Gut Microbiome Disruption Following SARS-CoV-2: A Review
et al., Microorganisms, doi:10.3390/microorganisms12010131, Jan 2024
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
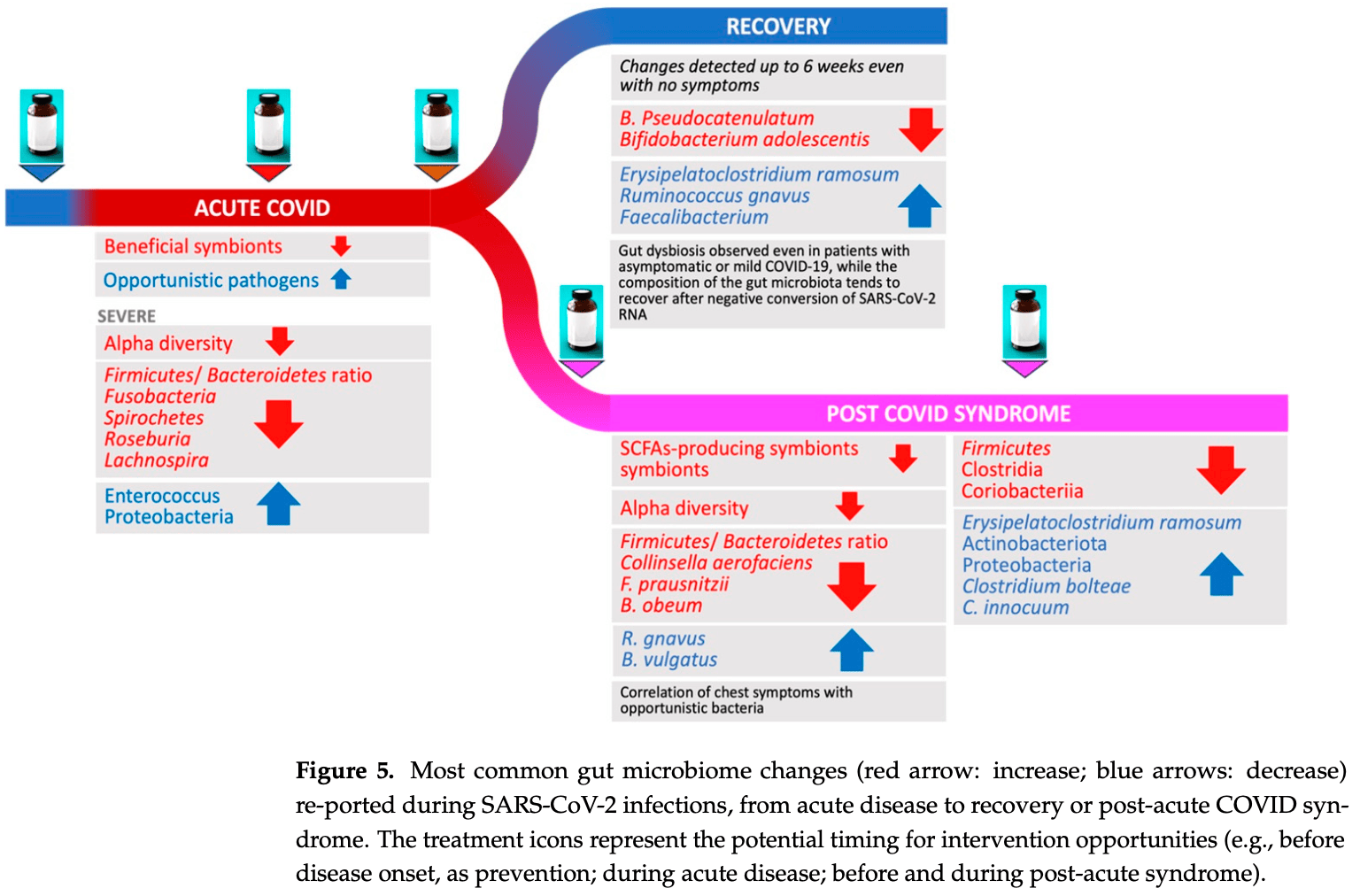
Review of gut microbiome changes associated with COVID-19 during acute infection and post-acute COVID syndrome (PCS). Authors report increased opportunistic pathogens and reduced beneficial symbionts in acute COVID-19 lead to dysbiosis and inflammation. Changes can persist for weeks after initial infection resolves. In PCS lasting 12+ months, reduced microbiome diversity and altered composition are seen, often with increased pathogens and reduced butyrate producers. Specific microbiome signatures associate with respiratory dysfunction. Probiotics, prebiotics, and microbiome-targeted dietary interventions may be beneficial before, during, and after infection.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
1.
Li et al., Large-scale genetic correlation studies explore the causal relationship and potential mechanism between gut microbiota and COVID-19-associated risks, BMC Microbiology, doi:10.1186/s12866-024-03423-0.
2.
Chau et al., Effectiveness of probiotics on COVID-19 prevention and treatment against mild COVID-19 in outpatient care: A systematic review, Nutrition and Health, doi:10.1177/02601060251378200.
3.
Bajić et al., Immunity's core reset: Synbiotics and gut microbiota in the COVID-19 era, Innate Immunity, doi:10.1177/17534259251362023.
4.
Bigman et al., A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery, Nutrients, doi:10.3390/nu17111802.
5.
Fazli et al., Possible Link between Gut Microbiota, Diet, and COVID-19 Infection, Journal of Medical Bacteriology, 12:4, jmb.tums.ac.ir/index.php/jmb/article/view/525.
6.
Santa et al., Comparative analysis of COVID-19 responses in Japan and Africa: diet, phytochemicals, vitamin D, and gut microbiota in reducing mortality—A systematic review and meta-analysis, Frontiers in Nutrition, doi:10.3389/fnut.2024.1465324.
7.
Kaushal, A., Nutraceuticals and pharmacological to balance the transitional microbiome to extend immunity during COVID-19 and other viral infections, Journal of Translational Medicine, doi:10.1186/s12967-024-05587-9.
8.
Mu et al., Anti-inflammatory and Nutritional Interventions Against SARS-CoV-2: A Comprehensive Review, Journal of Agriculture and Food Research, doi:10.1016/j.jafr.2024.101422.
9.
Taufer et al., Lactobacilli in COVID-19: A Systematic Review Based on Next-Generation Sequencing Studies, Microorganisms, doi:10.3390/microorganisms12020284.
10.
Righi et al., Gut Microbiome Disruption Following SARS-CoV-2: A Review, Microorganisms, doi:10.3390/microorganisms12010131.
11.
Petrariu et al., Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases, Frontiers in Microbiology, doi:10.3389/fmicb.2023.1296447.
12.
Taufer (B) et al., The Role of Bifidobacterium in COVID-19: A Systematic Review, Life, doi:10.3390/life13091847.
13.
Di Pierro, F., A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SARS-CoV-2, Minerva Medica, doi:10.23736/S0026-4806.20.06570-2.
14.
Kurian et al., Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects, Archives of Medical Research, doi:10.1016/j.arcmed.2021.03.002.
15.
Singh et al., Probiotics: A potential immunomodulator in COVID-19 infection management, Nutrition Research, doi:10.1016/j.nutres.2020.12.014.
16.
Stavropoulou et al., Probiotics as a Weapon in the Fight Against COVID-19, Frontiers in Nutrition, doi:10.3389/fnut.2020.614986.
Righi et al., 9 Jan 2024, peer-reviewed, 11 authors.
Contact: elda.righi@univr.it (corresponding author), anna.gorska@univr.it, concetta.sciammarella@univr.it, evelina.tacconelli@univr.it, assunta.sartor@asufc.sanita.fvg.it.
Gut Microbiome Disruption Following SARS-CoV-2: A Review
Microorganisms, doi:10.3390/microorganisms12010131
COVID-19 has been associated with having a negative impact on patients' gut microbiome during both active disease and in the post-acute phase. In acute COVID-19, rapid alteration of the gut microbiome composition was observed, showing on one side a reduction in beneficial symbionts (e.g., Roseburia, Lachnospiraceae) and on the other side an increase in opportunistic pathogens such as Enterococcus and Proteobacteria. Alpha diversity tends to decrease, especially initially with symptom onset and hospital admission. Although clinical recovery appears to align with improved gut homeostasis, this process could take several weeks, even in mild infections. Moreover, patients with COVID-19 post-acute syndrome showed changes in gut microbiome composition, with specific signatures associated with decreased respiratory function up to 12 months following acute disease. Potential treatments, especially probiotic-based therapy, are under investigation. Open questions remain on the possibility to use gut microbiome data to predict disease progression and on potential confounders that may impair result interpretation (e.g., concomitant therapies in the acute phase; reinfection, vaccines, and occurrence of novel conditions or diseases in the post-acute syndrome). Understanding the relationships between gut microbiome dynamics and disease progression may contribute to better understanding post-COVID syndrome pathogenesis or inform personalized treatment that can affect specific targets or microbiome markers.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Alenazy, Aljohar, Alruwaili, Daghestani, Alonazi et al., Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications, Metabolites, doi:10.3390/metabo12100912
Allie, Bradley, Mudunuru, Schultz, Graf et al., The establishment of resident memory B cells in the lung requires local antigen encounter, Nat. Immunol, doi:10.1038/s41590-018-0260-6
Baud, Dimopoulou Agri, Gibson, Reid, Giannoni, Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic, Front. Public Health, doi:10.3389/fpubh.2020.00186
Bili Ński, Winter, Jasi Ński, Szczęś, Bilinska et al., Rapid resolution of COVID-19 after faecal microbiota transplantation, Gut, doi:10.1136/gutjnl-2021-325010
Carneiro, Littlefield, Watson, Palmer, Lozupone, Inflammation-associated gut microbiome in postacute sequelae of SARS-CoV-2 points towards new therapeutic targets, Gut, doi:10.1136/gutjnl-2022-328757
Ceccarelli, Borrazzo, Pinacchio, Santinelli, Innocenti et al., Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study, Front. Nutr, doi:10.3389/fnut.2020.613928
Chen, Gu, Chen, Lu, Shi et al., Six-month follow-up of gut microbiota richness in patients with COVID-19, Gut, doi:10.1136/gutjnl-2021-324090
Cheng, Zhang, Li, Wu, Wu et al., Meta-analysis of 16S rRNA microbial data identified alterations of the gut microbiota in COVID-19 patients during the acute and recovery phases, BMC Microbiol, doi:10.1186/s12866-022-02686-9
Davani-Davari, Negahdaripour, Karimzadeh, Seifan, Mohkam et al., Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications, Foods, doi:10.3390/foods8030092
De Nies, Galata, Martin-Gallausiaux, Despotovic, Busi et al., Altered infective competence of the human gut microbiome in COVID-19, Microbiome, doi:10.1186/s40168-023-01472-7
Din, Mazhar, Waseem, Ahmad, Bibi et al., SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role, Biomed Pharmacother. Biomed. Pharmacother, doi:10.1016/j.biopha.2020.110947
Gaibani, D'amico, Bartoletti, Lombardo, Rampelli et al., The Gut Microbiota of Critically Ill Patients With COVID-19, Front. Cell Infect. Microbiol, doi:10.3389/fcimb.2021.670424
Gentilotti, Górska, Tami, Gusinow, Mirandola et al., Clinical phenotypes and quality of life to define post-COVID-19 syndrome: A cluster analysis of the multinational, prospective ORCHESTRA cohort, EClinicalMedicine, doi:10.1016/j.eclinm.2023.102107
Gutiérrez-Castrellón, Gandara-Martí, Abreu Yabreu, Nieto-Rufino, López-Orduña et al., Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial, Gut Microbes, doi:10.1080/19490976.2021.2018899
Hegazy, Ahmed Ashoush, Tharwat Hegazy, Wahba, Lithy et al., Beyond probiotic legend: ESSAP gut microbiota health score to delineate SARS-COV-2 infection severity, Br. J. Nutr, doi:10.1017/S0007114521001926
Hill, Guarner, Reid, Gibson, Merenstein et al., The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic, Nat. Rev. Gastroenterol. Hepatol, doi:10.1038/nrgastro.2014.66
Hu, Zhang, Lin, Tang, Chan et al., Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic, Trends Food Sci. Technol, doi:10.1016/j.tifs.2020.12.009
Huang, Huang, Wang, Li, Ren et al., 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study, Lancet Lond. Engl, doi:10.1016/S0140-6736(20)32656-8
Huerta-Cepas, Serra, Bork, ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data, Mol. Biol. Evol, doi:10.1093/molbev/msw046
Hunter, MATPLOTLIB: A 2D Graphics Environment, Comput. Sci. Eng, doi:10.1109/MCSE.2007.55
Huson, Beier, Flade, Górska, El-Hadidi et al., MEGAN Community Edition-Interactive Exploration and Analysis of Large-Scale Microbiome Sequencing Data, PLoS Comput. Biol, doi:10.1371/journal.pcbi.1004957
Johnson, Hage, Kalveram, Mears, Plante et al., Peptidoglycan-Associated Cyclic Lipopeptide Disrupts Viral Infectivity, J. Virol, doi:10.1128/JVI.01282-19
Jones, Oliphant, Peterson, SciPy: Open Source Scientific Tools for Python
Khan, Mathew, Gupta, Garg, Khadanga et al., Gut dysbiosis and il-21 response in patients with severe covid-19, Microorganisms, doi:10.3390/microorganisms9061292
Khanna, Voth, Therapeutics for Clostridioides difficile infection: Molecules and microbes, Expert. Rev. Gastroenterol. Hepatol, doi:10.1080/17474124.2023.2250716
Kim, Joo, Lee, Ahn, Kim et al., Reversion of Gut Microbiota during the Recovery Phase in Patients with Asymptomatic or Mild COVID-19: Longitudinal Study, Microorganisms, doi:10.3390/microorganisms9061237
Kim, Kim, Kim, Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy, Exp. Mol. Med, doi:10.1038/s12276-021-00627-6
Li, Minimap2: Pairwise alignment for nucleotide sequences, Bioinformatics, doi:10.1093/bioinformatics/bty191
Liu, Mak, Su, Yeoh, Lui et al., Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome, Gut, doi:10.1136/gutjnl-2021-325989
Liu, Su, Zhang, Tun, Mak et al., Multi-kingdom gut microbiota analyses define COVID-19 severity and post-acute COVID-19 syndrome, Nat. Commun, doi:10.1038/s41467-022-34535-8
Liu, Ye, Zhu, He, Wang et al., Gastrointestinal disturbance and effect of fecal microbiota transplantation in discharged COVID-19 patients, J. Med. Case Rep, doi:10.1186/s13256-020-02583-7
Lozupone, Knight, UniFrac: A new phylogenetic method for comparing microbial communities, Appl. Environ. Microbiol, doi:10.1128/AEM.71.12.8228-8235.2005
Martin, Cutadapt removes adapter sequences from high-throughput sequencing reads, EMBnet. J, doi:10.14806/ej.17.1.200
Mazzarelli, Giancola, Farina, Marchioni, Rueca et al., 16S rRNA gene sequencing of rectal swab in patients affected by COVID-19, PLoS ONE, doi:10.1371/journal.pone.0247041
Mcaleer, Kolls, Contributions of the intestinal microbiome in lung immunity, Eur. J. Immunol, doi:10.1002/eji.201646721
Moreira-Rosário, Marques, Pinheiro, Araújo, Ribeiro et al., Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients, Front. Microbiol, doi:10.3389/fmicb.2021.705020
Newsome, Gauthier, Hernandez, Abraham, Robinson et al., The gut microbiome of COVID-19 recovered patients returns to uninfected status in a minority-dominated United States cohort, Gut Microbes, doi:10.1080/19490976.2021.1926840
Oliva, Di Nardo, Ferrari, Mallardo, Rossi et al., Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis, Aliment. Pharmacol. Ther, doi:10.1111/j.1365-2036.2011.04939.x
Parker, Wearsch, Veloo, Rodriguez-Palacios, The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health, Front. Immunol, doi:10.3389/fimmu.2020.00906
Piscotta, Hoffmann, Choi, Small, Ashbrook et al., Metabolites with SARS-CoV-2 Inhibitory Activity Identified from Human Microbiome Commensals, Msphere, doi:10.1128/mSphere.00711-21
Quast, Pruesse, Yilmaz, Gerken, Schweer et al., The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools, Nucleic Acids Res, doi:10.1093/nar/gks1219
Reinold, Farahpour, Fehring, Dolff, Konik et al., A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates With Severe COVID-19, Front. Cell Infect. Microbiol, doi:10.3389/fcimb.2021.747816
Righi, Lambertenghi, Gorska, Sciammarella, Ivaldi et al., Impact of COVID-19 and Antibiotic Treatments on Gut Microbiome: A Role for Enterococcus spp, Biomedicines, doi:10.3390/biomedicines10112786
Righi, Mirandola, Mazzaferri, Dossi, Razzaboni et al., Determinants of persistence of symptoms and impact on physical and mental wellbeing in Long COVID: A prospective cohort study, J. Infect, doi:10.1016/j.jinf.2022.02.003
Righi, Mirandola, Mazzaferri, Razzaboni, Zaffagnini et al., Long-Term Patient-Centred Follow-up in a Prospective Cohort of Patients with COVID-19, Infect. Dis. Ther, doi:10.1007/s40121-021-00461-3
Su, Lau, Liu, Chan, Ng, Post-acute COVID-19 syndrome and gut dysbiosis linger beyond 1 year after SARS-CoV-2 clearance, Gut, doi:10.1136/gutjnl-2022-328319
Takahashi, Tomita, Nishioka, Hisada, Nishijima, Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing, PLoS ONE, doi:10.1371/journal.pone.0105592
Vestad, Ueland, Lerum, Dahl, Holm et al., Respiratory dysfunction three months after severe COVID-19 is associated with gut microbiota alterations, J. Intern. Med, doi:10.1111/joim.13458
Wischmeyer, Tang, Ren, Bohannon, Ramirez et al., Efficacy of Probiotic Treatment as Post-Exposure Prophylaxis for COVID-19: A Double-Blind, Placebo Controlled Randomized Tria
Wong, Zhang, Ching, Mak, Huang et al., Effects of Gut Microbiome Modulation on Reducing Adverse Health Outcomes among Elderly and Diabetes Patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study), Nutrients, doi:10.3390/nu15081982
Yeoh, Zuo, Lui, Zhang, Liu et al., Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut, doi:10.1136/gutjnl-2020-323020
Zhang, Han, Li, Chen, Xie et al., Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19, Ther. Adv. Gastroenterol, doi:10.1177/17562848211035670
Zhang, Lau, Liu, Su, Chan et al., Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications, Nat. Rev. Gastroenterol. Hepatol, doi:10.1038/s41575-022-00698-4
Zhang, Xu, Mak, Chow, Lui et al., Gut microbiota-derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID-19: An open-label pilot study, J. Gastroenterol. Hepatol, doi:10.1111/jgh.15796
Zhang, Zhou, Ma, Chen, Tang et al., Gut Microbiota Dysbiosis Correlates With Long COVID-19 at One-Year After Discharge, J. Korean Med. Sci, doi:10.3346/jkms.2023.38.e120
Zhou, Pang, Wu, Liu, Wang et al., Gut microbiota in COVID-19: New insights from inside, Gut Microbes, doi:10.1080/19490976.2023.2201157
Zhou, Zhang, Zhang, Ma, Wang, Linking the gut microbiota to persistent symptoms in survivors of COVID-19 after discharge, J. Microbiol, doi:10.1007/s12275-021-1206-5
Zuo, Zhan, Zhang, Liu, Tso et al., Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge, Gastroenterology, doi:10.1053/j.gastro.2020.06.048
DOI record:
{
"DOI": "10.3390/microorganisms12010131",
"ISSN": [
"2076-2607"
],
"URL": "http://dx.doi.org/10.3390/microorganisms12010131",
"abstract": "<jats:p>COVID-19 has been associated with having a negative impact on patients’ gut microbiome during both active disease and in the post-acute phase. In acute COVID-19, rapid alteration of the gut microbiome composition was observed, showing on one side a reduction in beneficial symbionts (e.g., Roseburia, Lachnospiraceae) and on the other side an increase in opportunistic pathogens such as Enterococcus and Proteobacteria. Alpha diversity tends to decrease, especially initially with symptom onset and hospital admission. Although clinical recovery appears to align with improved gut homeostasis, this process could take several weeks, even in mild infections. Moreover, patients with COVID-19 post-acute syndrome showed changes in gut microbiome composition, with specific signatures associated with decreased respiratory function up to 12 months following acute disease. Potential treatments, especially probiotic-based therapy, are under investigation. Open questions remain on the possibility to use gut microbiome data to predict disease progression and on potential confounders that may impair result interpretation (e.g., concomitant therapies in the acute phase; reinfection, vaccines, and occurrence of novel conditions or diseases in the post-acute syndrome). Understanding the relationships between gut microbiome dynamics and disease progression may contribute to better understanding post-COVID syndrome pathogenesis or inform personalized treatment that can affect specific targets or microbiome markers.</jats:p>",
"alternative-id": [
"microorganisms12010131"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8718-1200",
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"authenticated-orcid": false,
"family": "Righi",
"given": "Elda",
"sequence": "first"
},
{
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"family": "Dalla Vecchia",
"given": "Ilaria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"family": "Auerbach",
"given": "Nina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1684-0261",
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"authenticated-orcid": false,
"family": "Morra",
"given": "Matteo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3305-8711",
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"authenticated-orcid": false,
"family": "Górska",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"family": "Sciammarella",
"given": "Concetta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"family": "Lambertenghi",
"given": "Lorenza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"family": "Gentilotti",
"given": "Elisa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2342-5867",
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"authenticated-orcid": false,
"family": "Mirandola",
"given": "Massimo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IMID Laboratory, Department of Diagnostics and Public Health, Infectious Diseases Division, University of Verona, 37134 Verona, Italy"
}
],
"family": "Tacconelli",
"given": "Evelina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology Unit, Udine University Hospital, 33100 Udine, Italy"
}
],
"family": "Sartor",
"given": "Assunta",
"sequence": "additional"
}
],
"container-title": "Microorganisms",
"container-title-short": "Microorganisms",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
1,
10
]
],
"date-time": "2024-01-10T10:47:21Z",
"timestamp": 1704883641000
},
"deposited": {
"date-parts": [
[
2024,
1,
10
]
],
"date-time": "2024-01-10T12:11:55Z",
"timestamp": 1704888715000
},
"funder": [
{
"award": [
"ENACT Fund 2020"
],
"name": "Cariverona Foundation"
},
{
"award": [
"1010161671"
],
"name": "European Union Horizon 2020 research and innovation programme"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
11
]
],
"date-time": "2024-01-11T00:11:37Z",
"timestamp": 1704931897611
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
9
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
9
]
],
"date-time": "2024-01-09T00:00:00Z",
"timestamp": 1704758400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-2607/12/1/131/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "131",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
1,
9
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
9
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1053/j.gastro.2020.06.048",
"article-title": "Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge",
"author": "Zuo",
"doi-asserted-by": "crossref",
"first-page": "1302",
"journal-title": "Gastroenterology",
"key": "ref_1",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1080/19490976.2023.2201157",
"article-title": "Gut microbiota in COVID-19: New insights from inside",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "2201157",
"journal-title": "Gut Microbes",
"key": "ref_2",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1038/s41575-022-00698-4",
"article-title": "Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "323",
"journal-title": "Nat. Rev. Gastroenterol. Hepatol.",
"key": "ref_3",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.3390/biomedicines10112786",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Righi, E., Lambertenghi, L., Gorska, A., Sciammarella, C., Ivaldi, F., and Mirandola, M. (2022). Assunta Sartor, Evelina Tacconelli. Impact of COVID-19 and Antibiotic Treatments on Gut Microbiome: A Role for Enterococcus spp.. Biomedicines, 10."
},
{
"key": "ref_5",
"unstructured": "(2023, October 03). Post COVID-19 Condition (Long COVID) [Internet]. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition."
},
{
"DOI": "10.1136/gutjnl-2021-325989",
"article-title": "Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "544",
"journal-title": "Gut",
"key": "ref_6",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1136/gutjnl-2022-328319",
"article-title": "Post-acute COVID-19 syndrome and gut dysbiosis linger beyond 1 year after SARS-CoV-2 clearance",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "1230",
"journal-title": "Gut",
"key": "ref_7",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(20)32656-8",
"article-title": "6-month consequences of COVID-19 in patients discharged from hospital: A cohort study",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "220",
"journal-title": "Lancet Lond. Engl.",
"key": "ref_8",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00461-3",
"article-title": "Long-Term Patient-Centred Follow-up in a Prospective Cohort of Patients with COVID-19",
"author": "Righi",
"doi-asserted-by": "crossref",
"first-page": "1579",
"journal-title": "Infect. Dis. Ther.",
"key": "ref_9",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0105592",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Takahashi, S., Tomita, J., Nishioka, K., Hisada, T., and Nishijima, M. (2014). Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE, 9."
},
{
"DOI": "10.14806/ej.17.1.200",
"article-title": "Cutadapt removes adapter sequences from high-throughput sequencing reads",
"author": "Martin",
"doi-asserted-by": "crossref",
"first-page": "10",
"journal-title": "EMBnet. J.",
"key": "ref_11",
"volume": "17",
"year": "2011"
},
{
"DOI": "10.1093/bioinformatics/bty191",
"article-title": "Minimap2: Pairwise alignment for nucleotide sequences",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "3094",
"journal-title": "Bioinformatics",
"key": "ref_12",
"volume": "34",
"year": "2018"
},
{
"DOI": "10.1093/nar/gks1219",
"article-title": "The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools",
"author": "Quast",
"doi-asserted-by": "crossref",
"first-page": "D590",
"journal-title": "Nucleic Acids Res.",
"key": "ref_13",
"volume": "41",
"year": "2013"
},
{
"DOI": "10.1371/journal.pcbi.1004957",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Huson, D.H., Beier, S., Flade, I., Górska, A., El-Hadidi, M., Mitra, S., Ruscheweyh, H.J., and Tappu, R. (2016). MEGAN Community Edition—Interactive Exploration and Analysis of Large-Scale Microbiome Sequencing Data. PLoS Comput. Biol., 12."
},
{
"DOI": "10.1109/MCSE.2007.55",
"article-title": "MATPLOTLIB: A 2D Graphics Environment",
"author": "Hunter",
"doi-asserted-by": "crossref",
"first-page": "90",
"journal-title": "Comput. Sci. Eng.",
"key": "ref_15",
"volume": "9",
"year": "2007"
},
{
"key": "ref_16",
"unstructured": "Jones, E., Oliphant, T., and Peterson, P. (2023, June 23). SciPy: Open Source Scientific Tools for Python. Available online: https://scipy.org/."
},
{
"DOI": "10.1128/AEM.71.12.8228-8235.2005",
"article-title": "UniFrac: A new phylogenetic method for comparing microbial communities",
"author": "Lozupone",
"doi-asserted-by": "crossref",
"first-page": "8228",
"journal-title": "Appl. Environ. Microbiol.",
"key": "ref_17",
"volume": "71",
"year": "2005"
},
{
"key": "ref_18",
"unstructured": "(2023, April 20). scikit-bio: A Bioinformatics Library for Data Scientists, Students, and Developers. Available online: https://scikit.bio."
},
{
"DOI": "10.1093/molbev/msw046",
"article-title": "ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data",
"author": "Serra",
"doi-asserted-by": "crossref",
"first-page": "1635",
"journal-title": "Mol. Biol. Evol.",
"key": "ref_19",
"volume": "33",
"year": "2016"
},
{
"DOI": "10.3389/fcimb.2021.670424",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Gaibani, P., D’Amico, F., Bartoletti, M., Lombardo, D., Rampelli, S., Fornaro, G., Coladonato, S., Siniscalchi, A., Re, M.C., and Viale, P. (2021). The Gut Microbiota of Critically Ill Patients With COVID-19. Front. Cell Infect. Microbiol., 11."
},
{
"DOI": "10.1371/journal.pone.0247041",
"doi-asserted-by": "crossref",
"key": "ref_21",
"unstructured": "Mazzarelli, A., Giancola, M.L., Farina, A., Marchioni, L., Rueca, M., Gruber, C.E.M., Bartolini, B., Ascoli Bartoli, T., Maffongelli, G., and Capobianchi, M.R. (2021). 16S rRNA gene sequencing of rectal swab in patients affected by COVID-19. PLoS ONE, 16."
},
{
"DOI": "10.3389/fmicb.2021.705020",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Moreira-Rosário, A., Marques, C., Pinheiro, H., Araújo, J.R., Ribeiro, P., Rocha, R., Mota, I., Pestana, D., Ribeiro, R., and Pereira, A. (2021). Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients. Front. Microbiol., 12."
},
{
"DOI": "10.3390/microorganisms9061292",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Khan, M., Mathew, B.J., Gupta, P., Garg, G., Khadanga, S., Vyas, A.K., and Singh, A.K. (2021). Gut dysbiosis and il-21 response in patients with severe covid-19. Microorganisms, 9."
},
{
"DOI": "10.3389/fcimb.2021.747816",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Reinold, J., Farahpour, F., Fehring, C., Dolff, S., Konik, M., Korth, J., van Baal, L., Hoffmann, D., Buer, J., and Witzke, O. (2021). A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates With Severe COVID-19. Front. Cell Infect. Microbiol., 11."
},
{
"DOI": "10.1080/19490976.2021.1926840",
"article-title": "The gut microbiome of COVID-19 recovered patients returns to uninfected status in a minority-dominated United States cohort",
"author": "Newsome",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Gut Microbes",
"key": "ref_25",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/microorganisms9061237",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Kim, H.N., Joo, E.J., Lee, C.W., Ahn, K.S., Kim, H.L., Park, D.I., and Park, S.K. (2021). Reversion of Gut Microbiota during the Recovery Phase in Patients with Asymptomatic or Mild COVID-19: Longitudinal Study. Microorganisms, 9."
},
{
"DOI": "10.1186/s12866-022-02686-9",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "Cheng, X., Zhang, Y., Li, Y., Wu, Q., Wu, J., Park, S.K., Guo, C., and Lu, J. (2022). Meta-analysis of 16S rRNA microbial data identified alterations of the gut microbiota in COVID-19 patients during the acute and recovery phases. BMC Microbiol., 22."
},
{
"DOI": "10.1101/2022.10.20.512999",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "de Nies, L., Galata, V., Martin-Gallausiaux, C., Despotovic, M., Busi, S.B., Snoeck, C.J., Delacour, L., Budagavi, D.P., Laczny, C.C., and Habier, J. (2023). Altered infective competence of the human gut microbiome in COVID-19. Microbiome, 11."
},
{
"DOI": "10.3389/fimmu.2020.00906",
"article-title": "The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health",
"author": "Parker",
"doi-asserted-by": "crossref",
"first-page": "906",
"journal-title": "Front. Immunol.",
"key": "ref_29",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2023.102107",
"article-title": "Clinical phenotypes and quality of life to define post-COVID-19 syndrome: A cluster analysis of the multinational, prospective ORCHESTRA cohort",
"author": "Gentilotti",
"doi-asserted-by": "crossref",
"first-page": "102107",
"journal-title": "EClinicalMedicine",
"key": "ref_30",
"volume": "62",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2022.02.003",
"article-title": "Determinants of persistence of symptoms and impact on physical and mental wellbeing in Long COVID: A prospective cohort study",
"author": "Righi",
"doi-asserted-by": "crossref",
"first-page": "566",
"journal-title": "J. Infect.",
"key": "ref_31",
"volume": "84",
"year": "2022"
},
{
"DOI": "10.1136/gutjnl-2021-324090",
"article-title": "Six-month follow-up of gut microbiota richness in patients with COVID-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "Gut",
"key": "ref_32",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1007/s12275-021-1206-5",
"article-title": "Linking the gut microbiota to persistent symptoms in survivors of COVID-19 after discharge",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "941",
"journal-title": "J. Microbiol.",
"key": "ref_33",
"volume": "59",
"year": "2021"
},
{
"DOI": "10.1111/joim.13458",
"article-title": "Respiratory dysfunction three months after severe COVID-19 is associated with gut microbiota alterations",
"author": "Vestad",
"doi-asserted-by": "crossref",
"first-page": "801",
"journal-title": "J. Intern. Med.",
"key": "ref_34",
"volume": "291",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-34535-8",
"article-title": "Multi-kingdom gut microbiota analyses define COVID-19 severity and post-acute COVID-19 syndrome",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "6806",
"journal-title": "Nat. Commun.",
"key": "ref_35",
"volume": "13",
"year": "2022"
},
{
"article-title": "Inflammation-associated gut microbiome in postacute sequelae of SARS-CoV-2 points towards new therapeutic targets",
"author": "Carneiro",
"first-page": "376",
"journal-title": "Gut",
"key": "ref_36",
"volume": "73",
"year": "2023"
},
{
"DOI": "10.3346/jkms.2023.38.e120",
"article-title": "Gut Microbiota Dysbiosis Correlates With Long COVID-19 at One-Year After Discharge",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "e120",
"journal-title": "J. Korean Med. Sci.",
"key": "ref_37",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1038/nrgastro.2014.66",
"article-title": "The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic",
"author": "Hill",
"doi-asserted-by": "crossref",
"first-page": "506",
"journal-title": "Nat. Rev. Gastroenterol. Hepatol.",
"key": "ref_38",
"volume": "11",
"year": "2014"
},
{
"DOI": "10.3390/foods8030092",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Davani-Davari, D., Negahdaripour, M., Karimzadeh, I., Seifan, M., Mohkam, M., Masoumi, S.J., Berenjian, A., and Ghasemi, Y. (2019). Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods, 8."
},
{
"DOI": "10.1038/s12276-021-00627-6",
"article-title": "Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "907",
"journal-title": "Exp. Mol. Med.",
"key": "ref_40",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.3390/metabo12100912",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Alenazy, M.F., Aljohar, H.I., Alruwaili, A.R., Daghestani, M.H., Alonazi, M.A., Labban, R.S., El-Ansary, A.K., and Balto, H.A. (2022). Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites, 12."
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"article-title": "Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19",
"author": "Yeoh",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "Gut",
"key": "ref_42",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1017/S0007114521001926",
"article-title": "Beyond probiotic legend: ESSAP gut microbiota health score to delineate SARS-COV-2 infection severity",
"author": "Hegazy",
"doi-asserted-by": "crossref",
"first-page": "1180",
"journal-title": "Br. J. Nutr.",
"key": "ref_43",
"volume": "127",
"year": "2022"
},
{
"DOI": "10.1016/j.tifs.2020.12.009",
"article-title": "Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "187",
"journal-title": "Trends Food Sci. Technol.",
"key": "ref_44",
"volume": "108",
"year": "2021"
},
{
"DOI": "10.1111/j.1365-2036.2011.04939.x",
"article-title": "Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis",
"author": "Oliva",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Aliment. Pharmacol. Ther.",
"key": "ref_45",
"volume": "35",
"year": "2012"
},
{
"DOI": "10.3389/fpubh.2020.00186",
"article-title": "Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic",
"author": "Baud",
"doi-asserted-by": "crossref",
"first-page": "186",
"journal-title": "Front. Public Health",
"key": "ref_46",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2020.110947",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Din, A.U., Mazhar, M., Waseem, M., Ahmad, W., Bibi, A., Hassan, A., Ali, N., Gang, W., Qian, G., and Ullah, R. (2021). SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharmacother. Biomed. Pharmacother., 133."
},
{
"DOI": "10.1002/eji.201646721",
"article-title": "Contributions of the intestinal microbiome in lung immunity",
"author": "McAleer",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Eur. J. Immunol.",
"key": "ref_48",
"volume": "48",
"year": "2018"
},
{
"DOI": "10.1038/s41590-018-0260-6",
"article-title": "The establishment of resident memory B cells in the lung requires local antigen encounter",
"author": "Allie",
"doi-asserted-by": "crossref",
"first-page": "97",
"journal-title": "Nat. Immunol.",
"key": "ref_49",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.3390/nu15081982",
"doi-asserted-by": "crossref",
"key": "ref_50",
"unstructured": "Wong, M.C.S., Zhang, L., Ching, J.Y.L., Mak, J.W.Y., Huang, J., Wang, S., Mok, C.K.P., Wong, A., Chiu, Q.L., and Fung, J.T. (2023). Effects of Gut Microbiome Modulation on Reducing Adverse Health Outcomes among Elderly and Diabetes Patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study). Nutrients, 15."
},
{
"DOI": "10.1111/jgh.15796",
"article-title": "Gut microbiota-derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID-19: An open-label pilot study",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "823",
"journal-title": "J. Gastroenterol. Hepatol.",
"key": "ref_51",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-1930760/v1",
"doi-asserted-by": "crossref",
"key": "ref_52",
"unstructured": "Wischmeyer, P., Tang, H., Ren, Y., Bohannon, L., Ramirez, Z., Andermann, T., Messina, J.A., Sung, J.A., Jensen, D., and Jung, S. (2023, October 03). Efficacy of Probiotic Treatment as Post-Exposure Prophylaxis for COVID-19: A Double-Blind, Placebo Controlled Randomized Tria [Internet]. Available online: https://europepmc.org/article/PPR/PPR545983."
},
{
"DOI": "10.1128/JVI.01282-19",
"article-title": "Peptidoglycan-Associated Cyclic Lipopeptide Disrupts Viral Infectivity",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "e01282-19",
"journal-title": "J. Virol.",
"key": "ref_53",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1080/19490976.2021.2018899",
"article-title": "Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial",
"doi-asserted-by": "crossref",
"first-page": "2018899",
"journal-title": "Gut Microbes",
"key": "ref_54",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3389/fnut.2020.613928",
"article-title": "Oral Bacteriotherapy in Patients With COVID-19: A Retrospective Cohort Study",
"author": "Ceccarelli",
"doi-asserted-by": "crossref",
"first-page": "613928",
"journal-title": "Front. Nutr.",
"key": "ref_55",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1177/17562848211035670",
"article-title": "Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "17562848211035670",
"journal-title": "Ther. Adv. Gastroenterol.",
"key": "ref_56",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1128/mSphere.00711-21",
"article-title": "Metabolites with SARS-CoV-2 Inhibitory Activity Identified from Human Microbiome Commensals",
"author": "Piscotta",
"doi-asserted-by": "crossref",
"first-page": "e0071121",
"journal-title": "Msphere",
"key": "ref_57",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1080/17474124.2023.2250716",
"article-title": "Therapeutics for Clostridioides difficile infection: Molecules and microbes",
"author": "Khanna",
"doi-asserted-by": "crossref",
"first-page": "903",
"journal-title": "Expert. Rev. Gastroenterol. Hepatol.",
"key": "ref_58",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1136/gutjnl-2021-325010",
"article-title": "Rapid resolution of COVID-19 after faecal microbiota transplantation",
"author": "Winter",
"doi-asserted-by": "crossref",
"first-page": "230",
"journal-title": "Gut",
"key": "ref_59",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1186/s13256-020-02583-7",
"article-title": "Gastrointestinal disturbance and effect of fecal microbiota transplantation in discharged COVID-19 patients",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "60",
"journal-title": "J. Med. Case Rep.",
"key": "ref_60",
"volume": "15",
"year": "2021"
}
],
"reference-count": 60,
"references-count": 60,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-2607/12/1/131"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Microbiology (medical)",
"Microbiology"
],
"subtitle": [],
"title": "Gut Microbiome Disruption Following SARS-CoV-2: A Review",
"type": "journal-article",
"volume": "12"
}
