The Role of Bifidobacterium in COVID-19: A Systematic Review
et al., Life, doi:10.3390/life13091847, Aug 2023
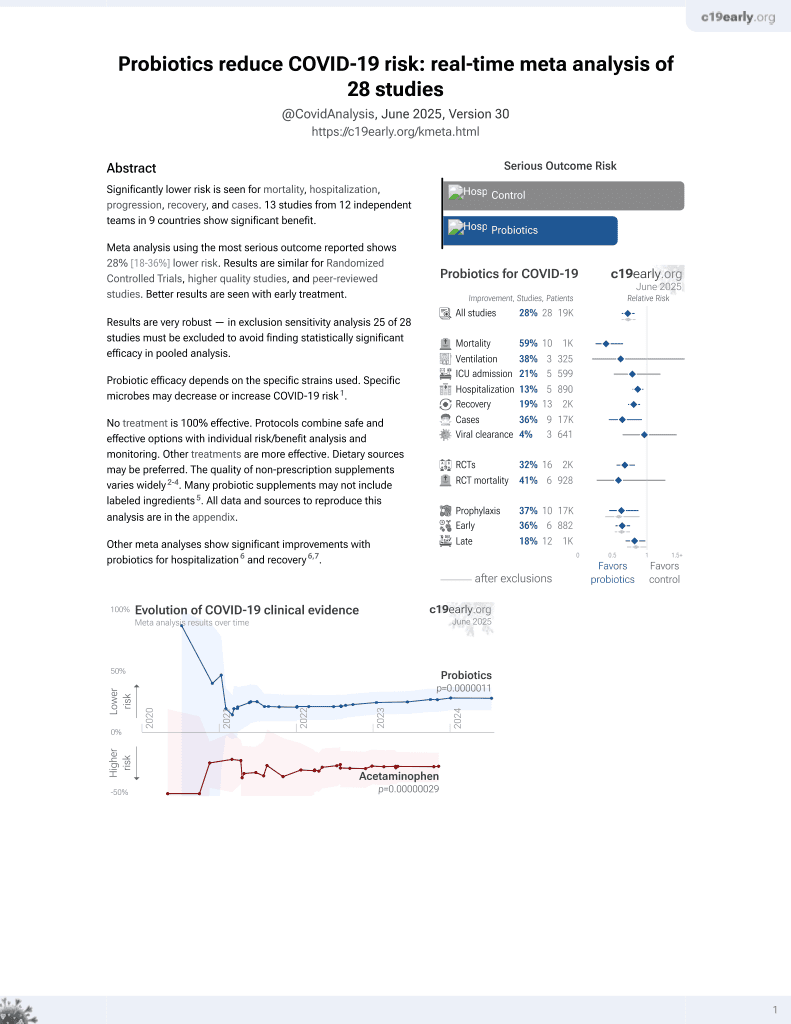
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of studies investigating the relationship between the gut bacteria genus Bifidobacterium and COVID-19. Observational studies found that lower abundance of Bifidobacterium was associated with more severe COVID-19. Interventional studies evaluating Bifidobacterium probiotics for COVID-19 patients showed benefits including reduced symptoms, inflammation, and mortality. Potential mechanisms include Bifidobacterium's ability to modulate immune response, reduce inflammation, outcompete pathogens, and maintain gut barrier integrity. See also1.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk2.
1.
Taufer et al., Lactobacilli in COVID-19: A Systematic Review Based on Next-Generation Sequencing Studies, Microorganisms, doi:10.3390/microorganisms12020284.
2.
Li et al., Large-scale genetic correlation studies explore the causal relationship and potential mechanism between gut microbiota and COVID-19-associated risks, BMC Microbiology, doi:10.1186/s12866-024-03423-0.
3.
Chau et al., Effectiveness of probiotics on COVID-19 prevention and treatment against mild COVID-19 in outpatient care: A systematic review, Nutrition and Health, doi:10.1177/02601060251378200.
4.
Bajić et al., Immunity's core reset: Synbiotics and gut microbiota in the COVID-19 era, Innate Immunity, doi:10.1177/17534259251362023.
5.
Bigman et al., A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery, Nutrients, doi:10.3390/nu17111802.
6.
Fazli et al., Possible Link between Gut Microbiota, Diet, and COVID-19 Infection, Journal of Medical Bacteriology, 12:4, jmb.tums.ac.ir/index.php/jmb/article/view/525.
7.
Santa et al., Comparative analysis of COVID-19 responses in Japan and Africa: diet, phytochemicals, vitamin D, and gut microbiota in reducing mortality—A systematic review and meta-analysis, Frontiers in Nutrition, doi:10.3389/fnut.2024.1465324.
8.
Kaushal, A., Nutraceuticals and pharmacological to balance the transitional microbiome to extend immunity during COVID-19 and other viral infections, Journal of Translational Medicine, doi:10.1186/s12967-024-05587-9.
9.
Mu et al., Anti-inflammatory and Nutritional Interventions Against SARS-CoV-2: A Comprehensive Review, Journal of Agriculture and Food Research, doi:10.1016/j.jafr.2024.101422.
10.
Righi et al., Gut Microbiome Disruption Following SARS-CoV-2: A Review, Microorganisms, doi:10.3390/microorganisms12010131.
11.
Petrariu et al., Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases, Frontiers in Microbiology, doi:10.3389/fmicb.2023.1296447.
12.
Taufer (B) et al., The Role of Bifidobacterium in COVID-19: A Systematic Review, Life, doi:10.3390/life13091847.
13.
Di Pierro, F., A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SARS-CoV-2, Minerva Medica, doi:10.23736/S0026-4806.20.06570-2.
14.
Kurian et al., Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects, Archives of Medical Research, doi:10.1016/j.arcmed.2021.03.002.
15.
Singh et al., Probiotics: A potential immunomodulator in COVID-19 infection management, Nutrition Research, doi:10.1016/j.nutres.2020.12.014.
16.
Stavropoulou et al., Probiotics as a Weapon in the Fight Against COVID-19, Frontiers in Nutrition, doi:10.3389/fnut.2020.614986.
Taufer et al., 31 Aug 2023, peer-reviewed, 2 authors.
Contact: prampelotto@hcpa.edu.br (corresponding author).
The Role of Bifidobacterium in COVID-19: A Systematic Review
Life, doi:10.3390/life13091847
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, mainly causes respiratory and intestinal symptoms and changes in the microbiota of patients. We performed a systematic search in major databases using "Bifidobacterium" and "COVID-19" or "SARS-CoV-2" as key terms to assess the relationship of the genus to COVID-19. After the selection steps, 25 articles were analyzed. Of these, eighteen were observational, and seven were interventional articles that evaluated the use of Bifidobacterium alone or in mix as probiotics for additional treatment of patients with COVID-19. All stages and severities were contemplated, including post-COVID-19 patients. Overall, Bifidobacterium was associated with both protective effects and reduced abundance in relation to the disease. The genus has been found to be abundant in some cases and linked to disease severity. The studies evaluating the use of Bifidobacterium as probiotics have demonstrated the potential of this genus in reducing symptoms, improving pulmonary function, reducing inflammatory markers, alleviating gastrointestinal symptoms, and even contributing to better control of mortality. In summary, Bifidobacterium may offer protection against COVID-19 through its ability to modulate the immune response, reduce inflammation, compete with pathogenic microbes, and maintain gut barrier function. The findings provide valuable insights into the relationship between the disease and the genus Bifidobacterium, highlighting the potential of microbiota modulation in the treatment of COVID-19.
Author Contributions: C.R.T.: Data collection and analysis, writing (original draft). P.H.R.: Conceptualization, supervision, analysis of results, and writing (revision). All authors have read and agreed to the published version of the manuscript. Funding: C.R.T. is the recipient of a CAPES grant (nº 88887.513461/2020-00). P.H.R. is the recipient of a CAPES grant (nº 88887.798411/2022-00). Institutional Review Board Statement: Not applicable. Informed Consent Statement: Not applicable.
References
Albrich, Ghosh, Ahearn-Ford, Mikaeloff, Lunjani et al., A High-Risk Gut Microbiota Configuration Associates with Fatal Hyperinflammatory Immune and Metabolic Responses to SARS-CoV-2, Gut Microbes, doi:10.1080/19490976.2022.2073131
Alessandri, Ossiprandi, Macsharry, Van Sinderen, Ventura, Bifidobacterial Dialogue With Its Human Host and Consequent Modulation of the Immune System, Front. Immunol, doi:10.3389/fimmu.2019.02348
Alessandri, Van Sinderen, Ventura, The Genus Bifidobacterium: From Genomics to Functionality of an Important Component of the Mammalian Gut Microbiota, Comput. Struct. Biotechnol. J, doi:10.1016/j.csbj.2021.03.006
Bozkurt, Bilen, Oral Booster Probiotic Bifidobacteria in SARS-CoV-2 Patients, Int. J. Immunopathol. Pharmacol, doi:10.1177/20587384211059677
Ceccarelli, Borrazzo, Pinacchio, Santinelli, Innocenti et al., Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study, Front. Nutr, doi:10.3389/fnut.2020.613928
Cui, Rao, Zeng, Wang, Ren et al., Characterization of Oral and Gut Microbiome and Plasma Metabolomics in COVID-19 Patients after 1-Year Follow-Up, Mil. Med. Res, doi:10.1186/s40779-022-00387-y
De Vrese, Winkler, Rautenberg, Harder, Noah et al., Effect of Lactobacillus Gasseri PA 16/8, Bifidobacterium Longum SP 07/3, B. bifidum MF 20/5 on Common Cold Episodes: A Double Blind, Randomized, Controlled Trial, Clin. Nutr, doi:10.1016/j.clnu.2005.02.006
Erdo Ǧan, Tanyeri, Torun, Gönüllü, Arslan et al., The Comparition of the Efficacy of Two Different Probiotics in Rotavirus Gastroenteritis in Children, J. Trop. Med, doi:10.1155/2012/787240
Ettorre, Ceccarelli, Marazzato, Campagna, Pinacchio et al., Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19, Front. Med, doi:10.3389/fmed.2020.00389
Ewaschuk, Diaz, Meddings, Diederichs, Dmytrash et al., Secreted Bioactive Factors from Bifidobacterium Infantis Enhance Epithelial Cell Barrier Function, Am. J. Physiol. Gastrointest. Liver Physiol, doi:10.1152/ajpgi.90227.2008
Fattahi, Heidari, Khosroushahi, Review of Short-Chain Fatty Acids Effects on the Immune System and Cancer, Food Biosci, doi:10.1016/j.fbio.2020.100793
Ferreira-Junior, Borgonovi, De Salis, Leite, Dantas et al., Detection of Intestinal Dysbiosis in Post-COVID-19 Patients One to Eight Months after Acute Disease Resolution, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph191610189
Fujiwara, Hashiba, Hirota, Forstner, Proteinaceous Factor(s) in Culture Supernatant Fluids of Bifidobacteria Which Prevents the Binding of Enterotoxigenic Escherichia Coli to Gangliotetraosylceramide, Appl. Environ. Microbiol, doi:10.1128/aem.63.2.506-512.1997
Gopal, Prasad, Smart, Gill, In Vitro Adherence Properties of Lactobacillus Rhamnosus DR20 and Bifidobacterium Lactis DR10 Strains and Their Antagonistic Activity against an Enterotoxigenic Escherichia Coli, Int. J. Food Microbiol, doi:10.1016/S0168-1605(01)00440-8
Groeger, Schiavi, Grant, Kurnik-Łucka, Michalovich et al., Intranasal Bifidobacterium Longum Protects against Viral-Induced Lung Inflammation and Injury in a Murine Model of Lethal Influenza Infection, eBioMedicine, doi:10.1016/j.ebiom.2020.102981
Gumenyuk, Golod, Silaeva, Sorokina, Ilyasov et al., Gut Microbiota Alterations and Their Relationship To the Disease Severity and Some Cytokine Profile Indicators in Patients With COVID-19, Bull. Russ. State Med. Univ, doi:10.24075/brsmu.2022.006
Hazan, Stollman, Bozkurt, Dave, Papoutsis et al., Lost Microbes of COVID-19: Bifidobacterium, Faecalibacterium Depletion and Decreased Microbiome Diversity Associated with SARS-CoV-2 Infection Severity, BMJ Open Gastroenterol, doi:10.1136/bmjgast-2022-000871
Hidalgo-Cantabrana, Delgado, Ruiz, Ruas-Madiedo, Sánchez et al., Bifidobacteria and Their Health-Promoting Effects, Microbiol. Spectr, doi:10.1128/microbiolspec.BAD-0010-2016
Islam, Foysal, Hoque, Mehedi, Rob et al., Dysbiosis of Oral and Gut Microbiomes in SARS-CoV-2 Infected Patients in Bangladesh: Elucidating the Role of Opportunistic Gut Microbes, Front. Med, doi:10.3389/fmed.2022.821777
Ivashkin, Fomin, Moiseev, Brovko, Maslennikov et al., Efficacy of a Probiotic Consisting of Lacticaseibacillus Rhamnosus PDV 1705, Bifidobacterium Bifidum PDV 0903, Bifidobacterium Longum Subsp. Infantis PDV 1911, and Bifidobacterium Longum Subsp. Longum PDV 2301 in the Treatment of Hospitalized Patients Wit, Probiotics Antimicrob. Proteins, doi:10.1007/s12602-021-09858-5
Kawahara, Takahashi, Oishi, Tanaka, Masuda et al., Consecutive Oral Administration of Bifidobacterium Longum MM-2 Improves the Defense System against Influenza Virus Infection by Enhancing Natural Killer Cell Activity in a Murine Model, Microbiol. Immunol, doi:10.1111/1348-0421.12210
Kim, Zhang, Rauseo, Goss, Mudd et al., The Salivary and Nasopharyngeal Microbiomes Are Associated with SARS-CoV-2 Infection and Disease Severity, J. Med. Virol, doi:10.1002/jmv.28445
Laterza, Putignani, Settanni, Petito, Varca et al., Ecology and Machine Learning-Based Classification Models of Gut Microbiota and Inflammatory Markers May Evaluate the Effects of Probiotic Supplementation in Patients Recently Recovered from COVID-19, Int. J. Mol. Sci, doi:10.3390/ijms24076623
Leyer, Li, Mubasher, Reifer, Ouwehand, Probiotic Effects on Cold and Influenza-like Symptom Incidence and Duration in Children, Pediatrics, doi:10.1542/peds.2008-2666
Li, Cheng, Xu, Su, Cai et al., The Role of Probiotics in Coronavirus Disease-19 Infection in Wuhan: A Retrospective Study of 311 Severe Patients, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.107531
Li, Yang, Zhou, Disoma, Dong et al., Microbiome Profiling Using Shotgun Metagenomic Sequencing Identified Unique Microorganisms in COVID-19 Patients with Altered Gut Microbiota, Front. Microbiol, doi:10.3389/fmicb.2021.712081
Lievin, Peiffer, Hudault, Rochat, Brassart et al., Bifidobacterium Strains from Resident Infant Human Gastrointestinal Microflora Exert Antimicrobial Activity, Gut, doi:10.1136/gut.47.5.646
Liu, Mak, Su, Yeoh, Lui et al., Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome, Gut, doi:10.1136/gutjnl-2021-325989
Liu, Zhang, Dong, Guo, Adhesion and Immunomodulatory Effects of Bifidobacterium Lactis HN019 on Intestinal Epithelial Cells INT-407, World J. Gastroenterol, doi:10.3748/wjg.v16.i18.2283
Lu, Fang, Liu, Li, Zhang et al., The Potential Role of Probiotics in Protection against Influenza a Virus Infection in Mice, Foods, doi:10.3390/foods10040902
Maddah, Goodarzi, Asadi-Yousefabad, Abbasluo, Shariati et al., Evaluation of the Gut Microbiome Associated with COVID-19, Inform. Med. Unlocked, doi:10.1016/j.imu.2023.101239
Mahooti, Abdolalipour, Salehzadeh, Mohebbi, Gorji et al., Immunomodulatory and Prophylactic Effects of Bifidobacterium Bifidum Probiotic Strain on Influenza Infection in Mice, World J. Microbiol. Biotechnol, doi:10.1007/s11274-019-2667-0
Marfia, Navone, Guarnaccia, Campanella, Mondoni et al., Decreased Serum Level of Sphingosine-1-phosphate: A Novel Predictor of Clinical Severity in COVID-19, EMBO Mol. Med, doi:10.15252/emmm.202013424
Mazzarelli, Giancola, Fontana, Piselli, Binda et al., Gut Microbiota Composition in COVID-19 Hospitalized Patients with Mild or Severe Symptoms, Front. Microbiol, doi:10.3389/fmicb.2022.1049215
Mirzayi, Renson, Furlanello, Sansone, Zohra et al., Reporting Guidelines for Human Microbiome Research: The STORMS Checklist, Nat. Med, doi:10.1038/s41591-021-01552-x
Moola, Munn, Tufanaru, Aromataris, Sears et al., Chapter 7: Systematic Reviews of Etiology and Risk
Ouzzani, Hammady, Fedorowicz, Elmagarmid, Rayyan-a Web and Mobile App for Systematic Reviews, Syst. Rev, doi:10.1186/s13643-016-0384-4
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews, BMJ, doi:10.1136/bmj.n71
Qin, Zheng, Yao, Guo, Zuo et al., Influence of H7N9 Virus Infection and Associated Treatment on Human Gut Microbiota, Sci. Rep, doi:10.1038/srep14771
Reinold, Farahpour, Fehring, Dolff, Konik et al., A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates With Severe COVID-19, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2021.747816
Romani, Del Chierico, Macari, Pane, Ristori et al., The Relationship Between Pediatric Gut Microbiota and SARS-CoV-2 Infection, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2022.908492
Rueca, Fontana, Bartolini, Piselli, Mazzarelli et al., Investigation of Nasal/Oropharyngeal Microbial Community of COVID-19 Patients by 16S RDNA Sequencing, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph18042174
Saviano, Potenza, Siciliano, Petruzziello, Tarli et al., COVID-19 Pneumonia and Gut Inflammation: The Role of a Mix of Three Probiotic Strains in Reducing Inflammatory Markers and Need for Oxygen Support, J. Clin. Med, doi:10.3390/jcm11133758
Segal, Mak, Mullish, Alexander, Ng et al., The Gut Microbiome: An under-Recognised Contributor to the COVID-19 Pandemic?, Therap. Adv. Gastroenterol, doi:10.1177/1756284820974914
Sgorbati, Biavati, Palenzona, The Genus Bifidobacterium
Sterne, Hernán, Reeves, Savović, Berkman et al., A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions, BMJ, doi:10.1136/bmj.i4919
Sterne, Savović, Page, Elbers, Blencowe et al., RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials, BMJ, doi:10.1136/bmj.l4898
Sun, Song, Liu, Tan, Lin et al., Gut Microbiome Alterations and Gut Barrier Dysfunction Are Associated with Host Immune Homeostasis in COVID-19 Patients, BMC Med, doi:10.1186/s12916-021-02212-0
Suskun, Kilic, Yilmaz Ciftdogan, Guven, Karbuz et al., Intestinal Microbiota Composition of Children with Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Multisystem Inflammatory Syndrome (MIS-C), Eur. J. Pediatr, doi:10.1007/s00431-022-04494-9
Tojo, Suárez, Clemente, De, Reyes-Gavilán et al., Intestinal Microbiota in Health and Disease: Role of Bifidobacteria in Gut Homeostasis, World J. Gastroenterol, doi:10.3748/wjg.v20.i41.15163
Turroni, Bifidobacteria: From Ecology to Genomics, Front. Biosci, doi:10.2741/3559
Usta-Gorgun, Yilmaz-Ersan, Short-Chain Fatty Acids Production by Bifidobacterium Species in the Presence of Salep, Electron. J. Biotechnol, doi:10.1016/j.ejbt.2020.06.004
Wang, Usyk, Sollecito, Qiu, Williams-Nguyen et al., Altered Gut Microbiota and Host Metabolite Profiles in Women with Human Immunodeficiency Virus, Clin. Infect. Dis, doi:10.1093/cid/ciz1117
Wang, Xiao, Yao, Guo, Lu et al., The Role of Bifidobacteria in Gut Barrier Function after Thermal Injury in Rats, J. Trauma Inj. Infect. Crit. Care, doi:10.1097/01.ta.0000196574.70614.27
Wang, Zhou, Lu, Hu, Xiao et al., Altered Gut Microbiota Composition in Children and Their Caregivers Infected with the SARS-CoV-2 Omicron Variant, World J. Pediatr, doi:10.1007/s12519-022-00659-6
Yan, Yang, Ross, Stanton, Zhang et al., Bifidobacterium Longum Subsp. Longum YS108R Fermented Milk Alleviates DSS Induced Colitis via Anti-Inflammation, Mucosal Barrier Maintenance and Gut Microbiota Modulation, J. Funct. Foods, doi:10.1016/j.jff.2020.104153
Yao, Zhao, Wang, Liu, Bifidobacterium Longum: Protection against Inflammatory Bowel Disease, J. Immunol. Res, doi:10.1155/2021/8030297
Yasui, Kiyoshima, Hori, Shida, Protection against Influenza Virus Infection of Mice Fed Bifidobacterium Breve YIT4064, Clin. Diagn. Lab. Immunol, doi:10.1128/CDLI.6.2.186-192.1999
Yeoh, Zuo, Lui, Zhang, Liu et al., Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19, Gut, doi:10.1136/gutjnl-2020-323020
Yitbarek, Weese, Alkie, Parkinson, Sharif, Influenza A Virus Subtype H9N2 Infection Disrupts the Composition of Intestinal Microbiota of Chickens, FEMS Microbiol. Ecol, doi:10.1093/femsec/fix165
Zhang, Hu, Feng, Hu, Wang et al., Influenza Infection Elicits an Expansion of Gut Population of Endogenous Bifidobacterium Animalis Which Protects Mice against Infection, Genome Biol, doi:10.1186/s13059-020-02007-1
DOI record:
{
"DOI": "10.3390/life13091847",
"ISSN": [
"2075-1729"
],
"URL": "http://dx.doi.org/10.3390/life13091847",
"abstract": "<jats:p>The COVID-19 pandemic, caused by the SARS-CoV-2 virus, mainly causes respiratory and intestinal symptoms and changes in the microbiota of patients. We performed a systematic search in major databases using “Bifidobacterium” and “COVID-19” or “SARS-CoV-2” as key terms to assess the relationship of the genus to COVID-19. After the selection steps, 25 articles were analyzed. Of these, eighteen were observational, and seven were interventional articles that evaluated the use of Bifidobacterium alone or in mix as probiotics for additional treatment of patients with COVID-19. All stages and severities were contemplated, including post-COVID-19 patients. Overall, Bifidobacterium was associated with both protective effects and reduced abundance in relation to the disease. The genus has been found to be abundant in some cases and linked to disease severity. The studies evaluating the use of Bifidobacterium as probiotics have demonstrated the potential of this genus in reducing symptoms, improving pulmonary function, reducing inflammatory markers, alleviating gastrointestinal symptoms, and even contributing to better control of mortality. In summary, Bifidobacterium may offer protection against COVID-19 through its ability to modulate the immune response, reduce inflammation, compete with pathogenic microbes, and maintain gut barrier function. The findings provide valuable insights into the relationship between the disease and the genus Bifidobacterium, highlighting the potential of microbiota modulation in the treatment of COVID-19.</jats:p>",
"alternative-id": [
"life13091847"
],
"author": [
{
"affiliation": [
{
"name": "Graduate Program in Genetics and Molecular Biology, Universidade Federal do Rio Grande do Sul, Porto Alegre 91501-970, Brazil"
}
],
"family": "Taufer",
"given": "Clarissa Reginato",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8992-9697",
"affiliation": [
{
"name": "Bioinformatics and Biostatistics Core Facility, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre 91501-970, Brazil"
},
{
"name": "Graduate Program in Biological Sciences: Pharmacology and Therapeutics, Universidade Federal do Rio Grande do Sul, Porto Alegre 91501-970, Brazil"
}
],
"authenticated-orcid": false,
"family": "Rampelotto",
"given": "Pabulo Henrique",
"sequence": "additional"
}
],
"container-title": "Life",
"container-title-short": "Life",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T15:33:25Z",
"timestamp": 1693496005000
},
"deposited": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T16:06:55Z",
"timestamp": 1693498015000
},
"funder": [
{
"DOI": "10.13039/501100002322",
"award": [
"88887.513461/2020-00",
"88887.798411/2022-00"
],
"doi-asserted-by": "publisher",
"name": "CAPES"
}
],
"indexed": {
"date-parts": [
[
2023,
9,
1
]
],
"date-time": "2023-09-01T13:46:26Z",
"timestamp": 1693575986486
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2023,
8,
31
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2023,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T00:00:00Z",
"timestamp": 1693440000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2075-1729/13/9/1847/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1847",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
8,
31
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
31
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1177/1756284820974914",
"article-title": "The Gut Microbiome: An under-Recognised Contributor to the COVID-19 Pandemic?",
"author": "Segal",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Therap. Adv. Gastroenterol.",
"key": "ref_1",
"volume": "13",
"year": "2020"
},
{
"article-title": "Bifidobacteria and Their Health-Promoting Effects",
"author": "Delgado",
"first-page": "1",
"journal-title": "Microbiol. Spectr.",
"key": "ref_2",
"volume": "5",
"year": "2017"
},
{
"DOI": "10.1186/s13643-016-0384-4",
"article-title": "Rayyan-a Web and Mobile App for Systematic Reviews",
"author": "Ouzzani",
"doi-asserted-by": "crossref",
"first-page": "210",
"journal-title": "Syst. Rev.",
"key": "ref_3",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.1136/bmj.n71",
"article-title": "The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews",
"author": "Page",
"doi-asserted-by": "crossref",
"first-page": "n71",
"journal-title": "BMJ",
"key": "ref_4",
"volume": "372",
"year": "2021"
},
{
"key": "ref_5",
"unstructured": "Aromataris, E., and Munn, Z. (2020). JBI Manual for Evidence Synthesis, JBI."
},
{
"DOI": "10.1136/bmj.l4898",
"article-title": "RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "l4898",
"journal-title": "BMJ",
"key": "ref_6",
"volume": "366",
"year": "2019"
},
{
"article-title": "ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions",
"author": "Sterne",
"first-page": "4",
"journal-title": "BMJ",
"key": "ref_7",
"volume": "355",
"year": "2016"
},
{
"DOI": "10.1080/19490976.2022.2073131",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Albrich, W.C., Ghosh, T.S., Ahearn-Ford, S., Mikaeloff, F., Lunjani, N., Forde, B., Suh, N., Kleger, G.-R., Pietsch, U., and Frischknecht, M. (2022). A High-Risk Gut Microbiota Configuration Associates with Fatal Hyperinflammatory Immune and Metabolic Responses to SARS-CoV-2. Gut Microbes, 14."
},
{
"article-title": "Characterization of Oral and Gut Microbiome and Plasma Metabolomics in COVID-19 Patients after 1-Year Follow-Up",
"author": "Cui",
"first-page": "32",
"journal-title": "Mil. Med. Res.",
"key": "ref_9",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.3390/ijerph191610189",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Ferreira-Junior, A.S., Borgonovi, T.F., De Salis, L.V.V., Leite, A.Z., Dantas, A.S., De Salis, G.V.V., Cruz, G.N.F., De Oliveira, L.F.V., Gomes, E., and Penna, A.L.B. (2022). Detection of Intestinal Dysbiosis in Post-COVID-19 Patients One to Eight Months after Acute Disease Resolution. Int. J. Environ. Res. Public Health, 19."
},
{
"DOI": "10.24075/brsmu.2022.006",
"doi-asserted-by": "crossref",
"key": "ref_11",
"unstructured": "Gumenyuk, L.N., Golod, M.V., Silaeva, N.V., Sorokina, L.E., Ilyasov, S.S., Androschyuk, N.A., Krivoshapko, O.R., Velilyaev, A.M., and Asanova, L.N. (2022). Gut Microbiota Alterations and Their Relationship To the Disease Severity and Some Cytokine Profile Indicators in Patients With COVID-19. Bull. Russ. State Med. Univ., 22–29."
},
{
"DOI": "10.1136/bmjgast-2022-000871",
"article-title": "Lost Microbes of COVID-19: Bifidobacterium, Faecalibacterium Depletion and Decreased Microbiome Diversity Associated with SARS-CoV-2 Infection Severity",
"author": "Hazan",
"doi-asserted-by": "crossref",
"first-page": "e000871",
"journal-title": "BMJ Open Gastroenterol.",
"key": "ref_12",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28445",
"article-title": "The Salivary and Nasopharyngeal Microbiomes Are Associated with SARS-CoV-2 Infection and Disease Severity",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Med. Virol.",
"key": "ref_13",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.3389/fmicb.2021.712081",
"article-title": "Microbiome Profiling Using Shotgun Metagenomic Sequencing Identified Unique Microorganisms in COVID-19 Patients with Altered Gut Microbiota",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "712081",
"journal-title": "Front. Microbiol.",
"key": "ref_14",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1136/gutjnl-2021-325989",
"article-title": "Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "544",
"journal-title": "Gut",
"key": "ref_15",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1016/j.imu.2023.101239",
"article-title": "Evaluation of the Gut Microbiome Associated with COVID-19",
"author": "Maddah",
"doi-asserted-by": "crossref",
"first-page": "101239",
"journal-title": "Inform. Med. Unlocked",
"key": "ref_16",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.3389/fmicb.2022.1049215",
"article-title": "Gut Microbiota Composition in COVID-19 Hospitalized Patients with Mild or Severe Symptoms",
"author": "Mazzarelli",
"doi-asserted-by": "crossref",
"first-page": "1049215",
"journal-title": "Front. Microbiol.",
"key": "ref_17",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2022.821777",
"article-title": "Dysbiosis of Oral and Gut Microbiomes in SARS-CoV-2 Infected Patients in Bangladesh: Elucidating the Role of Opportunistic Gut Microbes",
"author": "Foysal",
"doi-asserted-by": "crossref",
"first-page": "821777",
"journal-title": "Front. Med.",
"key": "ref_18",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.3389/fcimb.2021.747816",
"article-title": "A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates With Severe COVID-19",
"author": "Reinold",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "ref_19",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3389/fcimb.2022.908492",
"article-title": "The Relationship Between Pediatric Gut Microbiota and SARS-CoV-2 Infection",
"author": "Romani",
"doi-asserted-by": "crossref",
"first-page": "908492",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "ref_20",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/ijerph18042174",
"doi-asserted-by": "crossref",
"key": "ref_21",
"unstructured": "Rueca, M., Fontana, A., Bartolini, B., Piselli, P., Mazzarelli, A., Copetti, M., Binda, E., Perri, F., Gruber, C.E.M., and Nicastri, E. (2021). Investigation of Nasal/Oropharyngeal Microbial Community of COVID-19 Patients by 16S RDNA Sequencing. Int. J. Environ. Res. Public Health, 18."
},
{
"DOI": "10.1186/s12916-021-02212-0",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Sun, Z., Song, Z., Liu, C., Tan, S., Lin, S., Zhu, J., Dai, F., Gao, J., She, J., and Mei, Z. (2022). Gut Microbiome Alterations and Gut Barrier Dysfunction Are Associated with Host Immune Homeostasis in COVID-19 Patients. BMC Med., 20."
},
{
"DOI": "10.1007/s00431-022-04494-9",
"article-title": "Intestinal Microbiota Composition of Children with Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Multisystem Inflammatory Syndrome (MIS-C)",
"author": "Suskun",
"doi-asserted-by": "crossref",
"first-page": "3175",
"journal-title": "Eur. J. Pediatr.",
"key": "ref_23",
"volume": "181",
"year": "2022"
},
{
"DOI": "10.1007/s12519-022-00659-6",
"article-title": "Altered Gut Microbiota Composition in Children and Their Caregivers Infected with the SARS-CoV-2 Omicron Variant",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "478",
"journal-title": "World J. Pediatr.",
"key": "ref_24",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"article-title": "Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19",
"author": "Yeoh",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "Gut",
"key": "ref_25",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1177/20587384211059677",
"article-title": "Oral Booster Probiotic Bifidobacteria in SARS-CoV-2 Patients",
"author": "Bozkurt",
"doi-asserted-by": "crossref",
"first-page": "205873842110596",
"journal-title": "Int. J. Immunopathol. Pharmacol.",
"key": "ref_26",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.3389/fnut.2020.613928",
"article-title": "Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study",
"author": "Ceccarelli",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Front. Nutr.",
"key": "ref_27",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2020.00389",
"article-title": "Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19",
"author": "Ceccarelli",
"doi-asserted-by": "crossref",
"first-page": "389",
"journal-title": "Front. Med.",
"key": "ref_28",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1007/s12602-021-09858-5",
"article-title": "Efficacy of a Probiotic Consisting of Lacticaseibacillus Rhamnosus PDV 1705, Bifidobacterium Bifidum PDV 0903, Bifidobacterium Longum Subsp. Infantis PDV 1911, and Bifidobacterium Longum Subsp. Longum PDV 2301 in the Treatment of Hospitalized Patients Wit",
"author": "Ivashkin",
"doi-asserted-by": "crossref",
"first-page": "460",
"journal-title": "Probiotics Antimicrob. Proteins",
"key": "ref_29",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.3390/ijms24076623",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Laterza, L., Putignani, L., Settanni, C.R., Petito, V., Varca, S., De Maio, F., Macari, G., Guarrasi, V., Gremese, E., and Tolusso, B. (2023). Ecology and Machine Learning-Based Classification Models of Gut Microbiota and Inflammatory Markers May Evaluate the Effects of Probiotic Supplementation in Patients Recently Recovered from COVID-19. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.1016/j.intimp.2021.107531",
"article-title": "The Role of Probiotics in Coronavirus Disease-19 Infection in Wuhan: A Retrospective Study of 311 Severe Patients",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "107531",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_31",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.3390/jcm11133758",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Saviano, A., Potenza, A., Siciliano, V., Petruzziello, C., Tarli, C., Migneco, A., Nasella, F., Franceschi, F., and Ojetti, V. (2022). COVID-19 Pneumonia and Gut Inflammation: The Role of a Mix of Three Probiotic Strains in Reducing Inflammatory Markers and Need for Oxygen Support. J. Clin. Med., 11."
},
{
"key": "ref_33",
"unstructured": "Sgorbati, B., Biavati, B., and Palenzona, D. (1995). The Genera of Lactic Acid Bacteria, Springer US."
},
{
"DOI": "10.2741/3559",
"article-title": "Bifidobacteria: From Ecology to Genomics",
"author": "Turroni",
"doi-asserted-by": "crossref",
"first-page": "4673",
"journal-title": "Front. Biosci.",
"key": "ref_34",
"volume": "14",
"year": "2009"
},
{
"DOI": "10.1016/j.csbj.2021.03.006",
"article-title": "The Genus Bifidobacterium: From Genomics to Functionality of an Important Component of the Mammalian Gut Microbiota",
"author": "Alessandri",
"doi-asserted-by": "crossref",
"first-page": "1472",
"journal-title": "Comput. Struct. Biotechnol. J.",
"key": "ref_35",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1152/ajpgi.90227.2008",
"article-title": "Secreted Bioactive Factors from Bifidobacterium Infantis Enhance Epithelial Cell Barrier Function",
"author": "Ewaschuk",
"doi-asserted-by": "crossref",
"first-page": "1025",
"journal-title": "Am. J. Physiol. Gastrointest. Liver Physiol.",
"key": "ref_36",
"volume": "295",
"year": "2008"
},
{
"DOI": "10.3389/fimmu.2019.02348",
"article-title": "Bifidobacterial Dialogue With Its Human Host and Consequent Modulation of the Immune System",
"author": "Alessandri",
"doi-asserted-by": "crossref",
"first-page": "2348",
"journal-title": "Front. Immunol.",
"key": "ref_37",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.3748/wjg.v20.i41.15163",
"article-title": "Intestinal Microbiota in Health and Disease: Role of Bifidobacteria in Gut Homeostasis",
"author": "Tojo",
"doi-asserted-by": "crossref",
"first-page": "15163",
"journal-title": "World J. Gastroenterol.",
"key": "ref_38",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1016/j.ejbt.2020.06.004",
"article-title": "Short-Chain Fatty Acids Production by Bifidobacterium Species in the Presence of Salep",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Electron. J. Biotechnol.",
"key": "ref_39",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.1038/s41591-021-01552-x",
"article-title": "Reporting Guidelines for Human Microbiome Research: The STORMS Checklist",
"author": "Mirzayi",
"doi-asserted-by": "crossref",
"first-page": "1885",
"journal-title": "Nat. Med.",
"key": "ref_40",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1038/srep14771",
"article-title": "Influence of H7N9 Virus Infection and Associated Treatment on Human Gut Microbiota",
"author": "Qin",
"doi-asserted-by": "crossref",
"first-page": "14771",
"journal-title": "Sci. Rep.",
"key": "ref_41",
"volume": "5",
"year": "2015"
},
{
"DOI": "10.1093/cid/ciz1117",
"article-title": "Altered Gut Microbiota and Host Metabolite Profiles in Women with Human Immunodeficiency Virus",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "2345",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_42",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1093/femsec/fix165",
"article-title": "Influenza A Virus Subtype H9N2 Infection Disrupts the Composition of Intestinal Microbiota of Chickens",
"author": "Yitbarek",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "FEMS Microbiol. Ecol.",
"key": "ref_43",
"volume": "94",
"year": "2018"
},
{
"DOI": "10.1186/s13059-020-02007-1",
"article-title": "Influenza Infection Elicits an Expansion of Gut Population of Endogenous Bifidobacterium Animalis Which Protects Mice against Infection",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Genome Biol.",
"key": "ref_44",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1128/CDLI.6.2.186-192.1999",
"article-title": "Protection against Influenza Virus Infection of Mice Fed Bifidobacterium Breve YIT4064",
"author": "Yasui",
"doi-asserted-by": "crossref",
"first-page": "186",
"journal-title": "Clin. Diagn. Lab. Immunol.",
"key": "ref_45",
"volume": "6",
"year": "1999"
},
{
"DOI": "10.1016/j.ebiom.2020.102981",
"article-title": "Intranasal Bifidobacterium Longum Protects against Viral-Induced Lung Inflammation and Injury in a Murine Model of Lethal Influenza Infection",
"author": "Groeger",
"doi-asserted-by": "crossref",
"first-page": "102981",
"journal-title": "eBioMedicine",
"key": "ref_46",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1111/1348-0421.12210",
"article-title": "Consecutive Oral Administration of Bifidobacterium Longum MM-2 Improves the Defense System against Influenza Virus Infection by Enhancing Natural Killer Cell Activity in a Murine Model",
"author": "Kawahara",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Microbiol. Immunol.",
"key": "ref_47",
"volume": "59",
"year": "2015"
},
{
"article-title": "The Comparition of the Efficacy of Two Different Probiotics in Rotavirus Gastroenteritis in Children",
"author": "Tanyeri",
"first-page": "787240",
"journal-title": "J. Trop. Med.",
"key": "ref_48",
"volume": "2012",
"year": "2012"
},
{
"DOI": "10.1542/peds.2008-2666",
"article-title": "Probiotic Effects on Cold and Influenza-like Symptom Incidence and Duration in Children",
"author": "Leyer",
"doi-asserted-by": "crossref",
"first-page": "e172",
"journal-title": "Pediatrics",
"key": "ref_49",
"volume": "124",
"year": "2009"
},
{
"DOI": "10.1016/j.clnu.2005.02.006",
"article-title": "Effect of Lactobacillus Gasseri PA 16/8, Bifidobacterium Longum SP 07/3, B. bifidum MF 20/5 on Common Cold Episodes: A Double Blind, Randomized, Controlled Trial",
"author": "Winkler",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Clin. Nutr.",
"key": "ref_50",
"volume": "24",
"year": "2005"
},
{
"DOI": "10.3390/foods10040902",
"doi-asserted-by": "crossref",
"key": "ref_51",
"unstructured": "Lu, W., Fang, Z., Liu, X., Li, L., Zhang, P., Zhao, J., Zhang, H., and Chen, W. (2021). The Potential Role of Probiotics in Protection against Influenza a Virus Infection in Mice. Foods, 10."
},
{
"DOI": "10.1007/s11274-019-2667-0",
"article-title": "Immunomodulatory and Prophylactic Effects of Bifidobacterium Bifidum Probiotic Strain on Influenza Infection in Mice",
"author": "Mahooti",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "World J. Microbiol. Biotechnol.",
"key": "ref_52",
"volume": "35",
"year": "2019"
},
{
"DOI": "10.1016/j.fbio.2020.100793",
"article-title": "Review of Short-Chain Fatty Acids Effects on the Immune System and Cancer",
"author": "Fattahi",
"doi-asserted-by": "crossref",
"first-page": "100793",
"journal-title": "Food Biosci.",
"key": "ref_53",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.15252/emmm.202013424",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "Marfia, G., Navone, S., Guarnaccia, L., Campanella, R., Mondoni, M., Locatelli, M., Barassi, A., Fontana, L., Palumbo, F., and Garzia, E. (2021). Decreased Serum Level of Sphingosine-1-phosphate: A Novel Predictor of Clinical Severity in COVID-19. EMBO Mol. Med., 13."
},
{
"DOI": "10.1155/2021/8030297",
"article-title": "Bifidobacterium Longum: Protection against Inflammatory Bowel Disease",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "8030297",
"journal-title": "J. Immunol. Res.",
"key": "ref_55",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1016/S0168-1605(01)00440-8",
"article-title": "In Vitro Adherence Properties of Lactobacillus Rhamnosus DR20 and Bifidobacterium Lactis DR10 Strains and Their Antagonistic Activity against an Enterotoxigenic Escherichia Coli",
"author": "Gopal",
"doi-asserted-by": "crossref",
"first-page": "207",
"journal-title": "Int. J. Food Microbiol.",
"key": "ref_56",
"volume": "67",
"year": "2001"
},
{
"DOI": "10.1128/aem.63.2.506-512.1997",
"article-title": "Proteinaceous Factor(s) in Culture Supernatant Fluids of Bifidobacteria Which Prevents the Binding of Enterotoxigenic Escherichia Coli to Gangliotetraosylceramide",
"author": "Fujiwara",
"doi-asserted-by": "crossref",
"first-page": "506",
"journal-title": "Appl. Environ. Microbiol.",
"key": "ref_57",
"volume": "63",
"year": "1997"
},
{
"DOI": "10.3748/wjg.v16.i18.2283",
"article-title": "Adhesion and Immunomodulatory Effects of Bifidobacterium Lactis HN019 on Intestinal Epithelial Cells INT-407",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "2283",
"journal-title": "World J. Gastroenterol.",
"key": "ref_58",
"volume": "16",
"year": "2010"
},
{
"DOI": "10.1136/gut.47.5.646",
"article-title": "Bifidobacterium Strains from Resident Infant Human Gastrointestinal Microflora Exert Antimicrobial Activity",
"author": "Lievin",
"doi-asserted-by": "crossref",
"first-page": "646",
"journal-title": "Gut",
"key": "ref_59",
"volume": "47",
"year": "2000"
},
{
"DOI": "10.1097/01.ta.0000196574.70614.27",
"article-title": "The Role of Bifidobacteria in Gut Barrier Function after Thermal Injury in Rats",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "650",
"journal-title": "J. Trauma Inj. Infect. Crit. Care",
"key": "ref_60",
"volume": "61",
"year": "2006"
},
{
"DOI": "10.1016/j.jff.2020.104153",
"article-title": "Bifidobacterium Longum Subsp. Longum YS108R Fermented Milk Alleviates DSS Induced Colitis via Anti-Inflammation, Mucosal Barrier Maintenance and Gut Microbiota Modulation",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "104153",
"journal-title": "J. Funct. Foods",
"key": "ref_61",
"volume": "73",
"year": "2020"
}
],
"reference-count": 61,
"references-count": 61,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2075-1729/13/9/1847"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Paleontology",
"Space and Planetary Science",
"General Biochemistry, Genetics and Molecular Biology",
"Ecology, Evolution, Behavior and Systematics"
],
"subtitle": [],
"title": "The Role of Bifidobacterium in COVID-19: A Systematic Review",
"type": "journal-article",
"volume": "13"
}