Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: A randomized phase II clinical trial
et al., PLOS Medicine, doi:10.1371/journal.pmed.1003415, NCT04375098, Mar 2021
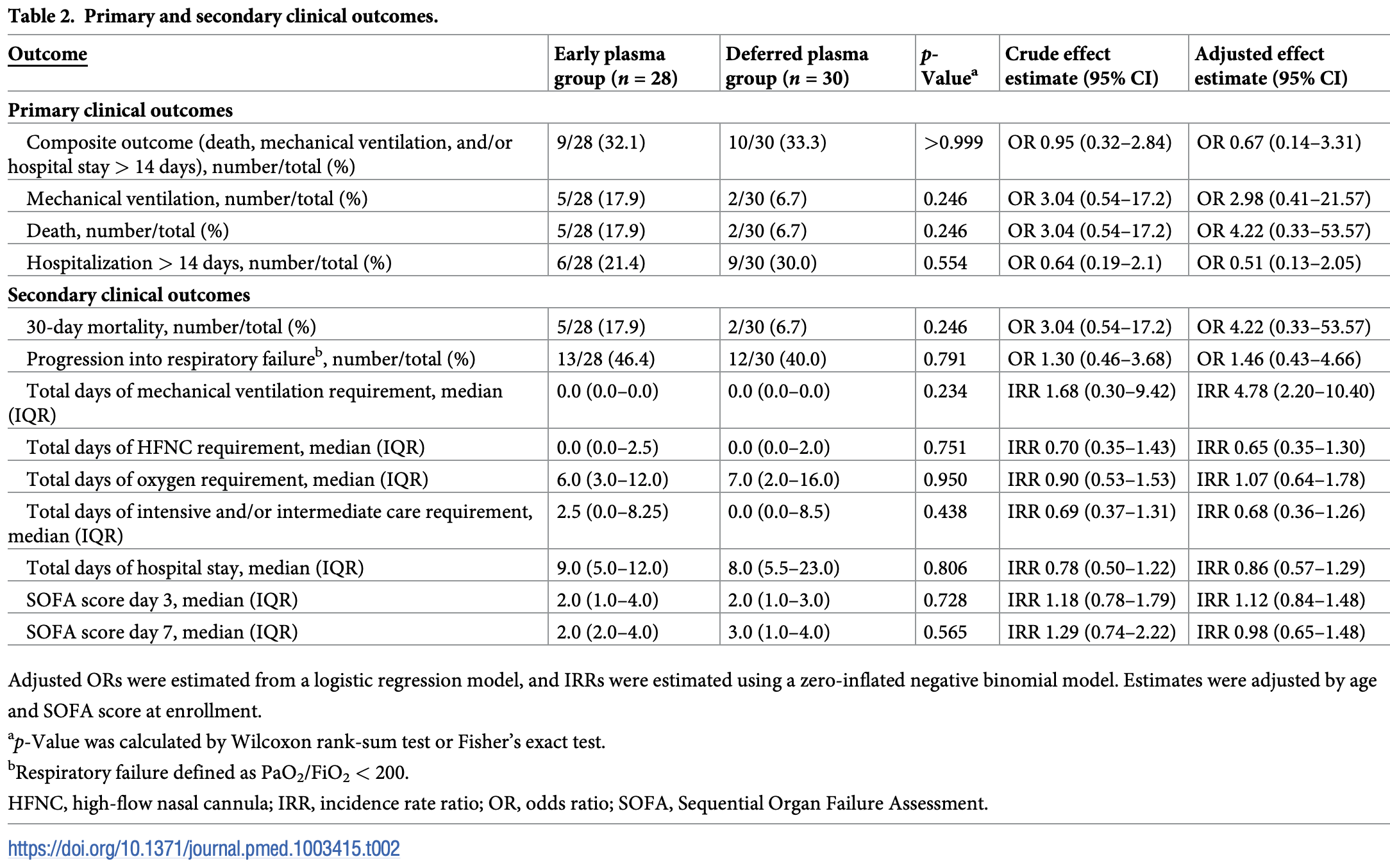
Small RCT with 28 early and 30 deferred (treated according to prespecified deterioration criteria) convalescent plasma patients, not showing significant differences. "Early" is relative, with a median of 5 days from symptom onset. 13 patients in the deferred group received plasma.
|
risk of death, 247.4% higher, RR 3.47, p = 0.17, treatment 5 of 28 (17.9%), control 2 of 30 (6.7%), adjusted per study, odds ratio converted to relative risk, logistic regression, early vs. deferred.
|
|
risk of mechanical ventilation, 163.3% higher, RR 2.63, p = 0.22, treatment 5 of 28 (17.9%), control 2 of 30 (6.7%), adjusted per study, odds ratio converted to relative risk, logistic regression, early vs. deferred.
|
|
risk of progression, 23.3% higher, RR 1.23, p = 0.51, treatment 13 of 28 (46.4%), control 12 of 30 (40.0%), adjusted per study, odds ratio converted to relative risk, logistic regression, early vs. deferred.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Balcells et al., 3 Mar 2021, Randomized Controlled Trial, Chile, peer-reviewed, 32 authors, study period 10 May, 2020 - 18 July, 2020, average treatment delay 5.0 days, trial NCT04375098 (history).
Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: A randomized phase II clinical trial
PLOS Medicine, doi:10.1371/journal.pmed.1003415
Background Convalescent plasma (CP), despite limited evidence on its efficacy, is being widely used as a compassionate therapy for hospitalized patients with COVID-19. We aimed to evaluate the efficacy and safety of early CP therapy in COVID-19 progression.
Methods and findings The study was an open-label, single-center randomized clinical trial performed in an academic medical center in Santiago, Chile, from May 10, 2020, to July 18, 2020, with final follow-up until August 17, 2020. The trial included patients hospitalized within the first 7 days of COVID-19 symptom onset, presenting risk factors for illness progression and not on mechanical ventilation. The intervention consisted of immediate CP (early plasma group)
Supporting information [22] ). (B) CT score 2 (Yang et al. [25] ). (C) CT score 3 (Pan et al. [23, 24] ). (TIF)
S1
References
Abraham, Passive antibody therapy in COVID-19, Nat Rev Immunol, doi:10.1038/s41577-020-0365-7
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Beltra ´n-Pavez, Riquelme-Barrios, Oyarzu ´n-Arrau, Gaete-Argel, Gonza ´lez-Stegmaier et al., Insights into neutralizing antibodies responses in individuals exposed to SARS-CoV-2 in Chile, Sci Adv
Borobia, Carcas, Arnalich, ´lvarez-Sala, Monserrat-Villatoro et al., A cohort of patients with COVID-19 in a major teaching hospital in Europe, J Clin Med, doi:10.3390/jcm9061733
Cheng, Wong, Soo, Wong, Lee et al., Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-004-1271-9
Duan, Liu, Li, Zhang, Yu et al., Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2004168117
Dzik, COVID-19 convalescent plasma: now is the time for better science, Transfus Med Rev, doi:10.1016/j.tmrv.2020.04.002
Garg, Kim, Whitaker, 'halloran, Cummings et al., Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019-COVID-NET, 14 states, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6915e3
Gharbharan, Jordans, Geurtsvankessel, Hollander, Karim et al., Convalescent plasma for COVID-19. A randomized clinical trial, medRxiv, doi:10.1101/2020.07.01.20139857
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Hung, To, Lee, Lee, Chan et al., Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection, Clin Infect Dis, doi:10.1093/cid/ciq106
Ji, Zhang, Xu, Chen, Yang et al., Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score, Clin Infect Dis, doi:10.1093/cid/ciaa414
Joyner, Bruno, Klassen, Kunze, Johnson et al., Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients, Mayo Clin Proc, doi:10.1016/j.mayocp.2020.06.028
Joyner, Senefeld, Klassen, Mills, Johnson et al., Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-2 month experience, medRxiv, doi:10.1101/2020.08.12.20169359
Ko, Seok, Cho, Ha, Baek et al., Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience, Antivir Ther, doi:10.3851/IMP3243
Li, Zhang, Hu, Tong, Zheng et al., Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.10044
Liu, Lin, Baine, Wajnberg, Gumprecht et al., Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study, Nat Med, doi:10.1038/s41591-020-1088-9
Luke, Kilbane, Jackson, Hoffman, Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?, Ann Intern Med, doi:10.7326/0003-4819-145-8-200610170-00139
Mair-Jenkins, Saavedra-Campos, Baillie, Cleary, Khaw et al., The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis, J Infect Dis, doi:10.1093/infdis/jiu396
Ministerio, de Salud de Chile. Esta ´ndares para la obtencio ´n de componentes sanguı ´neos y gestio ´n de stock
O ¨zdemir O ¨, Arsoy, Convalescent (immune) plasma therapy with all aspects: yesterday, today and COVID-19, Erciyes Med J, doi:10.14744/etd.2020.36528
Pan, Ye, Sun, Gui, Liang et al., Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID-19), Radiology, doi:10.1148/radiol.2020200370
Pan, Zheng, Ye, Li, Liu et al., Different computed tomography patterns of Coronavirus Disease 2019 (COVID-19) between survivors and non-survivors, Sci Rep, doi:10.1038/s41598-020-68057-4
Polidoro, Hagan, De, Santiago, Schmidt, Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19, Front Immunol, doi:10.3389/fimmu.2020.01626
Raoufi, Naini, Azizan, Zade, Shojaeian et al., Correlation between chest computed tomography scan findings and mortality of COVID-19 cases; a cross sectional study, Arch Acad Emerg Med, doi:10.22037/aaem.v8i1.719
Recovery Collaborative Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in hospitalized patients with Covid-19-preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA, doi:10.1001/jama.2020.6775
Roubinian, TACO and TRALI: biology, risk factors, and prevention strategies, Hematol Am Soc Hematol Educ Program, doi:10.1182/asheducation-2018.1.585
Sarzotti-Kelsoe, Bailer, Turk, Lin, Bilska et al., Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1, J Immunol Methods, doi:10.1016/j.jim.2013.11.022
Schmidt, Weisblum, Muecksch, Hoffmann, Michailidis et al., Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses, J Exp Med, doi:10.1084/jem.20201181
Schulz, Altman, Moher, Group, CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials, Ann Intern Med, doi:10.7326/0003-4819-152-11-201006010-00232
Shen, Wang, Zhao, Yang, Li et al., Treatment of 5 critically ill patients with COVID-19 with convalescent plasma, JAMA, doi:10.1001/jama.2020.4783
Thevarajan, Nguyen, Koutsakos, Druce, Caly et al., Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19, Nat Med, doi:10.1038/s41591-020-0819-2
Vlaar, Toy, Fung, Looney, Juffermans et al., A consensus redefinition of transfusion-related acute lung injury, Transfusion, doi:10.1111/trf.15311
Yang, Li, Liu, Zhen, Zhang et al., Chest CT severity score: an imaging tool for assessing severe COVID-19, Radiol Cardiothorac Imaging, doi:10.1148/ryct.2020200047
Ye, Fu, Ren, Wang, Wang et al., Treatment with convalescent plasma for COVID-19 patients in Wuhan, China, J Med Virol, doi:10.1002/jmv.25882
Zhang, Gan, Zhen, Hu, Li et al., Adaptive immune responses to SARS-CoV-2 infection in severe versus mild individuals, Signal Transduct Target Ther, doi:10.1038/s41392-020-00263-y
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736%2820%2930566-3
Zhou, Zhu, Wang, Xia, Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China, Eur Radiol, doi:10.1007/s00330-020-06879-6
DOI record:
{
"DOI": "10.1371/journal.pmed.1003415",
"ISSN": [
"1549-1676"
],
"URL": "http://dx.doi.org/10.1371/journal.pmed.1003415",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Convalescent plasma (CP), despite limited evidence on its efficacy, is being widely used as a compassionate therapy for hospitalized patients with COVID-19. We aimed to evaluate the efficacy and safety of early CP therapy in COVID-19 progression.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and findings</jats:title>\n<jats:p>The study was an open-label, single-center randomized clinical trial performed in an academic medical center in Santiago, Chile, from May 10, 2020, to July 18, 2020, with final follow-up until August 17, 2020. The trial included patients hospitalized within the first 7 days of COVID-19 symptom onset, presenting risk factors for illness progression and not on mechanical ventilation. The intervention consisted of immediate CP (early plasma group) versus no CP unless developing prespecified criteria of deterioration (deferred plasma group). Additional standard treatment was allowed in both arms. The primary outcome was a composite of mechanical ventilation, hospitalization for >14 days, or death. The key secondary outcomes included time to respiratory failure, days of mechanical ventilation, hospital length of stay, mortality at 30 days, and SARS-CoV-2 real-time PCR clearance rate. Of 58 randomized patients (mean age, 65.8 years; 50% male), 57 (98.3%) completed the trial. A total of 13 (43.3%) participants from the deferred group received plasma based on clinical aggravation. We failed to find benefit in the primary outcome (32.1% versus 33.3%, odds ratio [OR] 0.95, 95% CI 0.32–2.84, <jats:italic>p</jats:italic> > 0.999) in the early versus deferred CP group. The in-hospital mortality rate was 17.9% versus 6.7% (OR 3.04, 95% CI 0.54–17.17 <jats:italic>p =</jats:italic> 0.246), mechanical ventilation 17.9% versus 6.7% (OR 3.04, 95% CI 0.54–17.17, <jats:italic>p =</jats:italic> 0.246), and prolonged hospitalization 21.4% versus 30.0% (OR 0.64, 95% CI, 0.19–2.10, <jats:italic>p =</jats:italic> 0.554) in the early versus deferred CP group, respectively. The viral clearance rate on day 3 (26% versus 8%, <jats:italic>p =</jats:italic> 0.204) and day 7 (38% versus 19%, <jats:italic>p =</jats:italic> 0.374) did not differ between groups. Two patients experienced serious adverse events within 6 hours after plasma transfusion. The main limitation of this study is the lack of statistical power to detect a smaller but clinically relevant therapeutic effect of CP, as well as not having confirmed neutralizing antibodies in donor before plasma infusion.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Conclusions</jats:title>\n<jats:p>In the present study, we failed to find evidence of benefit in mortality, length of hospitalization, or mechanical ventilation requirement by immediate addition of CP therapy in the early stages of COVID-19 compared to its use only in case of patient deterioration.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Trial registration</jats:title>\n<jats:p><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04375098\" xlink:type=\"simple\">NCT04375098</jats:ext-link>.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7223-9665",
"affiliation": [],
"authenticated-orcid": true,
"family": "Balcells",
"given": "María Elvira",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0234-5876",
"affiliation": [],
"authenticated-orcid": true,
"family": "Rojas",
"given": "Luis",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9361-4049",
"affiliation": [],
"authenticated-orcid": true,
"family": "Le Corre",
"given": "Nicole",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2836-9817",
"affiliation": [],
"authenticated-orcid": true,
"family": "Martínez-Valdebenito",
"given": "Constanza",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5569-6485",
"affiliation": [],
"authenticated-orcid": true,
"family": "Ceballos",
"given": "María Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrés",
"given": "Marcela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Mayling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vizcaya",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mondaca",
"given": "Sebastián",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8746-1111",
"affiliation": [],
"authenticated-orcid": true,
"family": "Huete",
"given": "Álvaro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0978-9891",
"affiliation": [],
"authenticated-orcid": true,
"family": "Castro",
"given": "Ricardo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3715-9886",
"affiliation": [],
"authenticated-orcid": true,
"family": "Sarmiento",
"given": "Mauricio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9603-937X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Villarroel",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pizarro",
"given": "Alejandra",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8867-1970",
"affiliation": [],
"authenticated-orcid": true,
"family": "Ross",
"given": "Patricio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2156-8290",
"affiliation": [],
"authenticated-orcid": true,
"family": "Santander",
"given": "Jaime",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0265-808X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Lara",
"given": "Bárbara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrada",
"given": "Marcela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9369-2856",
"affiliation": [],
"authenticated-orcid": true,
"family": "Vargas-Salas",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beltrán-Pavez",
"given": "Carolina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0945-2970",
"affiliation": [],
"authenticated-orcid": true,
"family": "Soto-Rifo",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valiente-Echeverría",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caglevic",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahave",
"given": "Mauricio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Selman",
"given": "Carolina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1269-7322",
"affiliation": [],
"authenticated-orcid": true,
"family": "Gazitúa",
"given": "Raimundo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1140-2067",
"affiliation": [],
"authenticated-orcid": true,
"family": "Briones",
"given": "José Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villarroel-Espindola",
"given": "Franz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balmaceda",
"given": "Carlos",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9564-9512",
"affiliation": [],
"authenticated-orcid": true,
"family": "Espinoza",
"given": "Manuel A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pereira",
"given": "Jaime",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nervi",
"given": "Bruno",
"sequence": "additional"
}
],
"container-title": "PLOS Medicine",
"container-title-short": "PLoS Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosmedicine.org"
]
},
"created": {
"date-parts": [
[
2021,
3,
3
]
],
"date-time": "2021-03-03T21:05:33Z",
"timestamp": 1614805533000
},
"deposited": {
"date-parts": [
[
2021,
3,
3
]
],
"date-time": "2021-03-03T21:06:07Z",
"timestamp": 1614805567000
},
"editor": [
{
"affiliation": [],
"family": "Basu",
"given": "Sanjay",
"sequence": "first"
}
],
"funder": [
{
"name": "Fondo de Adopción Tecnológica SiEmpre, SOFOFA Hub, and Ministerio de Ciencia, Tecnología, Conocimiento e Innovación, Chile"
},
{
"name": "ENEL Chile S.A."
}
],
"indexed": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T06:35:53Z",
"timestamp": 1711694153702
},
"is-referenced-by-count": 62,
"issue": "3",
"issued": {
"date-parts": [
[
2021,
3,
3
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2021,
3,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
3
]
],
"date-time": "2021-03-03T00:00:00Z",
"timestamp": 1614729600000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pmed.1003415",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e1003415",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2021,
3,
3
]
]
},
"published-online": {
"date-parts": [
[
2021,
3,
3
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"author": "World Health Organization",
"key": "pmed.1003415.ref001",
"volume-title": "WHO coronavirus disease (COVID-19) dashboard",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "W Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "pmed.1003415.ref002",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "F Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "pmed.1003415.ref003",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area",
"author": "S Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"journal-title": "JAMA",
"key": "pmed.1003415.ref004",
"volume": "323",
"year": "2020"
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19—preliminary report",
"author": "RECOVERY Collaborative Group",
"journal-title": "N Engl J Med",
"key": "pmed.1003415.ref005",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "JH Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "pmed.1003415.ref006",
"volume": "383",
"year": "2020"
},
{
"article-title": "Convalescent (immune) plasma therapy with all aspects: yesterday, today and COVID-19",
"author": "Ö Özdemir",
"first-page": "252",
"journal-title": "Erciyes Med J",
"key": "pmed.1003415.ref007",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiu396",
"article-title": "The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis",
"author": "J Mair-Jenkins",
"doi-asserted-by": "crossref",
"first-page": "80",
"journal-title": "J Infect Dis",
"key": "pmed.1003415.ref008",
"volume": "211",
"year": "2015"
},
{
"DOI": "10.1007/s10096-004-1271-9",
"article-title": "Use of convalescent plasma therapy in SARS patients in Hong Kong",
"author": "Y Cheng",
"doi-asserted-by": "crossref",
"first-page": "44",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "pmed.1003415.ref009",
"volume": "24",
"year": "2005"
},
{
"DOI": "10.7326/0003-4819-145-8-200610170-00139",
"article-title": "Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?",
"author": "TC Luke",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "Ann Intern Med",
"key": "pmed.1003415.ref010",
"volume": "145",
"year": "2006"
},
{
"DOI": "10.3851/IMP3243",
"article-title": "Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience",
"author": "JH Ko",
"doi-asserted-by": "crossref",
"first-page": "617",
"journal-title": "Antivir Ther",
"key": "pmed.1003415.ref011",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1073/pnas.2004168117",
"article-title": "Effectiveness of convalescent plasma therapy in severe COVID-19 patients",
"author": "K Duan",
"doi-asserted-by": "crossref",
"first-page": "9490",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "pmed.1003415.ref012",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4783",
"article-title": "Treatment of 5 critically ill patients with COVID-19 with convalescent plasma",
"author": "C Shen",
"doi-asserted-by": "crossref",
"first-page": "1582",
"journal-title": "JAMA",
"key": "pmed.1003415.ref013",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25882",
"article-title": "Treatment with convalescent plasma for COVID-19 patients in Wuhan, China",
"author": "M Ye",
"doi-asserted-by": "crossref",
"first-page": "1890",
"journal-title": "J Med Virol",
"key": "pmed.1003415.ref014",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1088-9",
"article-title": "Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study.",
"author": "STH Liu",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "Nat Med.",
"key": "pmed.1003415.ref015",
"volume": "26",
"year": "2020"
},
{
"article-title": "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial",
"author": "L Li",
"first-page": "324460",
"journal-title": "JAMA",
"key": "pmed.1003415.ref016",
"year": "2020"
},
{
"article-title": "Convalescent plasma for COVID-19. A randomized clinical trial",
"author": "A Gharbharan",
"journal-title": "medRxiv",
"key": "pmed.1003415.ref017",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0365-7",
"article-title": "Passive antibody therapy in COVID-19",
"author": "J. Abraham",
"doi-asserted-by": "crossref",
"first-page": "401",
"journal-title": "Nat Rev Immunol",
"key": "pmed.1003415.ref018",
"volume": "20",
"year": "2020"
},
{
"article-title": "Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-2 month experience",
"author": "MJ Joyner",
"journal-title": "medRxiv",
"key": "pmed.1003415.ref019",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa414",
"article-title": "Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score",
"author": "D Ji",
"doi-asserted-by": "crossref",
"first-page": "1393",
"issue": "6",
"journal-title": "Clin Infect Dis",
"key": "pmed.1003415.ref020",
"volume": "71",
"year": "2020"
},
{
"author": "Ministerio de Salud de Chile",
"key": "pmed.1003415.ref021",
"volume-title": "Estándares para la obtención de componentes sanguíneos y gestión de stock",
"year": "2013"
},
{
"DOI": "10.1007/s00330-020-06879-6",
"article-title": "Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China",
"author": "S Zhou",
"doi-asserted-by": "crossref",
"first-page": "5446",
"journal-title": "Eur Radiol",
"key": "pmed.1003415.ref022",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1148/radiol.2020200370",
"article-title": "Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID-19)",
"author": "F Pan",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Radiology",
"key": "pmed.1003415.ref023",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-68057-4",
"article-title": "Different computed tomography patterns of Coronavirus Disease 2019 (COVID-19) between survivors and non-survivors",
"author": "F Pan",
"doi-asserted-by": "crossref",
"first-page": "11336",
"journal-title": "Sci Rep.",
"key": "pmed.1003415.ref024",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1148/ryct.2020200047",
"article-title": "Chest CT severity score: an imaging tool for assessing severe COVID-19",
"author": "R Yang",
"doi-asserted-by": "crossref",
"first-page": "e200047",
"journal-title": "Radiol Cardiothorac Imaging",
"key": "pmed.1003415.ref025",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1084/jem.20201181",
"article-title": "Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses",
"author": "F Schmidt",
"doi-asserted-by": "crossref",
"first-page": "e20201181",
"journal-title": "J Exp Med",
"key": "pmed.1003415.ref026",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1016/j.jim.2013.11.022",
"article-title": "Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1",
"author": "M Sarzotti-Kelsoe",
"doi-asserted-by": "crossref",
"first-page": "131",
"journal-title": "J Immunol Methods",
"key": "pmed.1003415.ref027",
"volume": "409",
"year": "2014"
},
{
"article-title": "Insights into neutralizing antibodies responses in individuals exposed to SARS-CoV-2 in Chile",
"author": "C Beltrán-Pavez",
"journal-title": "Sci Adv",
"key": "pmed.1003415.ref028"
},
{
"DOI": "10.1093/cid/ciq106",
"article-title": "Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection",
"author": "IFN Hung",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Clin Infect Dis",
"key": "pmed.1003415.ref029",
"volume": "52",
"year": "2011"
},
{
"DOI": "10.7326/0003-4819-152-11-201006010-00232",
"article-title": "CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials",
"author": "KF Schulz",
"doi-asserted-by": "crossref",
"first-page": "726",
"journal-title": "Ann Intern Med",
"key": "pmed.1003415.ref030",
"volume": "152",
"year": "2010"
},
{
"article-title": "Correlation between chest computed tomography scan findings and mortality of COVID-19 cases; a cross sectional study",
"author": "M Raoufi",
"first-page": "e57",
"journal-title": "Arch Acad Emerg Med",
"key": "pmed.1003415.ref031",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1111/trf.15311",
"article-title": "A consensus redefinition of transfusion-related acute lung injury",
"author": "APJ Vlaar",
"doi-asserted-by": "crossref",
"first-page": "2465",
"journal-title": "Transfusion",
"key": "pmed.1003415.ref032",
"volume": "59",
"year": "2019"
},
{
"DOI": "10.3389/fimmu.2020.01626",
"article-title": "Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19",
"author": "RB Polidoro",
"doi-asserted-by": "crossref",
"first-page": "1626",
"journal-title": "Front Immunol",
"key": "pmed.1003415.ref033",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0819-2",
"article-title": "Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19",
"author": "I Thevarajan",
"doi-asserted-by": "crossref",
"first-page": "453",
"journal-title": "Nat Med",
"key": "pmed.1003415.ref034",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1038/s41392-020-00263-y",
"article-title": "Adaptive immune responses to SARS-CoV-2 infection in severe versus mild individuals",
"author": "F Zhang",
"doi-asserted-by": "crossref",
"first-page": "156",
"journal-title": "Signal Transduct Target Ther",
"key": "pmed.1003415.ref035",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.tmrv.2020.04.002",
"article-title": "COVID-19 convalescent plasma: now is the time for better science",
"author": "S. Dzik",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Transfus Med Rev",
"key": "pmed.1003415.ref036",
"volume": "34",
"year": "2020"
},
{
"author": "US Food",
"key": "pmed.1003415.ref037",
"volume-title": "FDA issues emergency use authorization for convalescent plasma as potential promising COVID–19 treatment, another achievement in administration’s fight against pandemic",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.06.028",
"article-title": "Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients",
"author": "MJ Joyner",
"doi-asserted-by": "crossref",
"first-page": "1888",
"journal-title": "Mayo Clin Proc",
"key": "pmed.1003415.ref038",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1182/asheducation-2018.1.585",
"article-title": "TACO and TRALI: biology, risk factors, and prevention strategies",
"author": "N. Roubinian",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Hematol Am Soc Hematol Educ Program",
"key": "pmed.1003415.ref039",
"volume": "2018",
"year": "2018"
},
{
"DOI": "10.15585/mmwr.mm6915e3",
"article-title": "Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020",
"author": "S Garg",
"doi-asserted-by": "crossref",
"first-page": "458",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "pmed.1003415.ref040",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.3390/jcm9061733",
"article-title": "A cohort of patients with COVID-19 in a major teaching hospital in Europe",
"author": "A Borobia",
"doi-asserted-by": "crossref",
"first-page": "1733",
"journal-title": "J Clin Med",
"key": "pmed.1003415.ref041",
"volume": "9",
"year": "2020"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pmed.1003415"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: A randomized phase II clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pmed.corrections_policy",
"volume": "18"
}