Effectiveness of nirmatrelvir/ritonavir and molnupiravir on post-COVID-19 outcomes among outpatients: a target trial emulation investigation
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2025.2469648, Feb 2025
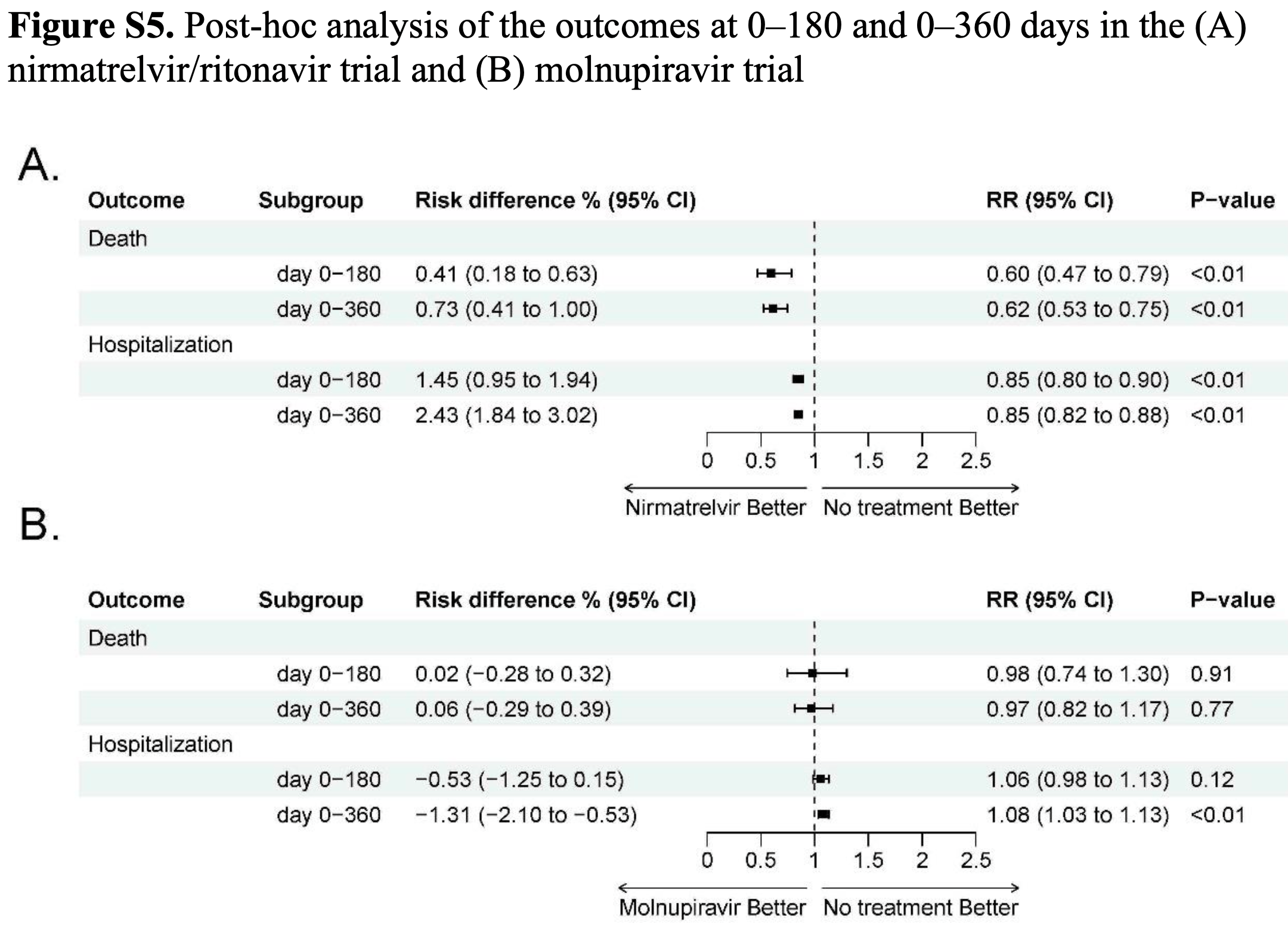
Retrospective target trial emulation of outpatients in Hong Kong showing reduced post-acute mortality and hospitalization with paxlovid, but no significant long-term benefit with molnupiravir. Figure 1 shows only 0.08% of patients were excluded for contraindications to paxlovid, which does not appear to be realistic for the population and suggests either a major limitation of the data or an error in the processing of contraindications, which will overestimate the efficacy of paxlovid.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending paxlovid also recommended them, or
because the patient seeking out paxlovid is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Malden et al. confirm significant bias in the use of paxlovid, showing that treated
patients are more likely to be from affluent neighborhoods, be more health-conscious, and
have better access to care. Campion et al. also show that female patients were more
likely to receive paxlovid, and studies show that female patients are significantly more
likely to be health-conscious, for example being more likely to take additional
non-prescription treatments.
Therefore, these kind of studies may
overestimate efficacy.
Resistance. Variants may be resistant to paxlovid6-13. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID14. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid15. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid16. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury17 and liver injury18,19. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound20-22.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments23.
Study covers molnupiravir and paxlovid.
|
risk of death, 38.0% lower, RR 0.62, p < 0.001, treatment 93,861, control 46,616, day 360.
|
|
risk of death, 40.0% lower, RR 0.60, p < 0.001, treatment 93,861, control 46,616, day 180.
|
|
risk of death, 61.0% lower, RR 0.39, p < 0.001, treatment 93,861, control 46,616, day 30.
|
|
risk of hospitalization, 15.0% lower, RR 0.85, p < 0.001, treatment 93,861, control 46,616, day 360.
|
|
risk of hospitalization, 15.0% lower, RR 0.85, p < 0.001, treatment 93,861, control 46,616, day 180.
|
|
risk of hospitalization, 11.0% lower, RR 0.89, p = 0.02, treatment 93,861, control 46,616, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Malden et al., Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system, Scientific Reports, doi:10.1038/s41598-024-57633-7.
5.
Campion et al., Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study, Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1809.
6.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
7.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
8.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
9.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
10.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
11.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
12.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
13.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
14.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
15.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
16.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
17.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
18.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
19.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
20.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
21.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Wei et al., 18 Feb 2025, retrospective, China, peer-reviewed, 19 authors.
Contact: marc@cuhk.edu.hk.
Effectiveness of nirmatrelvir/ritonavir and molnupiravir on post-COVID-19 outcomes among outpatients: a target trial emulation investigation
Emerging Microbes & Infections, doi:10.1080/22221751.2025.2469648
Limited studies compared the effectiveness of nirmatrelvir/ritonavir and molnupiravir against a control group on post-COVID-19 conditions. Our study examined the association of nirmatrelvir/ritonavir and molnupiravir with post-acute mortality and hospitalizations among outpatients using real-world outpatient records of COVID-19 designated clinics in Hong Kong. This is an observational study using a target trial emulation framework, involving nirmatrelvir-ritonavir versus no antiviral treatment (Trial 1) and molnupiravir versus no antiviral treatment (Trial 2). Outcomes included post-acute mortality, all-cause hospitalization, and hospitalization due to 13 selected sequelae. Relative effectiveness was assessed by comparing the cumulative incidence between two groups, reported as relative risk (RR), along with risk differences (RD) during day 0-30, 31-180, and 181-360. After screening, 140477 and 96030 patients were included in Trial 1 and 2, respectively. Compared with no treatment, nirmatrelvir/ritonavir-treated patients exhibited a significantly lower risk of post-acute mortality (31-180 days: RR, 0.71; 95% CI, 0.54-0.96; RD, 0.20%; 181-360 days: RR, 0.64; 95% CI, 0.50-0.82; RD, 0.32%) and all-cause hospitalization (31-180 days: RR, 0.82; 95% CI, 0.76-0.88; RD, 1.11%; 181-360 days: RR, 0.83; 95% CI, 0.78-0.89; RD, 1.18%). Patients receiving molnupiravir had a lower risk of 30-day mortality, but no significant beneficial effect was observed for the post-acute outcomes. In conclusion, this study demonstrated the effectiveness of nirmatrelvir/ritonavir in reducing post-COVID-19 outcomes among outpatients. While we observed the short-term effectiveness of molnupiravir in reducing mortality, no protective effect on long-term post-COVID-19 outcomes was observed.
Declarations
Ethics approval and consent to participate: This study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guideline. Ethics approval was obtained from the Joint Chinese University of Hong Kong and New Territories East Cluster clinical research ethics committee, Hong Kong (Ref No. 2023.006). As this study was a retrospective analysis using secondary data without any personal information, the requirement for informed consent was waived.
Consent for publication: Not applicable.
Competing interests: We declare that we have no conflicts of interest.
Contributions: Study design and conceptualisation: YWei, CB, KMJ, GL, KCC. Data collection and pre-processing: YWei, HW, CHKY, TYC, ZG, EKY. Data analysis and interpretation: YWei, CB, KMJ, KCC. Writing -Original Draft: YWei, CB, KMJ, HW, GL, CTH, XJ, CL, SZ, CKPM, DSCH, KCC. Writing -Review and Editing: HW, CHKY, TYC, KL, AY, EKY. EKY and KCC have accessed and verified all the data. All authors critically reviewed the manuscript and gave final approval for publication. Table S4 . Illustration of censoring and weighting during the 5-days grace period.
Person (i) Time Treatment Weight not being censored from the treatment arm within grace period Weight not being censored from the no treatment arm within grace period ) 0 p0 = probability of being censored from the treatment arm in Day 0, p1 = probability of being censored from the no treatment arm in day 1, etc...
References
Arbel, Sagy, Hoshen, Nirmatrelvir Use and Severe Covid -19 Outcomes during the Omicron Surge, N Engl J Med
Bajema, Berry, Streja, Rajeevan, Li et al., Effectiveness of COVID-19 Treatment With Nirmatrelvir-Ritonavir or Molnupiravir Among U.S. Veterans: Target Trial Emulation Studies With One-Month and Six-Month Outcomes, Ann Intern Med
Durstenfeld, Peluso, Lin, Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study, J Med Virol
Fung, Baye, Baik, Mcdonald, Nirmatrelvir and Molnupiravir and Post-COVID-19 Condition in Older Patients, JAMA Intern Med
Guo, Zhao, Mok, So, Yam et al., Comparing the incubation period, serial interval, and infectiousness profile between SARS-CoV-2 Omicron and Delta variants, J Med Virol
Hammond, Fountaine, Yunis, Fleishaker, Almas et al., Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl Med
Hsu, Shiau, Tsai, Wu, Liu et al., The effect of molnupiravir on post-acute outcome of COVID-19 survivors, J Infect
Ioannou, Berry, Rajeevan, Effectiveness of Nirmatrelvir-Ritonavir Against the Development of Post-COVID-19 Conditions Among U.S. Veterans : A Target Trial Emulation, Ann Intern Med
Kostev, Smith, Koyanagi, Konrad, Jacob, Post-COVID-19 conditions in children and adolescents diagnosed with COVID-19, Pediatr Res
Lam, Wong, Zhang, Long-term post-acute sequelae of COVID-19 infection: a retrospective, multi-database cohort study in Hong Kong and the UK, EClinicalMedicine
Lin, Wei, Wang, Boyer, Jia et al., Association of nirmatrelvir-ritonavir with post-acute sequelae and mortality among patients who are immunocompromised with COVID-19 in Hong Kong: a retrospective cohort study, Lancet Rheumatol
Sebők, Gyires, Long COVID and possible preventive options, Inflammopharmacology
Vangeel, Chiu, Jonghe, Maes, Slechten et al., Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Wang, Wei, Hung, Lin, Jiang et al., Association of nirmatrelvir-ritonavir with post-acute sequelae and mortality in patients admitted to hospital with COVID-19: a retrospective cohort study, Lancet Infect Dis
Wang, Wei, Lin, Boyer, Jia et al., COVID-19 vaccination modified the effect of nirmatrelvir-ritonavir on post-acute mortality and rehospitalization: a retrospective cohort study, Emerg Microbes Infect
Wei, Jia, Zhao, Hung, Mok et al., Estimation of Vaccine Effectiveness of CoronaVac and BNT162b2 Against Severe Outcomes Over Time Among Patients With SARS-CoV-2 Omicron, JAMA Netw Open
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis
Xie, Choi, Al-Aly, Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition, JAMA Intern Med
Xie, Choi, Al-Aly, Molnupiravir and risk of post-acute sequelae of covid-19: cohort study, BMJ
Zuo, Yu, Campbell, Yamamoto, Yuan, The implementation of target trial emulation for causal inference: a scoping review, J Clin Epidemiol
DOI record:
{
"DOI": "10.1080/22221751.2025.2469648",
"ISSN": [
"2222-1751"
],
"URL": "http://dx.doi.org/10.1080/22221751.2025.2469648",
"alternative-id": [
"10.1080/22221751.2025.2469648"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=temi20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=temi20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-02-18"
}
],
"author": [
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Wei",
"given": "Yuchen",
"sequence": "first",
"suffix": "PhD"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health",
"place": [
"Boston, Massachusetts, United States"
]
}
],
"family": "Boyer",
"given": "Christopher",
"sequence": "additional",
"suffix": "PhD"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health",
"place": [
"Boston, Massachusetts, United States"
]
}
],
"family": "Jia",
"given": "Katherine Min",
"sequence": "additional",
"suffix": "MSc"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Lin",
"given": "Guozhang",
"sequence": "additional",
"suffix": "MSc"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Wang",
"given": "Huwen",
"sequence": "additional",
"suffix": "MPhil"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Li",
"given": "Conglu",
"sequence": "additional",
"suffix": "MSc"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Hung",
"given": "Chi Tim",
"sequence": "additional",
"suffix": "MBBS"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Jiang",
"given": "Xiaoting",
"sequence": "additional",
"suffix": "MSc"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Kwan Yam",
"given": "Carrie Ho",
"sequence": "additional",
"suffix": "PhD"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Chow",
"given": "Tsz Yu",
"sequence": "additional",
"suffix": "BSc"
},
{
"affiliation": [
{
"name": "The University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Wang",
"given": "Yawen",
"sequence": "additional",
"suffix": "PhD"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
},
{
"name": "School of Public Health, Tianjin Medical University",
"place": [
"Tianjin, China"
]
}
],
"family": "Zhao",
"given": "Shi",
"sequence": "additional",
"suffix": "PhD"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Guo",
"given": "Zihao",
"sequence": "additional",
"suffix": "MSc"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Li",
"given": "Kehang",
"sequence": "additional",
"suffix": "MSc"
},
{
"affiliation": [
{
"name": "The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Yang",
"given": "Aimin",
"sequence": "additional",
"suffix": "PhD"
},
{
"affiliation": [
{
"name": "Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Pun Mok",
"given": "Chris Ka",
"sequence": "additional",
"suffix": "PhD"
},
{
"affiliation": [
{
"name": "S.H. Ho Research Centre for Infectious Diseases, Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Hui",
"given": "David SC",
"sequence": "additional",
"suffix": "MBBS"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Chong",
"given": "Ka Chun",
"sequence": "additional",
"suffix": "PhD"
},
{
"affiliation": [
{
"name": "School of Public Health and Primary Care, The Chinese University of Hong Kong",
"place": [
"Hong Kong"
]
}
],
"family": "Yeoh",
"given": "Eng Kiong",
"sequence": "additional",
"suffix": "MBBS"
}
],
"container-title": "Emerging Microbes & Infections",
"container-title-short": "Emerging Microbes & Infections",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2025,
2,
18
]
],
"date-time": "2025-02-18T13:19:16Z",
"timestamp": 1739884756000
},
"deposited": {
"date-parts": [
[
2025,
2,
18
]
],
"date-time": "2025-02-18T13:19:33Z",
"timestamp": 1739884773000
},
"funder": [
{
"DOI": "10.13039/501100005847",
"award": [
"COVID190105, COVID19F03, INF-CUHK-1, COVID1903003"
],
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/501100005847",
"id-type": "DOI"
}
],
"name": "Health and Medical Research Fund"
},
{
"award": [
"C6036-21GF"
],
"name": "RGC Collaborative Research Fund"
},
{
"award": [
"T11-705/21-N"
],
"name": "RGC theme-based research schemes"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
19
]
],
"date-time": "2025-02-19T05:14:51Z",
"timestamp": 1739942091801,
"version": "3.37.3"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
2,
18
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
18
]
],
"date-time": "2025-02-18T00:00:00Z",
"timestamp": 1739836800000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/22221751.2025.2469648",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"prefix": "10.1080",
"published": {
"date-parts": [
[
2025,
2,
18
]
]
},
"published-online": {
"date-parts": [
[
2025,
2,
18
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"key": "e_1_3_2_2_1",
"unstructured": "Fact sheet for healthcare providers: emergency use authorization for Paxlovid™. (www.fda.gov/media/155050/download)"
},
{
"key": "e_1_3_2_3_1",
"unstructured": "Fact sheet for healthcare providers: emergency use authorization for Lagevrio™ (molnupiravir) capsules. (www.fda.gov/media/155054/download)"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_4_1",
"unstructured": "Hammond J Leister-Tebbe H Gardner A Abreu P Bao W Wisemandle W Baniecki M Hendrick VM Damle B Simón-Campos A Pypstra R Rusnak JM; EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk Nonhospitalized Adults with Covid-19. N Engl J Med. 2022 Apr 14;386(15):1397-1408."
},
{
"DOI": "10.1056/NEJMoa2204919",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_5_1",
"unstructured": "Arbel R Wolff Sagy Y Hoshen M et al. Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge. N Engl J Med 2022; 387(9): 790-8."
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_6_1",
"unstructured": "Wong CKH Au ICH Lau KTK Lau EHY Cowling BJ Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis 2022; 22(12): 1681-93"
},
{
"DOI": "10.1056/NEJMoa2309003",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_7_1",
"unstructured": "Hammond J Fountaine RJ Yunis C Fleishaker D Almas M Bao W Wisemandle W Rusnak JM. Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19. N Engl J Med 2024;360:1186-1195."
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_8_1",
"unstructured": "Xie Y Choi T Al-Aly Z. Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern Med 2023; 183(6): 554-64."
},
{
"DOI": "10.1016/S1473-3099(24)00217-2",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_9_1",
"unstructured": "Wang H Wei Y Hung CT Lin G Jiang X Li C Jia KM Yam CHK Chow TY Ho JY Wang Y Zhao S Guo Z Li K Yang A Mok CKP Hui DSC Yeoh EK Chong KC. Association of nirmatrelvir-ritonavir with post-acute sequelae and mortality in patients admitted to hospital with COVID-19: a retrospective cohort study. Lancet Infect Dis. 2024 May 3:S1473-3099(24)00217-2."
},
{
"DOI": "10.7326/M23-1394",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_10_1",
"unstructured": "Ioannou GN Berry K Rajeevan N et al. Effectiveness of Nirmatrelvir-Ritonavir Against the Development of Post-COVID-19 Conditions Among U.S. Veterans : A Target Trial Emulation. Ann Intern Med 2023; 176(11): 1486-97."
},
{
"DOI": "10.1002/jmv.29333",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_11_1",
"unstructured": "Durstenfeld MS Peluso MJ Lin F et al. Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study. J Med Virol 2024; 96(1): e29333."
},
{
"DOI": "10.7326/M22-3565",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_12_1",
"unstructured": "Bajema KL Berry K Streja E Rajeevan N Li Y Mutalik P Yan L Cunningham F Hynes DM Rowneki M Bohnert A Boyko EJ Iwashyna TJ Maciejewski ML Osborne TF Viglianti EM Aslan M Huang GD Ioannou GN. Effectiveness of COVID-19 Treatment With Nirmatrelvir-Ritonavir or Molnupiravir Among U.S. Veterans: Target Trial Emulation Studies With One-Month and Six-Month Outcomes. Ann Intern Med. 2023 Jun;176(6):807-816."
},
{
"DOI": "10.1001/jamainternmed.2023.5099",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_13_1",
"unstructured": "Fung KW Baye F Baik SH McDonald CJ. Nirmatrelvir and Molnupiravir and Post-COVID-19 Condition in Older Patients. JAMA Intern Med. 2023 Dec 1;183(12):1404-1406."
},
{
"DOI": "10.1016/j.jinf.2023.03.016",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_14_1",
"unstructured": "Hsu WH Shiau BW Tsai YW Wu JY Liu TH Chuang MH Lai CC. The effect of molnupiravir on post-acute outcome of COVID-19 survivors. J Infect. 2023 Oct;87(4):339-343."
},
{
"DOI": "10.1136/bmj-2022-074572",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_15_1",
"unstructured": "Xie Y Choi T Al-Aly Z. Molnupiravir and risk of post-acute sequelae of covid-19: cohort study. BMJ. 2023 Apr 25;381:e074572."
},
{
"DOI": "10.1001/jamanetworkopen.2022.54777",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_16_1",
"unstructured": "Wei Y Jia KM Zhao S Hung CT Mok CKP Poon PKM Man Leung EY Wang MH Yam CHK Chow TY Guo Z Yeoh EK Chong KC. Estimation of Vaccine Effectiveness of CoronaVac and BNT162b2 Against Severe Outcomes Over Time Among Patients With SARS-CoV-2 Omicron. JAMA Netw Open. 2023 Feb 1;6(2):e2254777."
},
{
"key": "e_1_3_2_17_1",
"unstructured": "Lin G Wei Y Wang H Boyer C Jia KM Hung CT Jiang X Li C Yam CHK Chow TY Wang Y Zhao S Guo Z Li K Yang A Mok CKP Hui DSC Chong KC Yeoh EK. Association of nirmatrelvir-ritonavir with post-acute sequelae and mortality among patients who are immunocompromised with COVID-19 in Hong Kong: a retrospective cohort study. Lancet Rheumatol. 2024 Nov 8:S2665-9913(24)00224-8."
},
{
"DOI": "10.1016/j.eclinm.2023.102000",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_18_1",
"unstructured": "Lam ICH Wong CKH Zhang R et al. Long-term post-acute sequelae of COVID-19 infection: a retrospective multi-database cohort study in Hong Kong and the UK. EClinicalMedicine 2023; 60: 102000."
},
{
"DOI": "10.1007/s10787-023-01204-1",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_19_1",
"unstructured": "Sebők S Gyires K. Long COVID and possible preventive options. Inflammopharmacology 2023; 31(6): 2807-17."
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_20_1",
"unstructured": "Vangeel L Chiu W De Jonghe S Maes P Slechten B Raymenants J André E Leyssen P Neyts J Jochmans D. Remdesivir Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022 Feb;198:105252."
},
{
"DOI": "10.1038/s41390-022-02111-x",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_21_1",
"unstructured": "Kostev K Smith L Koyanagi A Konrad M Jacob L. Post-COVID-19 conditions in children and adolescents diagnosed with COVID-19. Pediatr Res. 2024 Jan;95(1):182-187."
},
{
"DOI": "10.1002/jmv.28648",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_22_1",
"unstructured": "Guo Z Zhao S Mok CKP So RTY Yam CHK Chow TY Chan TCP Wei Y Jia KM Wang MH Chong KC Yeoh EK. Comparing the incubation period serial interval and infectiousness profile between SARS-CoV-2 Omicron and Delta variants. J Med Virol. 2023 Mar;95(3):e28648."
},
{
"key": "e_1_3_2_23_1",
"unstructured": "National Institutes of Health. Therapeutic Management of Nonhospitalized Adults With COVID-19. Updated 20 April 2023. Accessed at www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeuticmanagement on 21 March 2023."
},
{
"DOI": "10.1080/22221751.2024.2421397",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_24_1",
"unstructured": "Wang H Wei Y Lin G Boyer C Jia KM Hung CT Jiang X Li C Yam CHK Chow TY Wang Y Zhao S Guo Z Li K Yang A Mok CKP Hui DSC Chong KC Yeoh EK. COVID-19 vaccination modified the effect of nirmatrelvir-ritonavir on post-acute mortality and rehospitalization: a retrospective cohort study. Emerg Microbes Infect. 2024 Dec;13(1):2421397."
},
{
"DOI": "10.1016/j.jclinepi.2023.08.003",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_25_1",
"unstructured": "Zuo H Yu L Campbell SM Yamamoto SS Yuan Y. The implementation of target trial emulation for causal inference: a scoping review. J Clin Epidemiol. 2023 Oct;162:29-37."
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/22221751.2025.2469648"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effectiveness of nirmatrelvir/ritonavir and molnupiravir on post-COVID-19 outcomes among outpatients: a target trial emulation investigation",
"type": "journal-article",
"update-policy": "https://doi.org/10.1080/tandf_crossmark_01"
}
wei5