fitzmaurice",
"key": "7560_CR25",
"unstructured": "Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. New York: Wiley; 2012.",
"volume-title": "Applied longitudinal analysis",
"year": "2012"
},
{
"author": "P Diggle",
"first-page": "13",
"journal-title": "New York Oxford Univ Press",
"key": "7560_CR26",
"unstructured": "Diggle P, Liang K-Y, Zeger SL. Longitudinal data analysis. New York Oxford Univ Press. 1994;5:13.",
"volume": "5",
"year": "1994"
},
{
"DOI": "10.1201/9781420011579",
"author": "G Fitzmaurice",
"doi-asserted-by": "publisher",
"key": "7560_CR27",
"unstructured": "Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G. Longitudinal data analysis. New York: CRC Press; 2008.",
"volume-title": "Longitudinal data analysis",
"year": "2008"
},
{
"DOI": "10.1080/15598608.2014.890983",
"author": "E Cepeda-Cuervo",
"doi-asserted-by": "publisher",
"first-page": "134",
"journal-title": "J Stat Theory Pract",
"key": "7560_CR28",
"unstructured": "Cepeda-Cuervo E. Beta regression models: Joint mean and variance modeling. J Stat Theory Pract. 2015;9:134–45.",
"volume": "9",
"year": "2015"
},
{
"DOI": "10.1056/NEJMoa2031893",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "1015",
"journal-title": "N Engl J Med",
"key": "7560_CR29",
"unstructured": "Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384:1015–27. https://doi.org/10.1056/NEJMoa2031893.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031304",
"doi-asserted-by": "crossref",
"key": "7560_CR30",
"unstructured": "Simonovich VA, Burgos Pratx LD, Scibona P, Beruto M V, Vallone MG, Vázquez C, et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2020;:NEJMoa2031304."
},
{
"DOI": "10.1001/jama.2020.4783",
"author": "C Shen",
"doi-asserted-by": "publisher",
"first-page": "1582",
"journal-title": "JAMA",
"key": "7560_CR31",
"unstructured": "Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2004168117",
"author": "K Duan",
"doi-asserted-by": "publisher",
"first-page": "9490",
"journal-title": "Proc Natl Acad Sci",
"key": "7560_CR32",
"unstructured": "Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117:9490–6.",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.10044",
"author": "L Li",
"doi-asserted-by": "publisher",
"first-page": "460",
"journal-title": "JAMA",
"key": "7560_CR33",
"unstructured": "Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–70.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1172/JCI152740",
"author": "C Avendaño-Solá",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Invest",
"key": "7560_CR34",
"unstructured": "Avendaño-Solá C, Ramos-Martínez A, Muñez-Rubio E, Ruiz-Antorán B, Malode Molina R, Torres F, et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021. https://doi.org/10.1172/JCI152740.",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m4232",
"author": "A Agarwal",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "7560_CR35",
"unstructured": "Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371: m4232. https://doi.org/10.1136/bmj.m4232.",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.12688/wellcomeopenres.16661.2",
"author": "Á O’Toole",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Wellcome Open Res.",
"key": "7560_CR36",
"unstructured": "O’Toole Á, Hill V, Pybus OG, Watts A, Bogoch II, Khan K, et al. Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2 with grinch. Wellcome Open Res. 2021;6:121. https://doi.org/10.12688/wellcomeopenres.16661.2.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01378-7",
"author": "FG Naveca",
"doi-asserted-by": "publisher",
"first-page": "1230",
"journal-title": "Nat Med",
"key": "7560_CR37",
"unstructured": "Naveca FG, Nascimento V, de Souza VC, Corado AL, Nascimento F, Silva G, et al. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P1 emergence. Nat Med. 2021;27:1230–8.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1371/journal.pcbi.1009726",
"author": "A Foix",
"doi-asserted-by": "publisher",
"journal-title": "PLoS Comput Biol",
"key": "7560_CR38",
"unstructured": "Foix A, López D, Díez-Fuertes F, McConnell MJ, Martín-Galiano AJ. Predicted impact of the viral mutational landscape on the cytotoxic response against SARS-CoV-2. PLoS Comput Biol. 2022;18: e1009726.",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.07.012",
"author": "Q Li",
"doi-asserted-by": "publisher",
"first-page": "1284",
"journal-title": "Cell",
"key": "7560_CR39",
"unstructured": "Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell. 2020;182:1284-1294.e9.",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"author": "WT Harvey",
"doi-asserted-by": "publisher",
"first-page": "409",
"journal-title": "Nat Rev Microbiol",
"key": "7560_CR40",
"unstructured": "Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–24.",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-28064-7",
"author": "Y Ray",
"doi-asserted-by": "publisher",
"first-page": "383",
"journal-title": "Nat Commun",
"key": "7560_CR41",
"unstructured": "Ray Y, Paul SR, Bandopadhyay P, D’Rozario R, Sarif J, Raychaudhuri D, et al. A phase 2 single center open label randomised control trial for convalescent plasma therapy in patients with severe COVID-19. Nat Commun. 2022;13:383. https://doi.org/10.1038/s41467-022-28064-7.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-89444-5",
"author": "M AlQahtani",
"doi-asserted-by": "publisher",
"first-page": "9927",
"journal-title": "Sci Rep",
"key": "7560_CR42",
"unstructured": "AlQahtani M, Abdulrahman A, Almadani A, Alali SY, Al Zamrooni AM, Hejab AH, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci Rep. 2021;11:9927. https://doi.org/10.1038/s41598-021-89444-5.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1101/2020.10.25.20219337",
"doi-asserted-by": "crossref",
"key": "7560_CR43",
"unstructured": "Bajpai M, Kumar S, Maheshwari A, Chhabra K, kale P, Gupta A, et al. Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients: A Pilot Randomized Controlled Trial. medRxiv. 2020;2020.10.25.20219337."
},
{
"DOI": "10.1038/s41467-021-23469-2",
"author": "A Gharbharan",
"doi-asserted-by": "publisher",
"first-page": "3189",
"journal-title": "Nat Commun",
"key": "7560_CR44",
"unstructured": "Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN, et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021;12:3189. https://doi.org/10.1038/s41467-021-23469-2.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1371/journal.pmed.1003415",
"author": "ME Balcells",
"doi-asserted-by": "publisher",
"journal-title": "PLoS Med",
"key": "7560_CR45",
"unstructured": "Balcells ME, Rojas L, Le Corre N, Martínez-Valdebenito C, Ceballos ME, Ferrés M, et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: A randomized phase II clinical trial. PLoS Med. 2021;18: e1003415. https://doi.org/10.1371/journal.pmed.1003415.",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033700",
"author": "R Libster",
"doi-asserted-by": "publisher",
"first-page": "610",
"journal-title": "N Engl J Med",
"key": "7560_CR46",
"unstructured": "Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N Engl J Med. 2021;384:610–8. https://doi.org/10.1056/NEJMoa2033700.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "2049",
"journal-title": "Lancet (London, England)",
"key": "7560_CR47",
"unstructured": "RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet (London, England). 2021;397:2049–59.",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2119657",
"author": "DJ Sullivan",
"doi-asserted-by": "publisher",
"first-page": "1700",
"journal-title": "N Engl J Med",
"key": "7560_CR48",
"unstructured": "Sullivan DJ, Gebo KA, Shoham S, Bloch EM, Lau B, Shenoy AG, et al. Early outpatient treatment for covid-19 with convalescent plasma. N Engl J Med. 2022;386:1700–11. https://doi.org/10.1056/NEJMoa2119657.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1136/bmjopen-2021-055189",
"author": "M Bajpai",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Open",
"key": "7560_CR49",
"unstructured": "Bajpai M, Maheshwari A, Dogra V, Kumar S, Gupta E, Kale P, et al. Efficacy of convalescent plasma therapy in the patient with COVID-19: a randomised control trial (COPLA-II trial). BMJ Open. 2022;12: e055189. https://doi.org/10.1136/bmjopen-2021-055189.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.47375",
"author": "H Park",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw open",
"key": "7560_CR50",
"unstructured": "Park H, Tarpey T, Liu M, Goldfeld K, Wu Y, Wu D, et al. Development and validation of a treatment benefit index to identify hospitalized patients with COVID-19 who may benefit from convalescent plasma. JAMA Netw open. 2022;5: e2147375.",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1038/s41467-021-25509-3",
"author": "SE Chang",
"doi-asserted-by": "publisher",
"first-page": "5417",
"journal-title": "Nat Commun",
"key": "7560_CR51",
"unstructured": "Chang SE, Feng A, Meng W, Apostolidis SA, Mack E, Artandi M, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12:5417.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.jtauto.2021.100091",
"author": "J-M Anaya",
"doi-asserted-by": "publisher",
"journal-title": "J Transl Autoimmun",
"key": "7560_CR52",
"unstructured": "Anaya J-M, Monsalve DM, Rojas M, Rodríguez Y, Montoya-García N, Mancera-Navarro LM, et al. Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. J Transl Autoimmun. 2021;4: 100091.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.3390/v13122553",
"author": "E Savvateeva",
"doi-asserted-by": "publisher",
"first-page": "89",
"journal-title": "Viruses",
"key": "7560_CR53",
"unstructured": "Savvateeva E, Filippova M, Valuev-Elliston V, Nuralieva N, Yukina M, Troshina E, et al. Microarray-based detection of antibodies against SARS-CoV-2 proteins, common respiratory viruses and type i interferons. Viruses. 2021;13:89.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1007/s10875-021-01168-3",
"author": "MP Raadsen",
"doi-asserted-by": "publisher",
"first-page": "232",
"journal-title": "J Clin Immunol",
"key": "7560_CR54",
"unstructured": "Raadsen MP, Gharbharan A, Jordans CCE, Mykytyn AZ, Lamers MM, van den Doel PB, et al. Interferon-α2 auto-antibodies in convalescent plasma therapy for COVID-19. J Clin Immunol. 2022;42:232–9.",
"volume": "42",
"year": "2022"
},
{
"DOI": "10.1111/tid.13477",
"author": "M Fung",
"doi-asserted-by": "publisher",
"first-page": "e13477",
"journal-title": "Transpl Infect Dis",
"key": "7560_CR55",
"unstructured": "Fung M, Nambiar A, Pandey S, Aldrich JM, Teraoka J, Freise C, et al. Treatment of immunocompromised COVID-19 patients with convalescent plasma. Transpl Infect Dis. 2021;23:e13477–e13477. https://doi.org/10.1111/tid.13477.",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1016/S2666-5247(21)00030-6",
"author": "RN Rodionov",
"doi-asserted-by": "publisher",
"journal-title": "The Lancet Microbe",
"key": "7560_CR56",
"unstructured": "Rodionov RN, Biener A, Spieth P, Achleitner M, Hölig K, Aringer M, et al. Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID-19. The Lancet Microbe. 2021;2: e138.",
"volume": "2",
"year": "2021"
}
],
"reference-count": 56,
"references-count": 56,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-022-07560-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Safety and efficacy of convalescent plasma for severe COVID-19: a randomized, single blinded, parallel, controlled clinical study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "22"
}