SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy
et al., medRxiv, doi:10.1101/2023.06.23.23288598, POSITIVES, Jun 2023
Prospective study of 127 COVID-19 patients in the USA showing higher risk of replication-competent virologic rebound with paxlovid treatment.
Authors note that rebound substantially increases the duration of shedding of replication-competent virus.
When compared with previous studies, authors believe the higher frequency of rebound detected is due to the frequent sampling and culture analysis. When authors restrict to 3 timepoints with PCR only, as in prior studies, they detect a similar rate of rebound as in previous studies, but miss 80% of rebound events detected in this study.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
viral rebound, 760.8% higher, RR 8.61, p = 0.04, treatment 15 of 72 (20.8%), control 1 of 55 (1.8%), adjusted per study, odds ratio converted to relative risk, replication-competent virological rebound, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Edelstein et al., 27 Jun 2023, prospective, USA, preprint, 22 authors, POSITIVES trial.
Contact: msiedner@mgh.harvard.edu.
SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy
doi:10.1101/2023.06.23.23288598
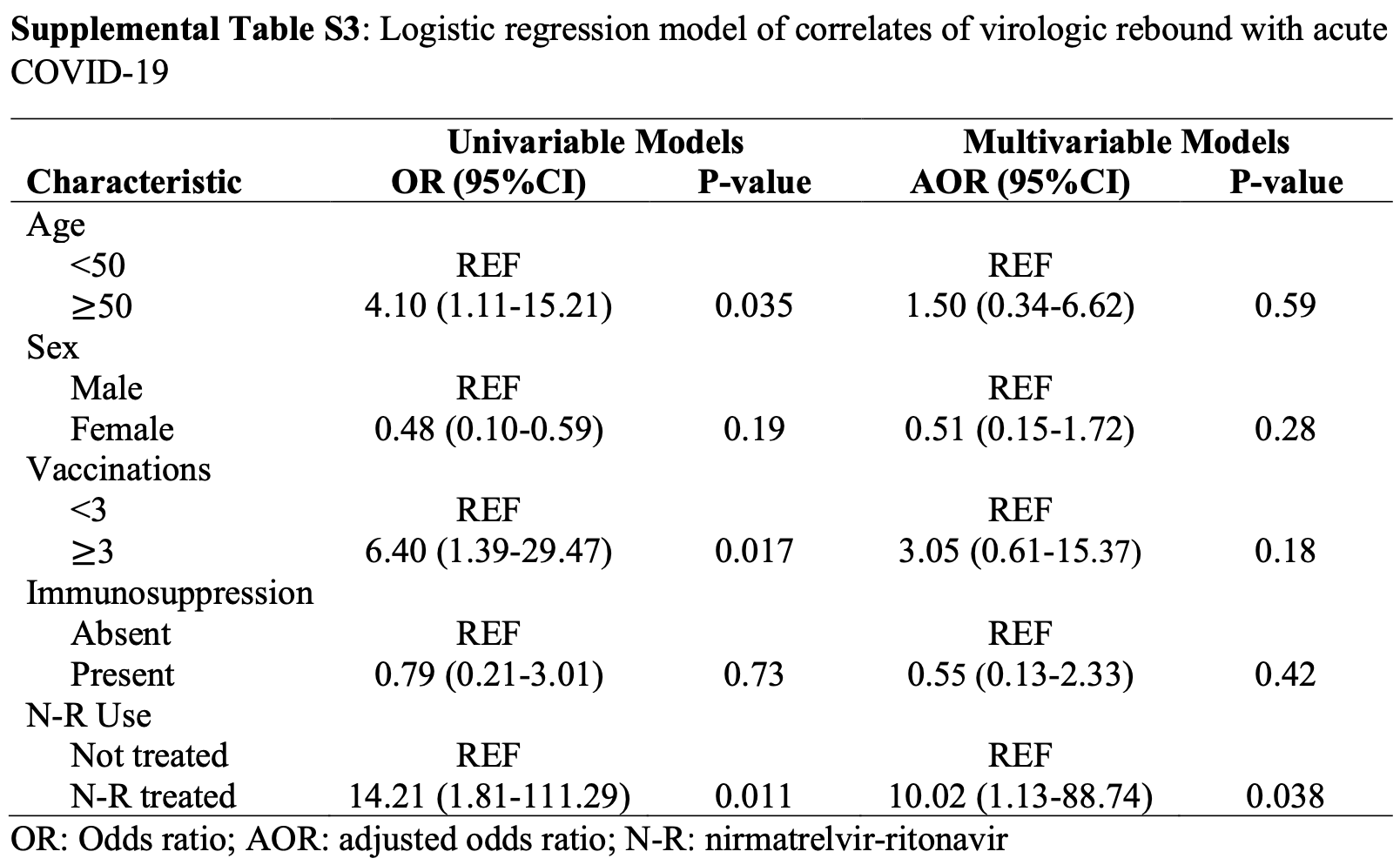
Objective: To compare the frequency of replication-competent virologic rebound with and without nirmatrelvir-ritonavir treatment for acute COVID-19. Secondary aims were to estimate the validity of symptoms to detect rebound and the incidence of emergent nirmatrelvir-resistance mutations after rebound. Design: Observational cohort study. Setting: Multicenter healthcare system in Boston, Massachusetts. Participants: We enrolled ambulatory adults with a positive COVID-19 test and/or a prescription for nirmatrelvir-ritonavir. Exposures: Receipt of 5 days of nirmatrelvir-ritonavir treatment versus no COVID-19 therapy. Main Outcome and Measures: The primary outcome was COVID-19 virologic rebound, defined as either (1) a positive SARS-CoV-2 viral culture following a prior negative culture or (2) two consecutive viral loads ≥4.0 log10 copies/milliliter after a prior reduction in viral load to <4.0 log10 copies/milliliter. Results: Compared with untreated individuals (n=55), those taking nirmatrelvir-ritonavir (n=72) were older, received more COVID-19 vaccinations, and were more commonly immunosuppressed. Fifteen individuals (20.8%) taking nirmatrelvir-ritonavir experienced virologic rebound versus one (1.8%) of the untreated (absolute difference 19.0% [95%CI 9.0-29.0%], P=0.001). In multivariable models, only N-R was associated with VR (AOR 10.02, 95%CI 1.13-88.74). VR occurred more commonly among those with earlier nirmatrelvirritonavir initiation (29.0%, 16.7% and 0% when initiated days 0, 1, and ≥2 after diagnosis, respectively, P=0.089). Among participants on N-R, those experiencing rebound had prolonged shedding of replication-competent virus compared to those that did not rebound (median: 14 vs 3 days). Only 8/16 with virologic rebound reported worsening symptoms (50%, 95%CI 25%-75%); 2 were completely asymptomatic. We detected no post-rebound nirmatrelvir-resistance mutations in the NSP5 protease gene. Conclusions and Relevance: Virologic rebound occurred in approximately one in five people taking nirmatrelvir-ritonavir and often occurred without worsening symptoms. Because it is associated with replication-competent viral shedding, close monitoring and potential isolation of those who rebound should be considered.
References
Anderson, Caubel, Rusnak, Nirmatrelvir-ritonavir and viral load rebound in COVID-19
Boucau, Uddin, Marino, Characterization of Virologic Rebound Following Nirmatrelvir-Ritonavir Treatment for Coronavirus Disease 2019 (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciac512
Charness, Gupta, Stack, Rebound of SARS-CoV-2 Infection after Nirmatrelvir-Ritonavir Treatment, N Engl J Med, doi:10.1056/NEJMc2206449
Deo, Choudhary, Moser, Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection, Ann Intern Med, doi:10.7326/M22-2381
Epling, Rocco, Boswell, Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment, Clin Infect Dis, doi:10.1093/cid/ciac663
Goyal, Reeves, Cardozo-Ojeda, Schiffer, Mayer, Viral load and contact heterogeneity predict SARS-CoV-2 transmission and super-spreading events, Walczak AM, doi:10.7554/eLife.63537
North, Barczak, Goldstein, Determining the Incidence of Asymptomatic SARS-CoV-2 Among Early Recipients of COVID-19 Vaccines (DISCOVER-COVID-19): A Prospective Cohort Study of Healthcare Workers Before, During and After Vaccination, Clin Infect Dis, doi:10.1093/cid/ciab643
Pandit, Radin, Chiang, The COVID-19 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated with Nirmatrelvir Plus Ritonavir Versus Untreated Controls, Clin Infect Dis. Published online February, doi:10.1093/cid/ciad102
Perelson, Ribeiro, Phan, National Institutes of Health. A Study to Learn About the Study Medicines (Nirmatrelvir Plus Ritonavir) in People Aged 12 Years or Older With COVID-19 and a Compromised Immune System (NCT05438602), doi:10.1101/2023.05.30.2329074713
Wong, Lau, Au, Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study, Lancet Infect Dis. Published online February, doi:10.1016/S1473-3099(22)00873-8
Wong, Yip, Lai, Wong, Hui et al., Incidence of Viral Rebound After Treatment With Nirmatrelvir-Ritonavir and Molnupiravir, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.45086
Wölfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
DOI record:
{
"DOI": "10.1101/2023.06.23.23288598",
"URL": "http://dx.doi.org/10.1101/2023.06.23.23288598",
"abstract": "<jats:p>Abstract Objective: To compare the frequency of replication-competent virologic rebound with and without nirmatrelvir-ritonavir treatment for acute COVID-19. Secondary aims were to estimate the validity of symptoms to detect rebound and the incidence of emergent nirmatrelvir-resistance mutations after rebound. Design: Observational cohort study. Setting: Multicenter healthcare system in Boston, Massachusetts. Participants: We enrolled ambulatory adults with a positive COVID-19 test and/or a prescription for nirmatrelvir-ritonavir. Exposures: Receipt of 5 days of nirmatrelvir-ritonavir treatment versus no COVID-19 therapy. Main Outcome and Measures: The primary outcome was COVID-19 virologic rebound, defined as either (1) a positive SARS-CoV-2 viral culture following a prior negative culture or (2) two consecutive viral loads ≥4.0 log10 copies/milliliter after a prior reduction in viral load to <4.0 log10 copies/milliliter. Results: Compared with untreated individuals (n=55), those taking nirmatrelvir-ritonavir (n=72) were older, received more COVID-19 vaccinations, and were more commonly immunosuppressed. Fifteen individuals (20.8%) taking nirmatrelvir-ritonavir experienced virologic rebound versus one (1.8%) of the untreated (absolute difference 19.0% [95%CI 9.0-29.0%], P=0.001). In multivariable models, only N-R was associated with VR (AOR 10.02, 95%CI 1.13-88.74). VR occurred more commonly among those with earlier nirmatrelvir-ritonavir initiation (29.0%, 16.7% and 0% when initiated days 0, 1, and ≥2 after diagnosis, respectively, P=0.089). Among participants on N-R, those experiencing rebound had prolonged shedding of replication-competent virus compared to those that did not rebound (median: 14 vs 3 days). Only 8/16 with virologic rebound reported worsening symptoms (50%, 95%CI 25%-75%); 2 were completely asymptomatic. We detected no post-rebound nirmatrelvir-resistance mutations in the NSP5 protease gene. Conclusions and Relevance: Virologic rebound occurred in approximately one in five people taking nirmatrelvir-ritonavir and often occurred without worsening symptoms. Because it is associated with replication-competent viral shedding, close monitoring and potential isolation of those who rebound should be considered.</jats:p>",
"accepted": {
"date-parts": [
[
2023,
6,
27
]
]
},
"author": [
{
"affiliation": [],
"family": "Edelstein",
"given": "Gregory E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Boucau",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uddin",
"given": "Rockib",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marino",
"given": "Caitlin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liew",
"given": "May Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barry",
"given": "Mamadou",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choudhary",
"given": "Manish C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gilbert",
"given": "Rebecca F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reynolds",
"given": "Zahra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yijia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tien",
"given": "Dessie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sagar",
"given": "Shruti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vyas",
"given": "Tammy D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kawano",
"given": "Yumeko",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5556-4618",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sparks",
"given": "Jeffrey A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hammond",
"given": "Sarah P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wallace",
"given": "Zachary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vyas",
"given": "Jatin M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barczak",
"given": "Amy K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lemieux",
"given": "Jacob E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Jonathan Z.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siedner",
"given": "Mark J.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
27
]
],
"date-time": "2023-06-27T19:30:16Z",
"timestamp": 1687894216000
},
"deposited": {
"date-parts": [
[
2023,
6,
27
]
],
"date-time": "2023-06-27T19:30:17Z",
"timestamp": 1687894217000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
6,
28
]
],
"date-time": "2023-06-28T04:34:37Z",
"timestamp": 1687926877196
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
6,
27
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2023.06.23.23288598",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
6,
27
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2023,
6,
27
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2023.06.23.23288598"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy",
"type": "posted-content"
}