Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial
et al., Lancet Infectious Diseases, doi:10.1016/S1473-3099(21)00485-0, DISCOVERY, NCT04315948, Sep 2021
RCT 857 hospitalized patients, showing no significant differences with remdesivir treatment. EudraCT2020-000936-23.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Remdesivir efficacy disappears with longer
followup. Mixed-effects meta-regression of efficacy as a function of
followup duration across all remdesivir studies shows decreasing efficacy with
longer followup15. This may reflect
antiviral efficacy being offset by serious adverse effects of treatment.
|
risk of death, 6.4% lower, RR 0.94, p = 0.77, treatment 34 of 414 (8.2%), control 37 of 418 (8.9%), NNT 156, adjusted per study, odds ratio converted to relative risk, day 28.
|
|
risk of death, 11.7% lower, RR 0.88, p = 0.76, treatment 21 of 414 (5.1%), control 24 of 418 (5.7%), NNT 149, day 15.
|
|
risk of 7-point scale, 9.9% lower, OR 0.90, p = 0.39, treatment 414, control 418, inverted to make OR<1 favor treatment, 28 days, RR approximated with OR.
|
|
risk of 7-point scale, 2.0% higher, OR 1.02, p = 0.85, treatment 414, control 418, inverted to make OR<1 favor treatment, 15 days, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Ader et al., 14 Sep 2021, Randomized Controlled Trial, multiple countries, peer-reviewed, 17 authors, study period 22 March, 2020 - 21 January, 2021, average treatment delay 9.0 days, trial NCT04315948 (history) (DISCOVERY).
Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(21)00485-0
Background The antiviral efficacy of remdesivir against SARS-CoV-2 is still controversial. We aimed to evaluate the clinical efficacy of remdesivir plus standard of care compared with standard of care alone in patients admitted to hospital with COVID-19, with indication of oxygen or ventilator support.
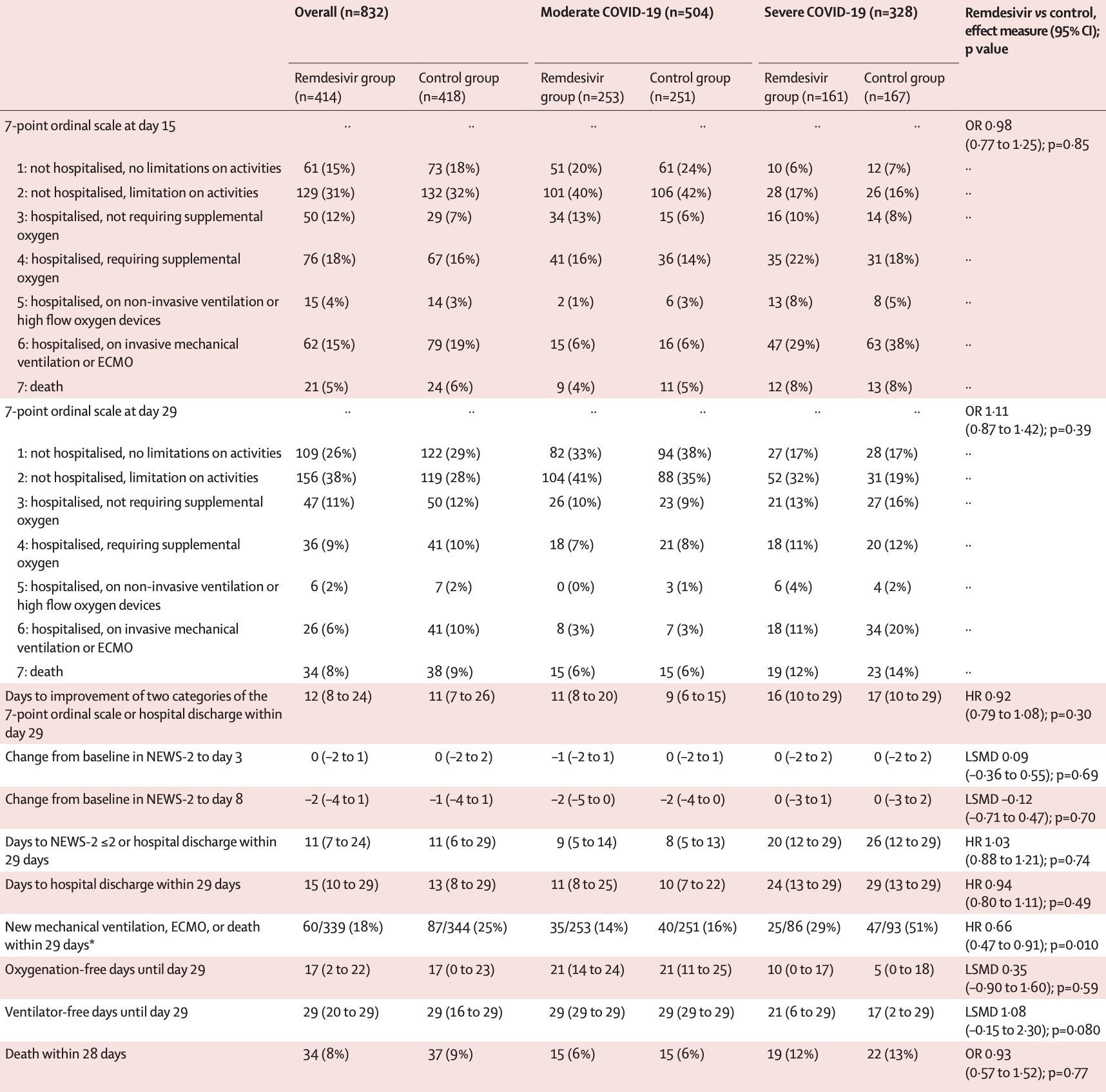
Methods DisCoVeRy was a phase 3, open-label, adaptive, multicentre, randomised, controlled trial conducted in 48 sites in Europe (France, Belgium, Austria, Portugal, Luxembourg). Adult patients (aged ≥18 years) admitted to hospital with laboratory-confirmed SARS-CoV-2 infection and illness of any duration were eligible if they had clinical evidence of hypoxaemic pneumonia, or required oxygen supplementation. Exclusion criteria included elevated liver enzymes, severe chronic kidney disease, any contraindication to one of the studied treatments or their use in the 29 days before random assignment, or use of ribavirin, as well as pregnancy or breastfeeding. Participants were randomly assigned (1:1:1:1:1) to receive standard of care alone or in combination with remdesivir, lopinavir-ritonavir, lopinavir-ritonavir and interferon beta-1a, or hydroxychloroquine. Randomisation used computer-generated blocks of various sizes; it was stratified on severity of disease at inclusion and on European administrative region. Remdesivir was administered as 200 mg intravenous infusion on day 1, followed by once daily, 1-h infusions of 100 mg up to 9 days, for a total duration of 10 days. It could be stopped after 5 days if the participant was discharged. The primary outcome was the clinical status at day 15 measured by the WHO seven-point ordinal scale, assessed in the intentionto-treat population. Safety was assessed in the modified intention-to-treat population and was one of the secondary outcomes. This trial is registered with the European Clinical Trials Database, EudraCT2020-000936-23, and ClinicalTrials.gov, NCT04315948. Findings Between March 22 , 2020, and Jan 21 , 2021, 857 participants were enrolled and randomly assigned to remdesivir plus standard of care (n=429) or standard of care only (n=428). 15 participants were excluded from analysis in the remdesivir group, and ten in the control group. At day 15, the distribution of the WHO ordinal scale was: (1) not hospitalised, no limitations on activities (61 [15%] of 414 in the remdesivir group vs 73 [17%] of 418 in the control group); (2) not hospitalised, limitation on activities (129 [31%] vs 132 [32%]); (3) hospitalised, not requiring supplemental oxygen (50 [12%] vs 29 [7%]); (4) hospitalised, requiring supplemental oxygen (76 [18%] vs 67 [16%]); (5) hospitalised, on non-invasive ventilation or high flow oxygen devices (15 [4%] vs 14 [3%]); (6) hospitalised, on invasive mechanical ventilation or extracorporeal membrane oxygenation (62 [15%] vs 79 [19%]); (7) death (21 [5%] vs 24 [6%]). The difference between treatment groups was not significant (odds ratio 0•98 [95% CI 0•77-1•25]; p=0•85). There was no..
Contributors FA, NP-S, JP, MB-D, GP, TS, RG, J-AP, DC, YY, CB, and FM were involved in the design, establishment, and day-to-day management and implementation of the trial. FA, MH, TS, RG, J-AP, DC, YY, and FM obtained funding for the trial. FA, MH, NP-S, JP, TS, RG, and J-AP included participants in the trial. MB-D was responsible for the virological analyses. M-PL and GP were responsible for the pharmacological analyses. FA, MB-D, DB, AD, M-PL, GP, CB, and FM were in charge of data curation and accessed and verified the data. DB, JG, DC, CB, and FM were involved in the statistical analyses. FA, MB-D, DB, AD, MH, JG, CB, and FM wrote the original draft of the manuscript, which was reviewed and edited by NP-S, JP, MPL, GP, DC, and YY. All authors contributed to refinement of and approved this manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests DC reports grants and lecture fees from Janssen and lecture fees from Gilead, outside the submitted work. FM reports grants and consulting fees from Da Volterra, grants from Sanofi, and consulting fees from Ipsen, outside the submitted work. MH reports grants from The Belgian Center for Knowledge (KCE), the Fonds Erasme-COVID-Université Libre de Bruxelles and the EU-Horizon programme, for the submitted work; and has received support for attending meetings from Pfizer; support for participation on an advisory board for..
References
Ader, Peiffer-Smadja, Poissy, An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.05.020
Ader, Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults, BMJ Open
Avataneo, De Nicolò, Cusato, Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: a tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease, J Antimicrob Chemother
Barratt-Due, Olsen, Nezvalova-Henriksen, Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19: a randomized trial, Ann Intern Med, doi:10.7326/M21-0653
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19-final report, N Engl J Med
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate COVID-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Etievant, Bal, Escuret, Performance assessment of SARS-CoV-2 PCR assays developed by WHO referral laboratories, J Clin Med
Gonçalves, Bertrand, Ke, Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load, CPT Pharmacometrics Syst Pharmacol
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19, N Engl J Med
Humeniuk, Mathias, Cao, Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects, Clin Transl Sci
Humeniuk, Mathias, Kirby, Pharmacokinetic, pharmacodynamic, and drug-interaction profile of remdesivir, a SARS-CoV-2 replication inhibitor, Clin Pharmacokinet
Libster, Marc, Wappner, Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med
Lê, Hingrat, Jaquet, Removal of remdesivir's metabolite GS-441524 by hemodialysis in a double lung transplant recipient with COVID-19, Antimicrob Agents Chemother
Nicholson, Aoki, Osterhaus, Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial, Lancet
Néant, Lingas, Hingrat, Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort, Proc Natl Acad Sci
Pan, Peto, Henao-Restrepo, Repurposed antiviral drugs for COVID-19-interim WHO Solidarity trial results, N Engl J Med
Paranjpe, Fuster, Lala, Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19, J Am Coll Cardiol
Rasmussen, Popescu, SARS-CoV-2 transmission without symptoms, Science
Sterne, Murthy, Diaz, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis, JAMA
Tang, Bai, Chen, Gong, Li et al., Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy, J Thromb Haemost
Villar, Ferrando, Martínez, Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial, Lancet Respir Med
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Warren, Jordan, Lo, Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys, Nature
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
Williamson, Feldmann, Schwarz, Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2, Nature
DOI record:
{
"DOI": "10.1016/s1473-3099(21)00485-0",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(21)00485-0",
"alternative-id": [
"S1473309921004850"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(21)00485-0"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(21)00559-4"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Ader",
"given": "Florence",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bouscambert-Duchamp",
"given": "Maude",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hites",
"given": "Maya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peiffer-Smadja",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poissy",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belhadi",
"given": "Drifa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diallo",
"given": "Alpha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lê",
"given": "Minh-Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peytavin",
"given": "Gilles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Staub",
"given": "Thérèse",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greil",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guedj",
"given": "Jérémie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paiva",
"given": "Jose-Artur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costagliola",
"given": "Dominique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yazdanpanah",
"given": "Yazdan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burdet",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mentré",
"given": "France",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Egle",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greil",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joannidis",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lamprecht",
"given": "Bernd",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Altdorfer",
"given": "Antoine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belkhir",
"given": "Leila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fraipont",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hites",
"given": "Maya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verschelden",
"given": "Gil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aboab",
"given": "Jérôme",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ader",
"given": "Florence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ait-Oufella",
"given": "Hafid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andrejak",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andreu",
"given": "Pascal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Argaud",
"given": "Laurent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bani-Sadr",
"given": "Firouzé",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benezit",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blot",
"given": "Mathieu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Botelho-Nevers",
"given": "Elisabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouadma",
"given": "Lila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouchaud",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bougon",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouiller",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bounes-Vardon",
"given": "Fanny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boutoille",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyer",
"given": "Alexandre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruel",
"given": "Cédric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cabié",
"given": "André",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Canet",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cazanave",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chabartier",
"given": "Cyrille",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chirouze",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clere-Jehl",
"given": "Raphaël",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Courjon",
"given": "Johan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crockett",
"given": "Flora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Danion",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delbove",
"given": "Agathe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dellamonica",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Djossou",
"given": "Félix",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubost",
"given": "Clément",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duvignaud",
"given": "Alexandre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Epaulard",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Epelboin",
"given": "Loïc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fartoukh",
"given": "Murielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faure",
"given": "Karine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faure",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferry",
"given": "Tristan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ficko",
"given": "Cécile",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Figueiredo",
"given": "Samy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaborit",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaci",
"given": "Rostane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gagneux-Brunon",
"given": "Amandine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallien",
"given": "Sébastien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garot",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geri",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibot",
"given": "Sébastien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goehringer",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gousseff",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gruson",
"given": "Didier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hansmann",
"given": "Yves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hinschberger",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaureguiberry",
"given": "Stéphane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jeanmichel",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kerneis",
"given": "Solen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kimmoun",
"given": "Antoine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klouche",
"given": "Kada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lachâtre",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lacombe",
"given": "Karine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laine",
"given": "Fabrice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lanoix",
"given": "Jean-Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Launay",
"given": "Odile",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laviolle",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Le Moing",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Le Pavec",
"given": "Jérôme",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Le Tulzo",
"given": "Yves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Le Turnier",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lebeaux",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lefevre",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leroy",
"given": "Sylvie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lescure",
"given": "François-Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lessire",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leveau",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loubet",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Makinson",
"given": "Alain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malvy",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marquette",
"given": "Charles-Hugo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martin-Blondel",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martinot",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mayaux",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mekontso-Dessap",
"given": "Armand",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meziani",
"given": "Ferhat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mira",
"given": "Jean-Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molina",
"given": "Jean-Michel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monnet",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mootien",
"given": "Joy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mourvillier",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murris-Espin",
"given": "Marlène",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Navellou",
"given": "Jean-Christophe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nseir",
"given": "Saad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oulehri",
"given": "Walid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peiffer-Smadja",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perpoint",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pialoux",
"given": "Gilles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pilmis",
"given": "Benoît",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piriou",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piroth",
"given": "Lionel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poissy",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pourcher",
"given": "Valérie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quenot",
"given": "Jean-Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raffi",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reignier",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Revest",
"given": "Matthieu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richard",
"given": "Jean-Christophe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Riu-Poulenc",
"given": "Béatrice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robert",
"given": "Céline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roger",
"given": "Pierre-Alexandre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roger",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rouveix-Nordon",
"given": "Elisabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruch",
"given": "Yvon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saidani",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sayre",
"given": "Naomi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Senneville",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sotto",
"given": "Albert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stefan",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tacquard",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Terzi",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Textoris",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thiery",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Timsit",
"given": "Jean-François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tolsma",
"given": "Violaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turmel",
"given": "Jean-Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valour",
"given": "Florent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wallet",
"given": "Florent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wattecamps",
"given": "Guilhem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yazdanpanah",
"given": "Yazdan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zerbib",
"given": "Yoann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berna",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reuter",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Staub",
"given": "Thérèse",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Braz",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferreira Ribeiro",
"given": "Joao-Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paiva",
"given": "José-Artur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roncon-Albuquerque",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouscambert-Duchamp",
"given": "Maude",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaymard",
"given": "Alexandre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lê",
"given": "Minh-Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lina",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peytavin",
"given": "Gilles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tubiana",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Couffin-Cadièrgues",
"given": "Sandrine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Esperou",
"given": "Hélène",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belhadi",
"given": "Drifa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burdet",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costagliola",
"given": "Dominique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dechanet",
"given": "Aline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delmas",
"given": "Christelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diallo",
"given": "Alpha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fougerou",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guedj",
"given": "Jérémie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mentré",
"given": "France",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mercier",
"given": "Noémie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noret",
"given": "Marion",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saillard",
"given": "Juliette",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Velou",
"given": "Priyanka",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
14
]
],
"date-time": "2021-09-14T22:31:01Z",
"timestamp": 1631658661000
},
"deposited": {
"date-parts": [
[
2023,
11,
8
]
],
"date-time": "2023-11-08T20:15:40Z",
"timestamp": 1699474540000
},
"funder": [
{
"DOI": "10.13039/100015400",
"doi-asserted-by": "publisher",
"name": "Fonds Erasme"
},
{
"DOI": "10.13039/501100000780",
"doi-asserted-by": "publisher",
"name": "European Commission"
},
{
"DOI": "10.13039/501100008530",
"doi-asserted-by": "publisher",
"name": "European Regional Development Fund"
},
{
"DOI": "10.13039/501100009242",
"doi-asserted-by": "publisher",
"name": "DGOS"
},
{
"DOI": "10.13039/501100019353",
"doi-asserted-by": "publisher",
"name": "Belgian Health Care Knowledge Centre"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T06:32:10Z",
"timestamp": 1712644330124
},
"is-referenced-by-count": 211,
"issue": "2",
"issued": {
"date-parts": [
[
2022,
2
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
},
{
"URL": "http://www.elsevier.com/open-access/userlicense/1.0/",
"content-version": "am",
"delay-in-days": 177,
"start": {
"date-parts": [
[
2022,
7,
28
]
],
"date-time": "2022-07-28T00:00:00Z",
"timestamp": 1658966400000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309921004850?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309921004850?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "209-221",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
2
]
]
},
"published-print": {
"date-parts": [
[
2022,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1136/bmjopen-2020-041437",
"article-title": "Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults",
"author": "Ader",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Open",
"key": "10.1016/S1473-3099(21)00485-0_bib1",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2021.05.020",
"article-title": "An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19",
"author": "Ader",
"doi-asserted-by": "crossref",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/S1473-3099(21)00485-0_bib2",
"year": "2021"
},
{
"DOI": "10.1128/AAC.01521-20",
"article-title": "Removal of remdesivir's metabolite GS-441524 by hemodialysis in a double lung transplant recipient with COVID-19",
"author": "Lê",
"doi-asserted-by": "crossref",
"first-page": "e01521",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/S1473-3099(21)00485-0_bib3",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1038/nature17180",
"article-title": "Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys",
"author": "Warren",
"doi-asserted-by": "crossref",
"first-page": "381",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(21)00485-0_bib5",
"volume": "531",
"year": "2016"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "10.1016/S1473-3099(21)00485-0_bib6",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2423-5",
"article-title": "Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(21)00485-0_bib7",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(21)00485-0_bib8",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00485-0_bib9",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed antiviral drugs for COVID-19—interim WHO Solidarity trial results",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00485-0_bib10",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00485-0_bib11",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(21)00485-0_bib12",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(19)30417-5",
"article-title": "Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial",
"author": "Villar",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S1473-3099(21)00485-0_bib13",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1111/jth.14817",
"article-title": "Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "1094",
"journal-title": "J Thromb Haemost",
"key": "10.1016/S1473-3099(21)00485-0_bib14",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.jacc.2020.05.001",
"article-title": "Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19",
"author": "Paranjpe",
"doi-asserted-by": "crossref",
"first-page": "122",
"journal-title": "J Am Coll Cardiol",
"key": "10.1016/S1473-3099(21)00485-0_bib15",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.3390/jcm9061871",
"article-title": "Performance assessment of SARS-CoV-2 PCR assays developed by WHO referral laboratories",
"author": "Etievant",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Med",
"key": "10.1016/S1473-3099(21)00485-0_bib16",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkaa152",
"article-title": "Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: a tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease",
"author": "Avataneo",
"doi-asserted-by": "crossref",
"first-page": "1772",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/S1473-3099(21)00485-0_bib17",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.7326/M21-0653",
"article-title": "Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID-19: a randomized trial",
"author": "Barratt-Due",
"doi-asserted-by": "crossref",
"journal-title": "Ann Intern Med",
"key": "10.1016/S1473-3099(21)00485-0_bib18",
"year": "2021"
},
{
"DOI": "10.1002/psp4.12543",
"article-title": "Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load",
"author": "Gonçalves",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "CPT Pharmacometrics Syst Pharmacol",
"key": "10.1016/S1473-3099(21)00485-0_bib19",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2017962118",
"article-title": "Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort",
"author": "Néant",
"doi-asserted-by": "crossref",
"journal-title": "Proc Natl Acad Sci USA",
"key": "10.1016/S1473-3099(21)00485-0_bib20",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1126/science.abf9569",
"article-title": "SARS-CoV-2 transmission without symptoms",
"author": "Rasmussen",
"doi-asserted-by": "crossref",
"first-page": "1206",
"journal-title": "Science",
"key": "10.1016/S1473-3099(21)00485-0_bib21",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(00)02288-1",
"article-title": "Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial",
"author": "Nicholson",
"doi-asserted-by": "crossref",
"first-page": "1845",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(21)00485-0_bib22",
"volume": "355",
"year": "2000"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(21)00485-0_bib23",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early high-titer plasma therapy to prevent severe COVID-19 in older adults",
"author": "Libster",
"doi-asserted-by": "crossref",
"first-page": "610",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00485-0_bib24",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate COVID-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00485-0_bib25",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(21)00485-0_bib26",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1111/cts.12840",
"article-title": "Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects",
"author": "Humeniuk",
"doi-asserted-by": "crossref",
"first-page": "896",
"journal-title": "Clin Transl Sci",
"key": "10.1016/S1473-3099(21)00485-0_bib27",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1007/s40262-021-00984-5",
"article-title": "Pharmacokinetic, pharmacodynamic, and drug-interaction profile of remdesivir, a SARS-CoV-2 replication inhibitor",
"author": "Humeniuk",
"doi-asserted-by": "crossref",
"first-page": "569",
"journal-title": "Clin Pharmacokinet",
"key": "10.1016/S1473-3099(21)00485-0_bib28",
"volume": "60",
"year": "2021"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309921004850"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "22"
}