Lactobacilli in COVID-19: A Systematic Review Based on Next-Generation Sequencing Studies
et al., Microorganisms, doi:10.3390/microorganisms12020284, Jan 2024
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
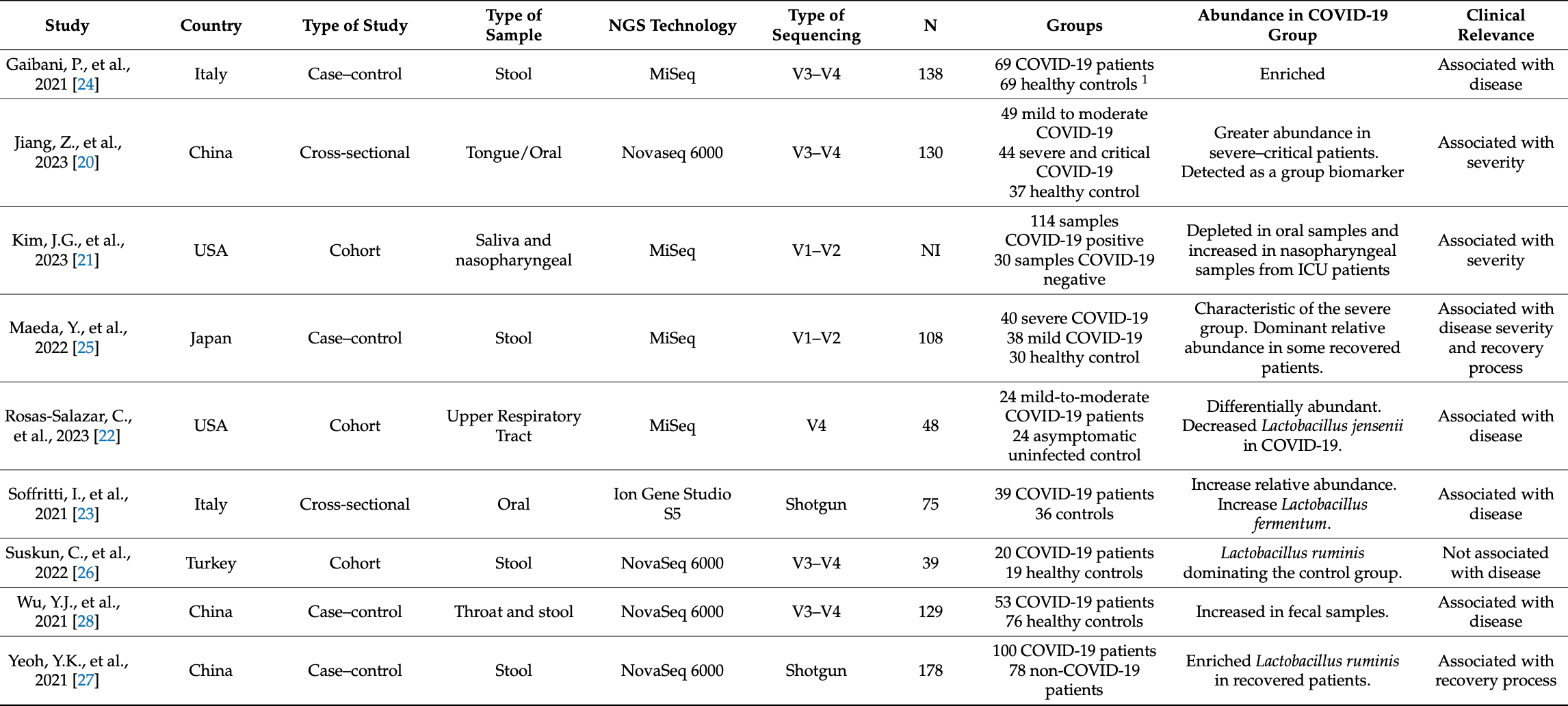
Review of nine studies using next generation sequencing to analyze the association between lactobacilli and COVID-19, focusing on the gut and upper respiratory tract microbiota. Studies showed that lactobacilli have reduced abundance in COVID-19 and are associated with disease severity. Proposed protective mechanisms are multifaceted, involving complex microbiota, immune system, and virus interactions. See also Taufer et al.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk2.
1.
Taufer et al., The Role of Bifidobacterium in COVID-19: A Systematic Review, Life, doi:10.3390/life13091847.
2.
Li et al., Large-scale genetic correlation studies explore the causal relationship and potential mechanism between gut microbiota and COVID-19-associated risks, BMC Microbiology, doi:10.1186/s12866-024-03423-0.
3.
Chau et al., Effectiveness of probiotics on COVID-19 prevention and treatment against mild COVID-19 in outpatient care: A systematic review, Nutrition and Health, doi:10.1177/02601060251378200.
4.
Bajić et al., Immunity's core reset: Synbiotics and gut microbiota in the COVID-19 era, Innate Immunity, doi:10.1177/17534259251362023.
5.
Bigman et al., A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery, Nutrients, doi:10.3390/nu17111802.
6.
Fazli et al., Possible Link between Gut Microbiota, Diet, and COVID-19 Infection, Journal of Medical Bacteriology, 12:4, jmb.tums.ac.ir/index.php/jmb/article/view/525.
7.
Santa et al., Comparative analysis of COVID-19 responses in Japan and Africa: diet, phytochemicals, vitamin D, and gut microbiota in reducing mortality—A systematic review and meta-analysis, Frontiers in Nutrition, doi:10.3389/fnut.2024.1465324.
8.
Kaushal, A., Nutraceuticals and pharmacological to balance the transitional microbiome to extend immunity during COVID-19 and other viral infections, Journal of Translational Medicine, doi:10.1186/s12967-024-05587-9.
9.
Mu et al., Anti-inflammatory and Nutritional Interventions Against SARS-CoV-2: A Comprehensive Review, Journal of Agriculture and Food Research, doi:10.1016/j.jafr.2024.101422.
10.
Taufer (B) et al., Lactobacilli in COVID-19: A Systematic Review Based on Next-Generation Sequencing Studies, Microorganisms, doi:10.3390/microorganisms12020284.
11.
Righi et al., Gut Microbiome Disruption Following SARS-CoV-2: A Review, Microorganisms, doi:10.3390/microorganisms12010131.
12.
Petrariu et al., Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases, Frontiers in Microbiology, doi:10.3389/fmicb.2023.1296447.
13.
Di Pierro, F., A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SARS-CoV-2, Minerva Medica, doi:10.23736/S0026-4806.20.06570-2.
14.
Kurian et al., Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects, Archives of Medical Research, doi:10.1016/j.arcmed.2021.03.002.
15.
Singh et al., Probiotics: A potential immunomodulator in COVID-19 infection management, Nutrition Research, doi:10.1016/j.nutres.2020.12.014.
16.
Stavropoulou et al., Probiotics as a Weapon in the Fight Against COVID-19, Frontiers in Nutrition, doi:10.3389/fnut.2020.614986.
Taufer et al., 29 Jan 2024, peer-reviewed, 2 authors.
Contact: prampelotto@hcpa.edu.br (corresponding author).
Lactobacilli in COVID-19: A Systematic Review Based on Next-Generation Sequencing Studies
Microorganisms, doi:10.3390/microorganisms12020284
The global pandemic was caused by the SARS-CoV-2 virus, known as COVID-19, which primarily affects the respiratory and intestinal systems and impacts the microbial communities of patients. This systematic review involved a comprehensive search across the major literature databases to explore the relationship between lactobacilli and COVID-19. Our emphasis was on investigations employing NGS technologies to explore this connection. Our analysis of nine selected studies revealed that lactobacilli have a reduced abundance in the disease and an association with disease severity. The protective mechanisms of lactobacilli in COVID-19 and other viral infections are likely to be multifaceted, involving complex interactions between the microbiota, the host immune system, and the virus itself. Moreover, upon closely examining the NGS methodologies and associated statistical analyses in each research study, we have noted concerns regarding the approach used to delineate the varying abundance of lactobacilli, which involves potential biases and the exclusion of pertinent data elements. These findings provide new insight into the relationship between COVID-19 and lactobacilli, highlighting the potential for microbiota modulation in COVID-19 treatment.
of lactobacilli in COVID-19 and other viral infections are expected to be multi-faceted, involving intricate interactions among the microbiome, the host immune system, and the virus. This combination of protective mechanisms, including direct antiviral effects, immunomodulation, and enhancement of mucosal immunity, makes this genus a promising avenue for interventions against viral infections, including COVID-19.
Supplementary Materials: The following supporting information can be downloaded at: https:// www.mdpi.com/article/10.3390/microorganisms12020284/s1, Supplementary File S1: Search strategy; Supplementary File S2: PRISMA 2020 Checklist; Supplementary File S3: Data extracted from the selected articles. Author Contributions: C.R.T.: data collection, analysis of results, and writing (original draft); P.H.R.: conceptualization, supervision, analysis of results, and writing (revision). All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Abreu, Fukata, Arditi, Tlr, Signaling in the Gut in Health and Disease, J. Immunol, doi:10.4049/jimmunol.174.8.4453
Al Kassaa, Hober, Hamze, Chihib, Drider, Antiviral Potential of Lactic Acid Bacteria and Their Bacteriocins, Probiot. Antimicrob. Proteins, doi:10.1007/s12602-014-9162-6
Amrouche, Chikindas, Probiotics for immunomodulation in prevention against respiratory viral infections with special emphasis on COVID-19, AIMS Microbiol, doi:10.3934/microbiol.2022024
Anwar, Altayb, Al-Abbasi, Al-Malki, Kamal et al., Antiviral effects of probiotic metabolites on COVID-19, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1775123
Balmeh, Mahmoudi, Fard, Manipulated bio antimicrobial peptides from probiotic bacteria as proposed drugs for COVID-19 disease, Inform. Med. Unlocked, doi:10.1016/j.imu.2021.100515
Berggren, Lazou Ahrén, Larsson, Önning, Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections, Eur. J. Nutr, doi:10.1007/s00394-010-0127-6
Castillo, Perdigán, De Moreno De Leblanc, Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice, BMC Microbiol, doi:10.1186/1471-2180-11-177
Cox, Pyne, Saunders, Fricker, Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes, Br. J. Sports Med, doi:10.1136/bjsm.2007.044628
Devika, Raman, Deciphering the metabolic capabilities of Bifidobacteria using genome-scale metabolic models, Sci. Rep, doi:10.1038/s41598-019-54696-9
Fernandez, Valenti, Rockel, Hermann, Pot et al., Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide, Gut, doi:10.1136/gut.2010.232918
Fujiwara, Hashiba, Hirota, Forstner, Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide, Appl. Environ. Microbiol, doi:10.1128/aem.63.2.506-512.1997
Gabryszewski, Bachar, Dyer, Percopo, Killoran et al., Lactobacillus-Mediated Priming of the Respiratory Mucosa Protects against Lethal Pneumovirus Infection, J. Immunol, doi:10.4049/jimmunol.1001751
Gaibani, D'amico, Bartoletti, Lombardo, Rampelli et al., The Gut Microbiota of Critically Ill Patients with COVID-19, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2021.670424
Geva-Zatorsky, Sefik, Kua, Pasman, Tan et al., Mining the Human Gut Microbiota for Immunomodulatory Organisms, Cell, doi:10.1016/j.cell.2017.01.022
Gu, Chen, Wu, Chen, Gao et al., Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza, Clin. Infect. Dis, doi:10.1093/cid/ciaa709
Heeney, Gareau, Marco, Intestinal Lactobacillus in health and disease, a driver or just along for the ride?, Curr. Opin. Biotechnol, doi:10.1016/j.copbio.2017.08.004
Jang, Hyam, Han, Kim, Lee et al., Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages, J. Appl. Microbiol, doi:10.1111/jam.12273
Jiang, Yang, Qian, Su, Huang et al., Tongue coating microbiome composition reflects disease severity in patients with COVID-19 in Nanjing, China, J. Oral Microbiol, doi:10.1080/20002297.2023.2236429
Johnson-Henry, Nadjafi, Avitzur, Mitchell, Ngan et al., Amelioration of the effects of Citrobacter rodentium infection in mice by pretreatment with probiotics, J. Infect. Dis, doi:10.1086/430318
Jung, Lee, Le Ngo, Cho, Ko et al., Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection, Sci. Rep, doi:10.1038/s41598-017-17487-8
Kaci, Lakhdari, Doré, Ehrlich, Renault et al., Inhibition of the NF-κB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius, Appl. Environ. Microbiol, doi:10.1128/AEM.03021-10
Kalantar-Zadeh, Ward, Kalantar-Zadeh, El-Omar, Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles, ACS Nano, doi:10.1021/acsnano.0c03402
Kim, Zhang, Rauseo, Goss, Mudd et al., The salivary and nasopharyngeal microbiomes are associated with SARS-CoV-2 infection and disease severity, J. Med. Virol, doi:10.1002/jmv.28445
Kobayashi, Saito, Uematsu, Kishi, Toba et al., Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice, Int. Immunopharmacol, doi:10.1016/j.intimp.2010.11.019
Lee, Hwang, Jun, Park, Lee, Antiinflammatory effect of lactic acid bacteria: Inhibition of cyclooxygenase-2 by suppressing nuclear factor-κB in Raw264.7 macrophage cells, J. Microbiol. Biotechnol
Lefterova, Suarez, Banaei, Pinsky, Next-Generation Sequencing for Infectious Disease Diagnosis and Management: A Report of the Association for Molecular Pathology, J. Mol. Diagn, doi:10.1016/j.jmoldx.2015.07.004
Lievin, Peiffer, Hudault, Rochat, Brassart et al., Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity, Gut
Lim, Shin, Antimicrobial and immunomodulatory effects of bifidobacterium strains: A review, J. Microbiol. Biotechnol, doi:10.4014/jmb.2007.07046
Liu, Fatheree, Mangalat, Rhoads, Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine, Am. J. Physiol.-Gastrointest. Liver Physiol, doi:10.1152/ajpgi.00266.2011
Liu, Katovich, Raizada, Shenoy, ACE2 and Microbiota:Emerging Targets for Cardiopulmonary Disease Therapy, J. Cardiovasc. Pharmacol, doi:10.1097/FJC.0000000000000307
Liu, Zhang, Dong, Guo, Adhesion and immunomodulatory effects of bifidobacterium lactis HN019 on intestinal epithelial cells INT-407, World J. Gastroenterol, doi:10.3748/wjg.v16.i18.2283
Mack, Ahrne, Hyde, Wei, Hollingsworth, Extracellular MUC3 mucin secretion follows adherence of, Gut, doi:10.1136/gut.52.6.827
Maeda, Motooka, Kawasaki, Oki, Noda et al., Longitudinal alterations of the gut mycobiota and microbiota on COVID-19 severity, BMC Infect. Dis, doi:10.1186/s12879-022-07358-7
Maeda, Nakamura, Hirose, Murosaki, Yamamoto et al., Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice, Int. Immunopharmacol, doi:10.1016/j.intimp.2009.04.015
Makino, Ikegami, Kume, Horiuchi, Sasaki et al., Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1, Br. J. Nutr, doi:10.1017/S000711451000173X
Makino, Sato, Goto, Nakamura, Ogawa et al., Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1, J. Dairy Sci, doi:10.3168/jds.2015-10376
Manor, Dai, Kornilov, Smith, Price et al., Health and disease markers correlate with gut microbiome composition across thousands of people, Nat. Commun, doi:10.1038/s41467-020-18871-1
Maragkoudakis, Chingwaru, Gradisnik, Tsakalidou, Cencic, Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection, Int. J. Food Microbiol, doi:10.1016/j.ijfoodmicro.2009.12.024
Mattar, Teitelbaum, Drongowski, Yongyi, Harmon et al., Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model, Pediatr. Surg. Int, doi:10.1007/s00383-002-0855-7
Merenstein, Murphy, Fokar, Hernandez, Park et al., Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: The DRINK study A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial, Eur. J. Clin. Nutr, doi:10.1038/ejcn.2010.65
Mirzayi, Renson, Furlanello, Sansone, Zohra et al., Reporting guidelines for human microbiome research: The STORMS checklist, Nat. Med, doi:10.1038/s41591-021-01552-x
Moola, Munn, Tufanaru, Aromataris, Sears et al., Chapter 7: Systematic reviews of etiology and risk, doi:10.46658/JBIMES-20-08
Nagai, Makino, Ikegami, Itoh, Yamada, Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice, Int. Immunopharmacol, doi:10.1016/j.intimp.2011.09.012
Ouzzani, Hammady, Fedorowicz, Elmagarmid, Rayyan-a web and mobile app for systematic reviews, Syst. Rev, doi:10.1186/s13643-016-0384-4
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 statement: An updated guideline for reporting systematic reviews, BMJ, doi:10.1136/bmj.n71
Park, Ngo, Kwon, Lee, Yoo et al., Lactobacillus plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity, PLoS ONE, doi:10.1371/journal.pone.0075368
Petrof, Claud, Sun, Abramova, Guo et al., Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function, Inflamm. Bowel Dis, doi:10.1002/ibd.20930
Pham, Yang, Kao, Gankhuyag, Zayabaatar et al., Gut probiotic Lactobacillus rhamnosus attenuates PDE4B-mediated interleukin-6 induced by SARS-CoV-2 membrane glycoprotein, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2021.108821
Purushothaman, Meola, Egli, Combination of Whole Genome Sequencing and Metagenomics for Microbiological Diagnostics, Int. J. Mol. Sci, doi:10.3390/ijms23179834
Ragab, Salah Eldin, Taeimah, Khattab, Salem, The COVID-19 Cytokine Storm; What We Know So Far, Front. Immunol, doi:10.3389/fimmu.2020.01446
Ranjan, Rani, Metwally, Mcgee, Perkins, Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2015.12.083
Rather, Choi, Kamli, Hakeem, Sabir et al., Potential adjuvant therapeutic effect of Lactobacillus plantarum probio-88 postbiotics against SARS-CoV-2, Vaccines, doi:10.3390/vaccines9101067
Rhoades, Pinski, Monsibais, Jankeel, Doratt et al., Acute SARS-CoV-2 infection is associated with an increased abundance of bacterial pathogens, including Pseudomonas aeruginosa in the nose, Cell Rep, doi:10.1016/j.celrep.2021.109637
Rizzardini, Eskesen, Calder, Capetti, Jespersen et al., Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12®and Lactobacillus paracasei ssp. paracasei, L. casei 431®in an influenza vaccination model: A randomised, double-blind, placebo-controlled study, Br. J. Nutr, doi:10.1017/S000711451100420X
Rosas-Salazar, Kimura, Shilts, Strickland, Freeman et al., Upper respiratory tract microbiota dynamics following COVID-19 in adults, Microb. Genom, doi:10.1099/mgen.0.000957
Rossi, Martínez-Martínez, Amaretti, Ulrici, Raimondi et al., Mining metagenomic whole genome sequences revealed subdominant but constant Lactobacillus population in the human gut microbiota, Environ. Microbiol. Rep, doi:10.1111/1758-2229.12405
Sanschagrin, Yergeau, Next-generation sequencing of 16S ribosomal RNA gene amplicons, J. Vis. Exp, doi:10.3791/51709
Shahi, Zarei, Guseva, Mangalam, Microbiota analysis using two-step pcr and next-generation 16s rrna gene sequencing, J. Vis. Exp, doi:10.3791/59980
Soffritti, D'accolti, Fabbri, Passaro, Manfredini et al., Oral Microbiome Dysbiosis Is Associated with Symptoms Severity and Local Immune/Inflammatory Response in COVID-19 Patients: A Cross-Sectional Study, Front. Microbiol, doi:10.3389/fmicb.2021.687513
Sun, Xu, Xie, Plummer, Tang et al., Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2dependent manner, Cytokine, doi:10.1016/j.cyto.2017.01.003
Suskun, Kilic, Yilmaz Ciftdogan, Guven, Karbuz et al., Intestinal microbiota composition of children with infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and multisystem inflammatory syndrome (MIS-C), Eur. J. Pediatr, doi:10.1007/s00431-022-04494-9
Synodinou, Nikolaki, Triantafyllou, Kasti, Immunomodulatory Effects of Probiotics on COVID-19 Infection by Targeting the Gut-Lung Axis Microbial Cross-Talk, Microorganisms, doi:10.3390/microorganisms10091764
Tang, Gu, Gong, Li, Lu et al., Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity, Engineering, doi:10.1016/j.eng.2020.05.013
Tien, Girardin, Regnault, Le Bourhis, Dillies et al., Anti-Inflammatory Effect of Lactobacillus casei on Shigella -Infected Human Intestinal Epithelial Cells, J. Immunol, doi:10.4049/jimmunol.176.2.1228
Tohno, Shimosato, Aso, Kitazawa, Immunobiotic Lactobacillus strains augment NLRP3 expression in newborn and adult porcine gut-associated lymphoid tissues, Vet. Immunol. Immunopathol, doi:10.1016/j.vetimm.2011.09.010
Van Der, Veer, Hertzberger, Bruisten, Tytgat et al., Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: Implications for in vivo dominance of the vaginal microbiota, Microbiome, doi:10.1186/s40168-019-0667-9
Wang, Hu, Zijlstra, Zheng, Gänzle, Metagenomic reconstructions of gut microbial metabolism in weanling pigs, Microbiome, doi:10.1186/s40168-019-0662-1
Wang, Moon, Huang, Sun, Qiu, Antiviral Effects and Underlying Mechanisms of Probiotics as Promising Antivirals, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2022.928050
Weiss, Raupach, Takeda, Akira, Zychlinsky, Toll-Like Receptors Are Temporally Involved in Host Defense, J. Immunol, doi:10.4049/jimmunol.172.7.4463
Wells, Immunomodulatory mechanisms of lactobacilli, Microb. Cell Fact, doi:10.1186/1475-2859-10-S1-S17
Wu, Cheng, Jiang, Tang, Ming et al., Altered oral and gut microbiota and its association with SARS-CoV-2 viral load in COVID-19 patients during hospitalization, NPJ Biofilms Microbiomes, doi:10.1038/s41522-021-00232-5
Yeoh, Zuo, Lui, Zhang, Liu et al., Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut, doi:10.1136/gutjnl-2020-323020
Youn, Lee, Lee, Park, Yuk et al., Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice, Antivir. Res, doi:10.1016/j.antiviral.2011.11.004
Zhang, Lau, Liu, Su, Chan et al., Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications, Nat. Rev. Gastroenterol. Hepatol, doi:10.1038/s41575-022-00698-4
Zhang, Li, Caicedo, Neu, Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α-induced interleukin-8 production in Caco-2 cells, J. Nutr, doi:10.1093/jn/135.7.1752
Zheng, Wittouck, Salvetti, Franz, Harris et al., A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae, Int. J. Syst. Evol. Microbiol, doi:10.1099/ijsem.0.004107
Zuo, Liu, Zhang, Lui, Tso et al., Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19, Gut, doi:10.1136/gutjnl-2020-322294
Zuo, Zhang, Lui, Yeoh, Li et al., Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization, Gastroenterology, doi:10.1053/j.gastro.2020.05.048
DOI record:
{
"DOI": "10.3390/microorganisms12020284",
"ISSN": [
"2076-2607"
],
"URL": "http://dx.doi.org/10.3390/microorganisms12020284",
"abstract": "<jats:p>The global pandemic was caused by the SARS-CoV-2 virus, known as COVID-19, which primarily affects the respiratory and intestinal systems and impacts the microbial communities of patients. This systematic review involved a comprehensive search across the major literature databases to explore the relationship between lactobacilli and COVID-19. Our emphasis was on investigations employing NGS technologies to explore this connection. Our analysis of nine selected studies revealed that lactobacilli have a reduced abundance in the disease and an association with disease severity. The protective mechanisms of lactobacilli in COVID-19 and other viral infections are likely to be multifaceted, involving complex interactions between the microbiota, the host immune system, and the virus itself. Moreover, upon closely examining the NGS methodologies and associated statistical analyses in each research study, we have noted concerns regarding the approach used to delineate the varying abundance of lactobacilli, which involves potential biases and the exclusion of pertinent data elements. These findings provide new insight into the relationship between COVID-19 and lactobacilli, highlighting the potential for microbiota modulation in COVID-19 treatment.</jats:p>",
"alternative-id": [
"microorganisms12020284"
],
"author": [
{
"affiliation": [
{
"name": "Graduate Program in Genetics and Molecular Biology, Universidade Federal do Rio Grande do Sul, Porto Alegre 91501-970, Brazil"
}
],
"family": "Taufer",
"given": "Clarissa Reginato",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8992-9697",
"affiliation": [
{
"name": "Bioinformatics and Biostatistics Core Facility, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre 91501-970, Brazil"
}
],
"authenticated-orcid": false,
"family": "Rampelotto",
"given": "Pabulo Henrique",
"sequence": "additional"
}
],
"container-title": "Microorganisms",
"container-title-short": "Microorganisms",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
1,
29
]
],
"date-time": "2024-01-29T13:54:03Z",
"timestamp": 1706536443000
},
"deposited": {
"date-parts": [
[
2024,
1,
29
]
],
"date-time": "2024-01-29T15:16:30Z",
"timestamp": 1706541390000
},
"funder": [
{
"award": [
"88887.513461/2020-00",
"88887.798411/2022-00"
],
"name": "CAPES"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
30
]
],
"date-time": "2024-01-30T00:22:44Z",
"timestamp": 1706574164857
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
1,
29
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2024,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
29
]
],
"date-time": "2024-01-29T00:00:00Z",
"timestamp": 1706486400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-2607/12/2/284/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "284",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
1,
29
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
29
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3389/fimmu.2020.01446",
"article-title": "The COVID-19 Cytokine Storm; What We Know So Far",
"author": "Ragab",
"doi-asserted-by": "crossref",
"first-page": "1446",
"journal-title": "Front. Immunol.",
"key": "ref_1",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1021/acsnano.0c03402",
"article-title": "Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles",
"author": "Ward",
"doi-asserted-by": "crossref",
"first-page": "5179",
"journal-title": "ACS Nano",
"key": "ref_2",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2021.109637",
"article-title": "Acute SARS-CoV-2 infection is associated with an increased abundance of bacterial pathogens, including Pseudomonas aeruginosa in the nose",
"author": "Rhoades",
"doi-asserted-by": "crossref",
"first-page": "109637",
"journal-title": "Cell Rep.",
"key": "ref_3",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-18871-1",
"article-title": "Health and disease markers correlate with gut microbiome composition across thousands of people",
"author": "Manor",
"doi-asserted-by": "crossref",
"first-page": "5206",
"journal-title": "Nat. Commun.",
"key": "ref_4",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa709",
"article-title": "Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza",
"author": "Gu",
"doi-asserted-by": "crossref",
"first-page": "2669",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_5",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1053/j.gastro.2020.05.048",
"article-title": "Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization",
"author": "Zuo",
"doi-asserted-by": "crossref",
"first-page": "944",
"journal-title": "Gastroenterology",
"key": "ref_6",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2017.01.022",
"article-title": "Mining the Human Gut Microbiota for Immunomodulatory Organisms",
"author": "Sefik",
"doi-asserted-by": "crossref",
"first-page": "928",
"journal-title": "Cell",
"key": "ref_7",
"volume": "168",
"year": "2017"
},
{
"article-title": "Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19",
"author": "Zuo",
"first-page": "276",
"journal-title": "Gut",
"key": "ref_8",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1016/j.eng.2020.05.013",
"article-title": "Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "1178",
"journal-title": "Engineering",
"key": "ref_9",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1111/1758-2229.12405",
"article-title": "Mining metagenomic whole genome sequences revealed subdominant but constant Lactobacillus population in the human gut microbiota",
"author": "Rossi",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "Environ. Microbiol. Rep.",
"key": "ref_10",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1016/j.copbio.2017.08.004",
"article-title": "Intestinal Lactobacillus in health and disease, a driver or just along for the ride?",
"author": "Heeney",
"doi-asserted-by": "crossref",
"first-page": "140",
"journal-title": "Curr. Opin. Biotechnol.",
"key": "ref_11",
"volume": "49",
"year": "2018"
},
{
"DOI": "10.1016/j.jmoldx.2015.07.004",
"article-title": "Next-Generation Sequencing for Infectious Disease Diagnosis and Management: A Report of the Association for Molecular Pathology",
"author": "Lefterova",
"doi-asserted-by": "crossref",
"first-page": "623",
"journal-title": "J. Mol. Diagn.",
"key": "ref_12",
"volume": "17",
"year": "2015"
},
{
"article-title": "Microbiota analysis using two-step pcr and next-generation 16s rrna gene sequencing",
"author": "Shahi",
"first-page": "e59980",
"journal-title": "J. Vis. Exp.",
"key": "ref_13",
"volume": "2019",
"year": "2019"
},
{
"article-title": "Next-generation sequencing of 16S ribosomal RNA gene amplicons",
"author": "Sanschagrin",
"first-page": "51709",
"journal-title": "J. Vis. Exp.",
"key": "ref_14",
"volume": "90",
"year": "2014"
},
{
"DOI": "10.1016/j.bbrc.2015.12.083",
"article-title": "Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing",
"author": "Ranjan",
"doi-asserted-by": "crossref",
"first-page": "967",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "ref_15",
"volume": "469",
"year": "2016"
},
{
"DOI": "10.3390/ijms23179834",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Purushothaman, S., Meola, M., and Egli, A. (2022). Combination of Whole Genome Sequencing and Metagenomics for Microbiological Diagnostics. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1038/s41575-022-00698-4",
"article-title": "Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "323",
"journal-title": "Nat. Rev. Gastroenterol. Hepatol.",
"key": "ref_17",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1186/s13643-016-0384-4",
"article-title": "Rayyan-a web and mobile app for systematic reviews",
"author": "Ouzzani",
"doi-asserted-by": "crossref",
"first-page": "210",
"journal-title": "Syst. Rev.",
"key": "ref_18",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.1136/bmj.n71",
"article-title": "The PRISMA 2020 statement: An updated guideline for reporting systematic reviews",
"author": "Page",
"doi-asserted-by": "crossref",
"first-page": "n71",
"journal-title": "BMJ",
"key": "ref_19",
"volume": "372",
"year": "2021"
},
{
"key": "ref_20",
"unstructured": "Aromataris, E., and Munn, Z. (2020). JBI Manual for Evidence Synthesis, JBI."
},
{
"DOI": "10.1080/20002297.2023.2236429",
"doi-asserted-by": "crossref",
"key": "ref_21",
"unstructured": "Jiang, Z., Yang, L., Qian, X., Su, K., Huang, Y., Qu, Y., Zhang, Z., and Liu, W. (2023). Tongue coating microbiome composition reflects disease severity in patients with COVID-19 in Nanjing, China. J. Oral Microbiol., 15."
},
{
"DOI": "10.1002/jmv.28445",
"article-title": "The salivary and nasopharyngeal microbiomes are associated with SARS-CoV-2 infection and disease severity",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "e28445",
"journal-title": "J. Med. Virol.",
"key": "ref_22",
"volume": "95",
"year": "2023"
},
{
"article-title": "Upper respiratory tract microbiota dynamics following COVID-19 in adults",
"author": "Kimura",
"first-page": "mgen000957",
"journal-title": "Microb. Genom.",
"key": "ref_23",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.3389/fmicb.2021.687513",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Soffritti, I., D’Accolti, M., Fabbri, C., Passaro, A., Manfredini, R., Zuliani, G., Libanore, M., Franchi, M., Contini, C., and Caselli, E. (2021). Oral Microbiome Dysbiosis Is Associated with Symptoms Severity and Local Immune/Inflammatory Response in COVID-19 Patients: A Cross-Sectional Study. Front. Microbiol., 12."
},
{
"DOI": "10.3389/fcimb.2021.670424",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Gaibani, P., D’Amico, F., Bartoletti, M., Lombardo, D., Rampelli, S., Fornaro, G., Coladonato, S., Siniscalchi, A., Re, M.C., and Viale, P. (2021). The Gut Microbiota of Critically Ill Patients with COVID-19. Front. Cell. Infect. Microbiol., 11."
},
{
"DOI": "10.1186/s12879-022-07358-7",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Maeda, Y., Motooka, D., Kawasaki, T., Oki, H., Noda, Y., Adachi, Y., Niitsu, T., Okamoto, S., Tanaka, K., and Fukushima, K. (2022). Longitudinal alterations of the gut mycobiota and microbiota on COVID-19 severity. BMC Infect. Dis., 22."
},
{
"DOI": "10.1007/s00431-022-04494-9",
"article-title": "Intestinal microbiota composition of children with infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and multisystem inflammatory syndrome (MIS-C)",
"author": "Suskun",
"doi-asserted-by": "crossref",
"first-page": "3175",
"journal-title": "Eur. J. Pediatr.",
"key": "ref_27",
"volume": "181",
"year": "2022"
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"article-title": "Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19",
"author": "Yeoh",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "Gut",
"key": "ref_28",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1038/s41522-021-00232-5",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Wu, Y., Cheng, X., Jiang, G., Tang, H., Ming, S., Tang, L., Lu, J., Guo, C., Shan, H., and Huang, X. (2021). Altered oral and gut microbiota and its association with SARS-CoV-2 viral load in COVID-19 patients during hospitalization. NPJ Biofilms Microbiomes, 7."
},
{
"DOI": "10.1099/ijsem.0.004107",
"article-title": "A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "2782",
"journal-title": "Int. J. Syst. Evol. Microbiol.",
"key": "ref_30",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.1186/s40168-019-0662-1",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Wang, W., Hu, H., Zijlstra, R.T., Zheng, J., and Gänzle, M.G. (2019). Metagenomic reconstructions of gut microbial metabolism in weanling pigs. Microbiome, 7."
},
{
"DOI": "10.1186/s40168-019-0667-9",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Van Der Veer, C., Hertzberger, R.Y., Bruisten, S.M., Tytgat, H.L.P., Swanenburg, J., Angelino-bart, A.D.K., Schuren, F., Molenaar, D., Reid, G., and De Vries, H. (2019). Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: Implications for in vivo dominance of the vaginal microbiota. Microbiome, 7."
},
{
"DOI": "10.1038/s41591-021-01552-x",
"article-title": "Reporting guidelines for human microbiome research: The STORMS checklist",
"author": "Mirzayi",
"doi-asserted-by": "crossref",
"first-page": "1885",
"journal-title": "Nat. Med.",
"key": "ref_33",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1007/s00394-010-0127-6",
"article-title": "Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections",
"author": "Berggren",
"doi-asserted-by": "crossref",
"first-page": "203",
"journal-title": "Eur. J. Nutr.",
"key": "ref_34",
"volume": "50",
"year": "2011"
},
{
"DOI": "10.1017/S000711451100420X",
"article-title": "Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study",
"author": "Rizzardini",
"doi-asserted-by": "crossref",
"first-page": "876",
"journal-title": "Br. J. Nutr.",
"key": "ref_35",
"volume": "107",
"year": "2012"
},
{
"DOI": "10.1136/bjsm.2007.044628",
"article-title": "Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes",
"author": "Cox",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "Br. J. Sports Med.",
"key": "ref_36",
"volume": "44",
"year": "2010"
},
{
"DOI": "10.1038/ejcn.2010.65",
"article-title": "Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: The DRINK study A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial",
"author": "Merenstein",
"doi-asserted-by": "crossref",
"first-page": "669",
"journal-title": "Eur. J. Clin. Nutr.",
"key": "ref_37",
"volume": "64",
"year": "2010"
},
{
"DOI": "10.1017/S000711451000173X",
"article-title": "Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1",
"author": "Makino",
"doi-asserted-by": "crossref",
"first-page": "998",
"journal-title": "Br. J. Nutr.",
"key": "ref_38",
"volume": "104",
"year": "2010"
},
{
"DOI": "10.1038/s41598-017-17487-8",
"article-title": "Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection",
"author": "Jung",
"doi-asserted-by": "crossref",
"first-page": "17360",
"journal-title": "Sci. Rep.",
"key": "ref_39",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1016/j.intimp.2009.04.015",
"article-title": "Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice",
"author": "Maeda",
"doi-asserted-by": "crossref",
"first-page": "1122",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_40",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1016/j.intimp.2011.09.012",
"article-title": "Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice",
"author": "Nagai",
"doi-asserted-by": "crossref",
"first-page": "2246",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_41",
"volume": "11",
"year": "2011"
},
{
"article-title": "Lactobacillus plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity",
"author": "Park",
"first-page": "26",
"journal-title": "PLoS ONE",
"key": "ref_42",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1016/j.intimp.2010.11.019",
"article-title": "Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice",
"author": "Kobayashi",
"doi-asserted-by": "crossref",
"first-page": "199",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_43",
"volume": "11",
"year": "2011"
},
{
"DOI": "10.1016/j.antiviral.2011.11.004",
"article-title": "Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice",
"author": "Youn",
"doi-asserted-by": "crossref",
"first-page": "138",
"journal-title": "Antivir. Res.",
"key": "ref_44",
"volume": "93",
"year": "2012"
},
{
"DOI": "10.4049/jimmunol.1001751",
"article-title": "Lactobacillus-Mediated Priming of the Respiratory Mucosa Protects against Lethal Pneumovirus Infection",
"author": "Gabryszewski",
"doi-asserted-by": "crossref",
"first-page": "1151",
"journal-title": "J. Immunol.",
"key": "ref_45",
"volume": "186",
"year": "2011"
},
{
"DOI": "10.1007/s12602-014-9162-6",
"article-title": "Antiviral Potential of Lactic Acid Bacteria and Their Bacteriocins",
"author": "Hober",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "Probiot. Antimicrob. Proteins",
"key": "ref_46",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.3389/fcimb.2022.928050",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Wang, Y., Moon, A., Huang, J., Sun, Y., and Qiu, H.J. (2022). Antiviral Effects and Underlying Mechanisms of Probiotics as Promising Antivirals. Front. Cell. Infect. Microbiol., 12."
},
{
"DOI": "10.1007/s00383-002-0855-7",
"article-title": "Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model",
"author": "Mattar",
"doi-asserted-by": "crossref",
"first-page": "586",
"journal-title": "Pediatr. Surg. Int.",
"key": "ref_48",
"volume": "18",
"year": "2002"
},
{
"DOI": "10.1136/gut.52.6.827",
"article-title": "Extracellular MUC3 mucin secretion follows adherence of",
"author": "Mack",
"doi-asserted-by": "crossref",
"first-page": "827",
"journal-title": "Gut",
"key": "ref_49",
"volume": "52",
"year": "2003"
},
{
"DOI": "10.1097/FJC.0000000000000307",
"article-title": "ACE2 and Microbiota:Emerging Targets for Cardiopulmonary Disease Therapy",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "540",
"journal-title": "J. Cardiovasc. Pharmacol.",
"key": "ref_50",
"volume": "66",
"year": "2015"
},
{
"DOI": "10.1080/07391102.2020.1775123",
"article-title": "Antiviral effects of probiotic metabolites on COVID-19",
"author": "Anwar",
"doi-asserted-by": "crossref",
"first-page": "4175",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_51",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1016/j.imu.2021.100515",
"article-title": "Manipulated bio antimicrobial peptides from probiotic bacteria as proposed drugs for COVID-19 disease",
"author": "Balmeh",
"doi-asserted-by": "crossref",
"first-page": "100515",
"journal-title": "Inform. Med. Unlocked",
"key": "ref_52",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.3390/vaccines9101067",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Rather, I.A., Choi, S.-B., Kamli, M.R., Hakeem, K.R., Sabir, J.S.M., Park, Y.-H., and Hor, Y.-Y. (2021). Potential adjuvant therapeutic effect of Lactobacillus plantarum probio-88 postbiotics against SARS-CoV-2. Vaccines, 9."
},
{
"DOI": "10.3168/jds.2015-10376",
"article-title": "Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1",
"author": "Makino",
"doi-asserted-by": "crossref",
"first-page": "915",
"journal-title": "J. Dairy Sci.",
"key": "ref_54",
"volume": "99",
"year": "2016"
},
{
"DOI": "10.4049/jimmunol.174.8.4453",
"article-title": "TLR Signaling in the Gut in Health and Disease",
"author": "Abreu",
"doi-asserted-by": "crossref",
"first-page": "4453",
"journal-title": "J. Immunol.",
"key": "ref_55",
"volume": "174",
"year": "2005"
},
{
"DOI": "10.1186/1471-2180-11-177",
"doi-asserted-by": "crossref",
"key": "ref_56",
"unstructured": "Castillo, N.A., Perdigán, G., and De Moreno De Leblanc, A. (2011). Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol., 11."
},
{
"DOI": "10.4049/jimmunol.172.7.4463",
"article-title": "Toll-Like Receptors Are Temporally Involved in Host Defense",
"author": "Weiss",
"doi-asserted-by": "crossref",
"first-page": "4463",
"journal-title": "J. Immunol.",
"key": "ref_57",
"volume": "172",
"year": "2004"
},
{
"DOI": "10.1016/j.vetimm.2011.09.010",
"article-title": "Immunobiotic Lactobacillus strains augment NLRP3 expression in newborn and adult porcine gut-associated lymphoid tissues",
"author": "Tohno",
"doi-asserted-by": "crossref",
"first-page": "410",
"journal-title": "Vet. Immunol. Immunopathol.",
"key": "ref_58",
"volume": "144",
"year": "2011"
},
{
"DOI": "10.1136/gut.2010.232918",
"article-title": "Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide",
"author": "Fernandez",
"doi-asserted-by": "crossref",
"first-page": "1050",
"journal-title": "Gut",
"key": "ref_59",
"volume": "60",
"year": "2011"
},
{
"DOI": "10.3934/microbiol.2022024",
"article-title": "Probiotics for immunomodulation in prevention against respiratory viral infections with special emphasis on COVID-19",
"author": "Amrouche",
"doi-asserted-by": "crossref",
"first-page": "338",
"journal-title": "AIMS Microbiol.",
"key": "ref_60",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/j.ijfoodmicro.2009.12.024",
"article-title": "Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection",
"author": "Maragkoudakis",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Int. J. Food Microbiol.",
"key": "ref_61",
"volume": "141",
"year": "2010"
},
{
"DOI": "10.3390/microorganisms10091764",
"doi-asserted-by": "crossref",
"key": "ref_62",
"unstructured": "Synodinou, K.D., Nikolaki, M.D., Triantafyllou, K., and Kasti, A.N. (2022). Immunomodulatory Effects of Probiotics on COVID-19 Infection by Targeting the Gut–Lung Axis Microbial Cross-Talk. Microorganisms, 10."
},
{
"DOI": "10.1186/1475-2859-10-S1-S17",
"article-title": "Immunomodulatory mechanisms of lactobacilli",
"author": "Wells",
"doi-asserted-by": "crossref",
"first-page": "S17",
"journal-title": "Microb. Cell Fact.",
"key": "ref_63",
"volume": "10",
"year": "2011"
},
{
"DOI": "10.1093/jn/135.7.1752",
"article-title": "Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α-induced interleukin-8 production in Caco-2 cells",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1752",
"journal-title": "J. Nutr.",
"key": "ref_64",
"volume": "135",
"year": "2005"
},
{
"DOI": "10.1016/j.jnutbio.2021.108821",
"doi-asserted-by": "crossref",
"key": "ref_65",
"unstructured": "Pham, M.T., Yang, A.J., Kao, M., Gankhuyag, U., Zayabaatar, E., Jin, S.C., and Huang, C. (2021). Gut probiotic Lactobacillus rhamnosus attenuates PDE4B-mediated interleukin-6 induced by SARS-CoV-2 membrane glycoprotein. J. Nutr. Biochem., 98."
},
{
"DOI": "10.4049/jimmunol.176.2.1228",
"article-title": "Anti-Inflammatory Effect of Lactobacillus casei on Shigella -Infected Human Intestinal Epithelial Cells",
"author": "Tien",
"doi-asserted-by": "crossref",
"first-page": "1228",
"journal-title": "J. Immunol.",
"key": "ref_66",
"volume": "176",
"year": "2006"
},
{
"DOI": "10.1086/430318",
"article-title": "Amelioration of the effects of Citrobacter rodentium infection in mice by pretreatment with probiotics",
"author": "Nadjafi",
"doi-asserted-by": "crossref",
"first-page": "2106",
"journal-title": "J. Infect. Dis.",
"key": "ref_67",
"volume": "191",
"year": "2005"
},
{
"DOI": "10.1128/AEM.03021-10",
"article-title": "Inhibition of the NF-κB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius",
"author": "Kaci",
"doi-asserted-by": "crossref",
"first-page": "4681",
"journal-title": "Appl. Environ. Microbiol.",
"key": "ref_68",
"volume": "77",
"year": "2011"
},
{
"DOI": "10.1152/ajpgi.00266.2011",
"article-title": "Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "608",
"journal-title": "Am. J. Physiol.-Gastrointest. Liver Physiol.",
"key": "ref_69",
"volume": "302",
"year": "2012"
},
{
"article-title": "Antiinflammatory effect of lactic acid bacteria: Inhibition of cyclooxygenase-2 by suppressing nuclear factor-κB in Raw264.7 macrophage cells",
"author": "Lee",
"first-page": "1683",
"journal-title": "J. Microbiol. Biotechnol.",
"key": "ref_70",
"volume": "18",
"year": "2008"
},
{
"DOI": "10.1016/j.cyto.2017.01.003",
"article-title": "Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Cytokine",
"key": "ref_71",
"volume": "92",
"year": "2017"
},
{
"DOI": "10.1002/ibd.20930",
"article-title": "Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function",
"author": "Petrof",
"doi-asserted-by": "crossref",
"first-page": "1537",
"journal-title": "Inflamm. Bowel Dis.",
"key": "ref_72",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.1111/jam.12273",
"article-title": "Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "888",
"journal-title": "J. Appl. Microbiol.",
"key": "ref_73",
"volume": "115",
"year": "2013"
},
{
"DOI": "10.4014/jmb.2007.07046",
"article-title": "Antimicrobial and immunomodulatory effects of bifidobacterium strains: A review",
"author": "Lim",
"doi-asserted-by": "crossref",
"first-page": "1793",
"journal-title": "J. Microbiol. Biotechnol.",
"key": "ref_74",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1038/s41598-019-54696-9",
"article-title": "Deciphering the metabolic capabilities of Bifidobacteria using genome-scale metabolic models",
"author": "Devika",
"doi-asserted-by": "crossref",
"first-page": "18222",
"journal-title": "Sci. Rep.",
"key": "ref_75",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1128/aem.63.2.506-512.1997",
"article-title": "Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide",
"author": "Fujiwara",
"doi-asserted-by": "crossref",
"first-page": "506",
"journal-title": "Appl. Environ. Microbiol.",
"key": "ref_76",
"volume": "63",
"year": "1997"
},
{
"DOI": "10.3748/wjg.v16.i18.2283",
"article-title": "Adhesion and immunomodulatory effects of bifidobacterium lactis HN019 on intestinal epithelial cells INT-407",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "2283",
"journal-title": "World J. Gastroenterol.",
"key": "ref_77",
"volume": "16",
"year": "2010"
},
{
"DOI": "10.1136/gut.47.5.646",
"article-title": "Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity",
"author": "Lievin",
"doi-asserted-by": "crossref",
"first-page": "646",
"journal-title": "Gut",
"key": "ref_78",
"volume": "47",
"year": "2000"
}
],
"reference-count": 78,
"references-count": 78,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-2607/12/2/284"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Microbiology (medical)",
"Microbiology"
],
"subtitle": [],
"title": "Lactobacilli in COVID-19: A Systematic Review Based on Next-Generation Sequencing Studies",
"type": "journal-article",
"volume": "12"
}
