Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0, Jul 2024
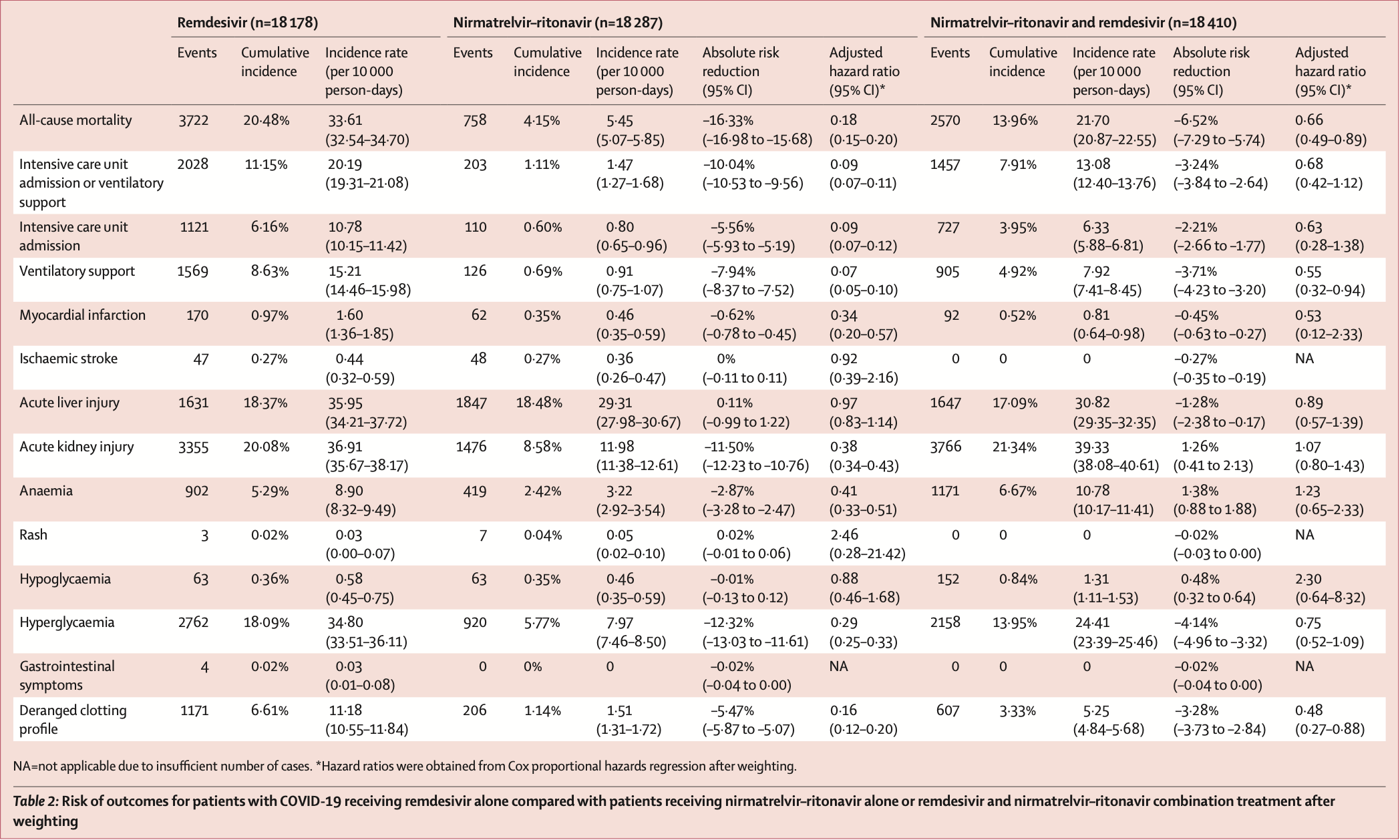
Target trial emulation study of 18,196 hospitalized COVID-19 patients in Hong Kong showing significantly higher ICU admission and AKI with remdesivir + paxlovid compared with paxlovid alone, and lower mortality and ventilatory support with remdesivir + paxlovid compared with remdesivir alone. Patients were treated within 5 days of diagnosis, however the time from onset is not known.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
Study covers remdesivir and paxlovid.
|
risk of death, 34.0% lower, HR 0.66, p = 0.006, treatment 308, control 4,232, remdesivir+paxlovid vs. remdesivir, day 90.
|
|
risk of ICU admission, 37.0% lower, HR 0.63, p = 0.26, treatment 308, control 4,232, remdesivir+paxlovid vs. remdesivir, day 90.
|
|
ventilatory support, 45.0% lower, HR 0.55, p = 0.03, treatment 308, control 4,232, remdesivir+paxlovid vs. remdesivir, day 90.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Choi et al., 15 Jul 2024, retrospective, China, peer-reviewed, mean age 74.0, 8 authors, study period 16 March, 2022 - 31 December, 2022.
Contact: ivanhung@hku.hk.
Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(24)00353-0
Background Remdesivir (Veklury, Gilead Sciences, Foster
References
Ader, Bouscambert-Duchamp, Hites, Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial, Lancet Infect Dis
Agarwal, Hunt, Stegemann, A living WHO guideline on drugs for COVID-19, BMJ
Aggarwal, Molina, Beaty, Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study, Lancet Infect Dis
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19-final report, N Engl J Med
Bhimraj, Morgan, Shumaker, Infectious Diseases Society of America guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciaa478
Chan, Lui, Wong, Safety profile and clinical and virological outcomes of nirmatrelvir-ritonavir treatment in patients with advanced chronic kidney disease and coronavirus disease 2019, Clin Infect Dis
Chesnaye, Stel, Tripepi, An introduction to inverse probability of treatment weighting in observational research, Clin Kidney J
Cianci, Massaro, Santis, Changes in lymphocyte subpopulations after remdesivir therapy for COVID-19: a brief report, Int J Mol Sci
Gentile, Foggia, Silvitelli, Sardanelli, Cattaneo et al., Optimizing COVID-19 treatment in immunocompromised patients: early combination therapy with remdesivir, nirmatrelvir/ritonavir and sotrovimab, Virol J
Gidari, Sabbatini, Schiaroli, Synergistic activity of remdesivir-nirmatrelvir combination on a SARS-CoV-2 in vitro model and a case report, Viruses
Grundeis, Ansems, Dahms, Remdesivir for the treatment of COVID-19, Cochrane Database Syst Rev
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
Jeong, Chokkakula, Min, Combination therapy with nirmatrelvir and molnupiravir improves the survival of SARS-CoV-2 infected mice, Antiviral Res
Kourjian, Rucevic, Berberich, HIV protease inhibitorinduced cathepsin modulation alters antigen processing and crosspresentation, J Immunol
Lai, Huang, Chui, Multimorbidity and adverse events of special interest associated with COVID-19 vaccines in Hong Kong, Nat Commun
Li, Liclican, Xu, Key metabolic enzymes involved in remdesivir activation in human lung cells, Antimicrob Agents Chemother
Liu, Pan, Zhang, Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomised controlled study, Lancet Reg Health West Pac
Mansournia, Nazemipour, Etminan, A practical guide to handling competing events in etiologic time-to-event studies, Glob Epidemiol
Marshall, Murthy, Diaz, A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis
Marzolini, Kuritzkes, Marra, Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications, Clin Pharmacol Ther
Mikulska, Sepulcri, Dentone, Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients, Clin Infect Dis
Mozaffari, Chandak, Gottlieb, Remdesivir is associated with reduced mortality in COVID-19 patients requiring supplemental oxygen including invasive mechanical ventilation across SARS-CoV-2 variants, Open Forum Infect Dis
Pasquini, Toschi, Casadei, Dual combined antiviral treatment with remdesivir and nirmatrelvir/ritonavir in patients with impaired humoral immunity and persistent SARS-CoV-2 infection, Hematol Oncol
To, Sridhar, Chiu, Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic, Emerg Microbes Infect
Vanderweele, Ding, Sensitivity analysis in observational research: introducing the E-value, Ann Intern Med
Wan, Chui, Lai, Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study, Lancet Infect Dis
Wang, Sacramento, Jockusch, Combination of antiviral drugs inhibits SARS-CoV-2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture, Commun Biol
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Wong, Jiang, Tang, Lam, Fung et al., Health services research in the public healthcare system in Hong Kong: an analysis of over 1 million antihypertensive prescriptions between 2004-2007 as an example of the potential and pitfalls of using routinely collected electronic patient data, BMC Health Serv Res
Wong, Root, Douglas, Cardiovascular outcomes associated with use of clarithromycin: population based study, BMJ
DOI record:
{
"DOI": "10.1016/s1473-3099(24)00353-0",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(24)00353-0",
"alternative-id": [
"S1473309924003530"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(24)00353-0"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(24)00407-9"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Elsevier Ltd. All rights are reserved, including those for text and data mining, AI training, and similar technologies."
}
],
"author": [
{
"affiliation": [],
"family": "Choi",
"given": "Ming Hong",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wan",
"given": "Eric Yuk Fai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Ian Chi Kei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chan",
"given": "Esther Wai Yin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chu",
"given": "Wing Ming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tam",
"given": "Anthony Raymond",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yuen",
"given": "Kwok Yung",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hung",
"given": "Ivan Fan Ngai",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
7,
15
]
],
"date-time": "2024-07-15T22:32:52Z",
"timestamp": 1721082772000
},
"deposited": {
"date-parts": [
[
2024,
7,
15
]
],
"date-time": "2024-07-15T22:33:13Z",
"timestamp": 1721082793000
},
"funder": [
{
"DOI": "10.13039/501100005847",
"doi-asserted-by": "publisher",
"name": "HMRF"
}
],
"indexed": {
"date-parts": [
[
2024,
7,
16
]
],
"date-time": "2024-07-16T00:22:31Z",
"timestamp": 1721089351019
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2024,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309924003530?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309924003530?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
7
]
]
},
"published-print": {
"date-parts": [
[
2024,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/S1473-3099(24)00353-0_bib2",
"series-title": "Interim recommendation on clinical management of adult cases with coronavirus disease 2019 (COVID-19)",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00353-0_bib3",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(22)00519-0",
"article-title": "Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(24)00353-0_bib4",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(24)00353-0_bib5",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofad482",
"article-title": "Remdesivir is associated with reduced mortality in COVID-19 patients requiring supplemental oxygen including invasive mechanical ventilation across SARS-CoV-2 variants",
"author": "Mozaffari",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/S1473-3099(24)00353-0_bib6",
"volume": "10",
"year": "2023"
},
{
"article-title": "Infectious Diseases Society of America guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19)",
"author": "Bhimraj",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S1473-3099(24)00353-0_bib7",
"year": "2020"
},
{
"key": "10.1016/S1473-3099(24)00353-0_bib8",
"series-title": "Coronavirus disease 2019 (COVID-19) treatment guidelines",
"year": "2021"
},
{
"article-title": "A living WHO guideline on drugs for COVID-19",
"author": "Agarwal",
"journal-title": "BMJ",
"key": "10.1016/S1473-3099(24)00353-0_bib9",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00353-0_bib10",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3390/v15071577",
"article-title": "Synergistic activity of remdesivir–nirmatrelvir combination on a SARS-CoV-2 in vitro model and a case report",
"author": "Gidari",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/S1473-3099(24)00353-0_bib11",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1186/s12985-023-02269-8",
"article-title": "Optimizing COVID-19 treatment in immunocompromised patients: early combination therapy with remdesivir, nirmatrelvir/ritonavir and sotrovimab",
"author": "Gentile",
"doi-asserted-by": "crossref",
"first-page": "301",
"journal-title": "Virol J",
"key": "10.1016/S1473-3099(24)00353-0_bib12",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciad181",
"article-title": "Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients",
"author": "Mikulska",
"doi-asserted-by": "crossref",
"first-page": "280",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S1473-3099(24)00353-0_bib13",
"volume": "77",
"year": "2023"
},
{
"DOI": "10.1002/hon.3206",
"article-title": "Dual combined antiviral treatment with remdesivir and nirmatrelvir/ritonavir in patients with impaired humoral immunity and persistent SARS-CoV-2 infection",
"author": "Pasquini",
"doi-asserted-by": "crossref",
"first-page": "904",
"journal-title": "Hematol Oncol",
"key": "10.1016/S1473-3099(24)00353-0_bib14",
"volume": "41",
"year": "2023"
},
{
"DOI": "10.1186/1472-6963-8-138",
"article-title": "Health services research in the public healthcare system in Hong Kong: an analysis of over 1 million antihypertensive prescriptions between 2004–2007 as an example of the potential and pitfalls of using routinely collected electronic patient data",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "138",
"journal-title": "BMC Health Serv Res",
"key": "10.1016/S1473-3099(24)00353-0_bib16",
"volume": "8",
"year": "2008"
},
{
"article-title": "Cardiovascular outcomes associated with use of clarithromycin: population based study",
"author": "Wong",
"journal-title": "BMJ",
"key": "10.1016/S1473-3099(24)00353-0_bib17",
"volume": "352",
"year": "2016"
},
{
"DOI": "10.1016/S1473-3099(21)00451-5",
"article-title": "Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study",
"author": "Wan",
"doi-asserted-by": "crossref",
"first-page": "64",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(24)00353-0_bib18",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-28068-3",
"article-title": "Multimorbidity and adverse events of special interest associated with COVID-19 vaccines in Hong Kong",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "411",
"journal-title": "Nat Commun",
"key": "10.1016/S1473-3099(24)00353-0_bib19",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1093/ckj/sfab158",
"article-title": "An introduction to inverse probability of treatment weighting in observational research",
"author": "Chesnaye",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Clin Kidney J",
"key": "10.1016/S1473-3099(24)00353-0_bib20",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"author": "Marshall",
"doi-asserted-by": "crossref",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(24)00353-0_bib21",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(21)00485-0",
"article-title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial",
"author": "Ader",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(24)00353-0_bib22",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00011-7",
"article-title": "Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "696",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(24)00353-0_bib23",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.7326/M16-2607",
"article-title": "Sensitivity analysis in observational research: introducing the E-value",
"author": "VanderWeele",
"doi-asserted-by": "crossref",
"first-page": "268",
"journal-title": "Ann Intern Med",
"key": "10.1016/S1473-3099(24)00353-0_bib24",
"volume": "167",
"year": "2017"
},
{
"article-title": "Remdesivir for the treatment of COVID-19",
"author": "Grundeis",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/S1473-3099(24)00353-0_bib25",
"volume": "1",
"year": "2023"
},
{
"DOI": "10.1080/22221751.2021.1898291",
"article-title": "Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic",
"author": "To",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Emerg Microbes Infect",
"key": "10.1016/S1473-3099(24)00353-0_bib26",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3390/ijms241914973",
"article-title": "Changes in lymphocyte subpopulations after remdesivir therapy for COVID-19: a brief report",
"author": "Cianci",
"doi-asserted-by": "crossref",
"journal-title": "Int J Mol Sci",
"key": "10.1016/S1473-3099(24)00353-0_bib27",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1128/AAC.00602-21",
"article-title": "Key metabolic enzymes involved in remdesivir activation in human lung cells",
"author": "Li",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/S1473-3099(24)00353-0_bib28",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.4049/jimmunol.1600055",
"article-title": "HIV protease inhibitor-induced cathepsin modulation alters antigen processing and cross-presentation",
"author": "Kourjian",
"doi-asserted-by": "crossref",
"first-page": "3595",
"journal-title": "J Immunol",
"key": "10.1016/S1473-3099(24)00353-0_bib29",
"volume": "196",
"year": "2016"
},
{
"DOI": "10.1016/j.antiviral.2022.105430",
"article-title": "Combination therapy with nirmatrelvir and molnupiravir improves the survival of SARS-CoV-2 infected mice",
"author": "Jeong",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/S1473-3099(24)00353-0_bib30",
"volume": "208",
"year": "2022"
},
{
"DOI": "10.1038/s42003-022-03101-9",
"article-title": "Combination of antiviral drugs inhibits SARS-CoV-2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "154",
"journal-title": "Commun Biol",
"key": "10.1016/S1473-3099(24)00353-0_bib31",
"volume": "5",
"year": "2022"
},
{
"article-title": "Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomised controlled study",
"author": "Liu",
"first-page": "1",
"journal-title": "Lancet Reg Health West Pac",
"key": "10.1016/S1473-3099(24)00353-0_bib32",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciad371",
"article-title": "Safety profile and clinical and virological outcomes of nirmatrelvir–ritonavir treatment in patients with advanced chronic kidney disease and coronavirus disease 2019",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "1406",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S1473-3099(24)00353-0_bib33",
"volume": "77",
"year": "2023"
},
{
"DOI": "10.1002/cpt.2646",
"article-title": "Recommendations for the management of drug–drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/S1473-3099(24)00353-0_bib34",
"volume": "112",
"year": "2022"
},
{
"article-title": "A practical guide to handling competing events in etiologic time-to-event studies",
"author": "Mansournia",
"journal-title": "Glob Epidemiol",
"key": "10.1016/S1473-3099(24)00353-0_bib35",
"volume": "4",
"year": "2022"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309924003530"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
choi7