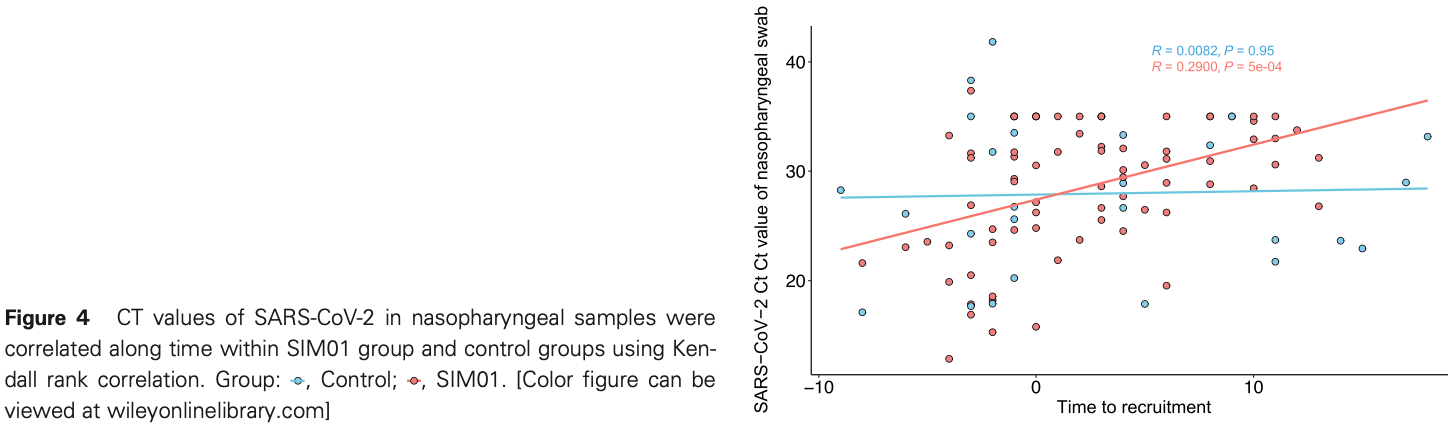
Gut microbiota-derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID-19: An open-label pilot study
et al., Journal of Gastroenterology and Hepatology, doi:10.1111/jgh.15796, NCT04581018, Mar 2022
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Pilot study of probiotic SIM01 with 25 consecutive COVID-19 patients in Hong Kong and 30 control patients treated by a different team during the same time period, showing improved antibody formation, reduced viral load and pro-inflammatory responses, and improvements for gut dysbiosis. SIM01 contains bifidobacteria strains, galactooligosaccharides, xylooligosaccharide, and resistant dextrin (derived from metagenomic databases of COVID-19 patients and healthy patients).
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments2.
|
risk of mechanical ventilation, 64.7% lower, RR 0.35, p = 1.00, treatment 0 of 25 (0.0%), control 1 of 30 (3.3%), NNT 30, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no antibody formation, 67.3% lower, RR 0.33, p = 0.06, treatment 3 of 25 (12.0%), control 11 of 30 (36.7%), NNT 4.1.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zhang et al., 2 Mar 2022, retrospective, China, peer-reviewed, 12 authors, trial NCT04581018 (history).
Contact: siewchienng@cuhk.edu.hk, fklchan@cuhk.edu.hk.
Gut microbiota‐derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID‐19: An open‐label pilot study
Journal of Gastroenterology and Hepatology, doi:10.1111/jgh.15796
Background and Aim: Gut dysbiosis is associated with immune dysfunction and severity of COVID-19. Whether targeting dysbiosis will improve outcomes of COVID-19 is unknown. This study aimed to assess the effects of a novel gut microbiota-derived synbiotic formula (SIM01) as an adjuvant therapy on immunological responses and changes in gut microbiota of hospitalized COVID-19 patients.
Methods: This was an open-label, proof-of-concept study. Consecutive COVID-19 patients admitted to an infectious disease referral center in Hong Kong were given a novel formula of Bifidobacteria strains, galactooligosaccharides, xylooligosaccharide, and resistant dextrin (SIM01). The latter was derived from metagenomic databases of COVID-19 patients and healthy population. COVID-19 patients who were admitted under another independent infectious disease team during the same period without receiving SIM01 acted as controls. All patients received standard treatments for COVID-19 according to the hospital protocol. We assessed antibody response, plasma proinflammatory markers, nasopharyngeal SARS-CoV-2 viral load, and fecal microbiota profile from admission up to week 5. Results: Twenty-five consecutive COVID-19 patients received SIM01 for 28 days; 30 patients who did not receive the formula acted as controls. Significantly more patients receiving SIM01 than controls developed SARS-CoV-2 IgG antibody (88% vs 63.3%; P = 0.037) by Day 16. One (4%) and 8 patients (26.7%) in the SIM01 and control group, respectively, failed to develop positive IgG antibody upon discharge. At week 5, plasma levels of interleukin (IL)-6, monocyte chemoattractant protein-1 (MCP-1), macrophage colony-stimulating factor (M-CSF), tumor necrosis factor (TNF-α), and IL-1RA reduced significantly in the SIM01 but not in the control group. There was a significant negative correlation of nasopharyngeal SARS-CoV-2 viral load and SIM01 intervention. Metagenomic analysis showed that bacterial species in SIM01 formula were found in greater abundance leading to enrichment of commensal bacteria and suppression of opportunistic pathogens in COVID-19 patients by week 4 and week 5. Conclusions: This proof-of-concept study suggested that the use of a novel gut microbiota-derived synbiotic formula, SIM01, hastened antibody formation against SARS-CoV-2, reduced nasopharyngeal viral load, reduced pro-inflammatory immune markers, and restored gut dysbiosis in hospitalised COVID-19 patients. TW110115155). Prof. Siew Ng and Prof. Francis Chan are inventors of patent applications for "Therapeutic and Diagnostic Use of Microorganisms for COVID-19" (US63/016,759, US63/015,310, US63/064,821, PCT/CN2021/090488, and TW110115153). No other potential conflict of interest relevant to this article was reported. No other potential conflict of interest relevant to this article was reported. Financial support: This study was supported by a generous donation from The DH Chen Foundation. Clinical Trial Registry: The trial..
References
Aachary, Prapulla, Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications, Comprehensive Reviews in Food Science and Food Safety
Amaretti, Bernardi, Leonardi, Raimondi, Zanoni et al., Fermentation of xylo-oligosaccharides by Bifidobacterium adolescentis DSMZ 18350: kinetics, metabolism, and beta-xylosidase activities, Appl. Microbiol. Biotechnol
Barczynska, Jurgonski, Slizewska, Juskiewicz, Kapusniak, Corn starch dextrin changes intestinal microbiota and its metabolic activity in rats fed a basal and high-fat diet, British Food Journal
Bolger, Lohse, Usadel, Trimmomatic: a flexible trimmer for Illumina sequence data, Bioinformatics
Chen, Gu, Chen, Six-month follow-up of gut microbiota richness in patients with COVID-19, Gut, doi:10.1136/gutjnl-2021-324090
Grimm, Radulovic, Riedel, Colonization of C57BL/6 Mice by a potential probiotic Bifidobacterium bifidum strain under germ-free and specific pathogen-free conditions and during experimental colitis, PLoS One
Gu, Chen, Wu, Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza, Clin. Infect. Dis
Hu, Zhang, Lin, Tang, Chan et al., Probiotics, prebiotics and dietary approaches during COVID-19 pandemic, Trends Food Sci Technol
Hughes, Davoodi-Semiromi, Colee, Galactooligosaccharide supplementation reduces stress-induced gastrointestinal dysfunction and days of cold or flu: a randomized, double-blind, controlled trial in healthy university students, American Journal of Clinical Nutrition
King, Glanville, Sanders, Fitzgerald, Varley, Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis, Br. J. Nutr
Klaenhammer, Kleerebezem, Kopp, Rescigno, The impact of probiotics and prebiotics on the immune system, Nat. Rev. Immunol
Lai, Chen, Lui, Prospective study comparing deep-throat saliva with other respiratory tract specimens in the diagnosis of novel coronavirus disease (COVID-19), J Infect Dis
Li, Limenitakis, Greiff, Mucosal or systemic microbiota exposures shape the B cell repertoire, Nature
Lucas, Wong, Klein, Longitudinal analyses reveal immunological misfiring in severe COVID-19, Nature
Maldonado-Gomez, Martinez, Bottacini, Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome, Cell Host Microbe
Marina, Beatriz, Roberto, Oteo, Hernan et al., Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study, Lancet
Riviere, Selak, Lantin, Leroy, Vuyst, Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut, Front. Microbiol
Rizzardini, Eskesen, Calder, Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12(R) and Lactobacillus paracasei ssp. paracasei, L. casei 431(R) in an influenza vaccination model: a randomised, double-blind, placebo-controlled study, Br. J. Nutr
Van Laere, Hartemink, Bosveld, Schols, Voragen, Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria, J. Agric. Food Chem
Van Puyenbroeck, Hens, Coenen, Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents, Am. J. Clin. Nutr
Wronkowska, Soral-Smietana, Biedrzycka, Utilization of resistant starch of native tapioca, corn and waxy corn starches and their retrograded preparations by Bifidobacterium, Int. J. Food Sci. Nutr
Wu, Liu, Zhao, Liu, Wang, Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study, Clin. Infect. Dis
Yan, Polk, Probiotics and immune health, Curr. Opin. Gastroenterol
Yeoh, Zuo, Lui, Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut
Zhang, Ai, Yang, Metatranscriptomic characterization of COVID-19 identified a host transcriptional classifier associated with immune signaling, Clin. Infect. Dis, doi:10.1093/cid/ciaa663
Zuo, Zhang, Lui, Alterations in gut microbiota of patients with COVID-19 during time of hospitalization, Gastroenterology
DOI record:
{
"DOI": "10.1111/jgh.15796",
"ISSN": [
"0815-9319",
"1440-1746"
],
"URL": "http://dx.doi.org/10.1111/jgh.15796",
"alternative-id": [
"10.1111/jgh.15796"
],
"archive": [
"Portico"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1634-3780",
"affiliation": [
{
"name": "Microbiota I‐Center (MagIC) The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "State Key Laboratory of Digestive Disease, Institute of Digestive Disease, Li Ka Shing Institute of Health Sciences, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Lin",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5552-7534",
"affiliation": [
{
"name": "Microbiota I‐Center (MagIC) The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "State Key Laboratory of Digestive Disease, Institute of Digestive Disease, Li Ka Shing Institute of Health Sciences, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"authenticated-orcid": false,
"family": "Xu",
"given": "Zhilu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5221-7349",
"affiliation": [
{
"name": "Microbiota I‐Center (MagIC) The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "State Key Laboratory of Digestive Disease, Institute of Digestive Disease, Li Ka Shing Institute of Health Sciences, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"authenticated-orcid": false,
"family": "Mak",
"given": "Joyce W Y",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"family": "Chow",
"given": "Kai Ming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "Stanley Ho Centre for Emerging Infectious Diseases, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"family": "Lui",
"given": "Grace",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"family": "Li",
"given": "Timothy C M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Chemical Pathology, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"family": "Wong",
"given": "Chun Kwok",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6360-4608",
"affiliation": [
{
"name": "Department of Microbiology, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"authenticated-orcid": false,
"family": "Chan",
"given": "Paul K S",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8257-2843",
"affiliation": [
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"authenticated-orcid": false,
"family": "Ching",
"given": "Jessica Y L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology Osaka City University Graduate School of Medicine Osaka Japan"
}
],
"family": "Fujiwara",
"given": "Yasuhiro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7388-2436",
"affiliation": [
{
"name": "Microbiota I‐Center (MagIC) The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "State Key Laboratory of Digestive Disease, Institute of Digestive Disease, Li Ka Shing Institute of Health Sciences, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"authenticated-orcid": false,
"family": "Chan",
"given": "Francis K L",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6850-4454",
"affiliation": [
{
"name": "Microbiota I‐Center (MagIC) The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "Department of Medicine and Therapeutics, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
},
{
"name": "State Key Laboratory of Digestive Disease, Institute of Digestive Disease, Li Ka Shing Institute of Health Sciences, Faculty of Medicine The Chinese University of Hong Kong Hong Kong SAR China"
}
],
"authenticated-orcid": false,
"family": "Ng",
"given": "Siew C",
"sequence": "additional"
}
],
"container-title": [
"Journal of Gastroenterology and Hepatology"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
16
]
],
"date-time": "2022-02-16T05:55:05Z",
"timestamp": 1644990905000
},
"deposited": {
"date-parts": [
[
2022,
3,
3
]
],
"date-time": "2022-03-03T02:45:12Z",
"timestamp": 1646275512000
},
"indexed": {
"date-parts": [
[
2022,
3,
30
]
],
"date-time": "2022-03-30T00:38:11Z",
"timestamp": 1648600691271
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0815-9319"
},
{
"type": "electronic",
"value": "1440-1746"
}
],
"issued": {
"date-parts": [
[
2022,
3,
2
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
2
]
],
"date-time": "2022-03-02T00:00:00Z",
"timestamp": 1646179200000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
2
]
],
"date-time": "2022-03-02T00:00:00Z",
"timestamp": 1646179200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jgh.15796",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/jgh.15796",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jgh.15796",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
3,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
2
]
]
},
"publisher": "Wiley",
"reference": [
{
"article-title": "Alterations in gut microbiota of patients with COVID‐19 during time of hospitalization",
"author": "Zuo T",
"first-page": "e8",
"journal-title": "Gastroenterology",
"key": "e_1_2_6_2_1",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"article-title": "Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID‐19",
"author": "Yeoh YK",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "Gut",
"key": "e_1_2_6_3_1",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa709",
"article-title": "Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza",
"author": "Gu S",
"doi-asserted-by": "crossref",
"first-page": "2669",
"journal-title": "Clin. Infect. Dis.",
"key": "e_1_2_6_4_1",
"volume": "71",
"year": "2020"
},
{
"article-title": "Metatranscriptomic characterization of COVID‐19 identified a host transcriptional classifier associated with immune signaling",
"author": "Zhang H",
"first-page": "ciaa663",
"journal-title": "Clin. Infect. Dis.",
"key": "e_1_2_6_5_1",
"year": "2020"
},
{
"article-title": "Six‐month follow‐up of gut microbiota richness in patients with COVID‐19",
"author": "Chen Y",
"first-page": "gutjnl‐2021‐324",
"journal-title": "Gut",
"key": "e_1_2_6_6_1",
"year": "2021"
},
{
"DOI": "10.1038/nri3312",
"article-title": "The impact of probiotics and prebiotics on the immune system",
"author": "Klaenhammer TR",
"doi-asserted-by": "crossref",
"first-page": "728",
"journal-title": "Nat. Rev. Immunol.",
"key": "e_1_2_6_7_1",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1017/S0007114514000075",
"article-title": "Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta‐analysis",
"author": "King S",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Br. J. Nutr.",
"key": "e_1_2_6_8_1",
"volume": "112",
"year": "2014"
},
{
"DOI": "10.3945/ajcn.111.026831",
"article-title": "Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double‐blind, placebo‐controlled trial in healthy elderly nursing home residents",
"author": "Van Puyenbroeck K",
"doi-asserted-by": "crossref",
"first-page": "1165",
"journal-title": "Am. J. Clin. Nutr.",
"key": "e_1_2_6_9_1",
"volume": "95",
"year": "2012"
},
{
"key": "e_1_2_6_10_1",
"unstructured": "National Health Committee of the People's Republic of China.National Administration of Traditional Chinese Medicine Diagnostic and therapeutic guidance for 2019 novel coronavirus disease (version 5).http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf"
},
{
"DOI": "10.1111/j.1541-4337.2010.00135.x",
"article-title": "Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications",
"author": "Aachary AA",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Comprehensive Reviews in Food Science and Food Safety 2011",
"key": "e_1_2_6_11_1",
"volume": "10",
"year": "2011"
},
{
"DOI": "10.1007/s00253-012-4509-y",
"article-title": "Fermentation of xylo‐oligosaccharides by Bifidobacterium adolescentis DSMZ 18350: kinetics, metabolism, and beta‐xylosidase activities",
"author": "Amaretti A",
"doi-asserted-by": "crossref",
"first-page": "3109",
"journal-title": "Appl. Microbiol. Biotechnol.",
"key": "e_1_2_6_12_1",
"volume": "97",
"year": "2013"
},
{
"DOI": "10.1108/BFJ-02-2019-0083",
"article-title": "Corn starch dextrin changes intestinal microbiota and its metabolic activity in rats fed a basal and high‐fat diet",
"author": "Barczynska R",
"doi-asserted-by": "crossref",
"first-page": "2219",
"journal-title": "British Food Journal",
"key": "e_1_2_6_13_1",
"volume": "121",
"year": "2019"
},
{
"DOI": "10.3945/ajcn.111.014126",
"article-title": "Galactooligosaccharide supplementation reduces stress‐induced gastrointestinal dysfunction and days of cold or flu: a randomized, double‐blind, controlled trial in healthy university students",
"author": "Hughes C",
"doi-asserted-by": "crossref",
"first-page": "1305",
"journal-title": "American Journal of Clinical Nutrition",
"key": "e_1_2_6_14_1",
"volume": "93",
"year": "2011"
},
{
"DOI": "10.1021/jf990519i",
"article-title": "Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria",
"author": "Van Laere KMJ",
"doi-asserted-by": "crossref",
"first-page": "1644",
"journal-title": "J. Agric. Food Chem.",
"key": "e_1_2_6_15_1",
"volume": "48",
"year": "2000"
},
{
"DOI": "10.1080/09637480701663862",
"article-title": "Utilization of resistant starch of native tapioca, corn and waxy corn starches and their retrograded preparations by Bifidobacterium",
"author": "Wronkowska M",
"doi-asserted-by": "crossref",
"first-page": "80",
"journal-title": "Int. J. Food Sci. Nutr.",
"key": "e_1_2_6_16_1",
"volume": "59",
"year": "2009"
},
{
"DOI": "10.1093/cid/ciaa199",
"article-title": "Clinical characteristics of imported cases of coronavirus disease 2019 (COVID‐19) in Jiangsu province: a multicenter descriptive study",
"author": "Wu J",
"doi-asserted-by": "crossref",
"first-page": "706",
"journal-title": "Clin. Infect. Dis.",
"key": "e_1_2_6_17_1",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31483-5",
"article-title": "Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study",
"author": "Marina P",
"doi-asserted-by": "crossref",
"first-page": "535",
"journal-title": "Lancet",
"key": "e_1_2_6_18_1",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa487",
"article-title": "Prospective study comparing deep‐throat saliva with other respiratory tract specimens in the diagnosis of novel coronavirus disease (COVID‐19)",
"author": "Lai CKC",
"doi-asserted-by": "crossref",
"first-page": "1612",
"journal-title": "J Infect Dis",
"key": "e_1_2_6_19_1",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.1093/bioinformatics/btu170",
"article-title": "Trimmomatic: a flexible trimmer for Illumina sequence data",
"author": "Bolger AM",
"doi-asserted-by": "crossref",
"first-page": "2114",
"journal-title": "Bioinformatics",
"key": "e_1_2_6_20_1",
"volume": "30",
"year": "2014"
},
{
"DOI": "10.1038/s41586-020-2564-6",
"article-title": "Mucosal or systemic microbiota exposures shape the B cell repertoire",
"author": "Li H",
"doi-asserted-by": "crossref",
"first-page": "274",
"journal-title": "Nature",
"key": "e_1_2_6_21_1",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1097/MOG.0b013e32834baa4d",
"article-title": "Probiotics and immune health",
"author": "Yan F",
"doi-asserted-by": "crossref",
"first-page": "496",
"journal-title": "Curr. Opin. Gastroenterol.",
"key": "e_1_2_6_22_1",
"volume": "27",
"year": "2011"
},
{
"DOI": "10.1017/S000711451100420X",
"article-title": "Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB‐12(R) and Lactobacillus paracasei ssp. paracasei, L. casei 431(R) in an influenza vaccination model: a randomised, double‐blind, placebo‐controlled study",
"author": "Rizzardini G",
"doi-asserted-by": "crossref",
"first-page": "876",
"journal-title": "Br. J. Nutr.",
"key": "e_1_2_6_23_1",
"volume": "107",
"year": "2012"
},
{
"DOI": "10.1038/s41586-020-2588-y",
"article-title": "Longitudinal analyses reveal immunological misfiring in severe COVID‐19",
"author": "Lucas C",
"doi-asserted-by": "crossref",
"first-page": "463",
"journal-title": "Nature",
"key": "e_1_2_6_24_1",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1016/j.tifs.2020.12.009",
"article-title": "Probiotics, prebiotics and dietary approaches during COVID‐19 pandemic",
"author": "Hu J",
"doi-asserted-by": "crossref",
"first-page": "187",
"journal-title": "Trends Food Sci Technol",
"key": "e_1_2_6_25_1",
"volume": "108",
"year": "2021"
},
{
"DOI": "10.3389/fmicb.2016.00979",
"article-title": "Bifidobacteria and butyrate‐producing colon bacteria: importance and strategies for their stimulation in the human gut",
"author": "Riviere A",
"doi-asserted-by": "crossref",
"first-page": "979",
"journal-title": "Front. Microbiol.",
"key": "e_1_2_6_26_1",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0139935",
"article-title": "Colonization of C57BL/6 Mice by a potential probiotic Bifidobacterium bifidum strain under germ‐free and specific pathogen‐free conditions and during experimental colitis",
"author": "Grimm V",
"doi-asserted-by": "crossref",
"first-page": "e0139935",
"journal-title": "PLoS One",
"key": "e_1_2_6_27_1",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1016/j.chom.2016.09.001",
"article-title": "Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome",
"author": "Maldonado‐Gomez MX",
"doi-asserted-by": "crossref",
"first-page": "515",
"journal-title": "Cell Host Microbe",
"key": "e_1_2_6_28_1",
"volume": "20",
"year": "2016"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/jgh.15796"
}
},
"score": 1,
"short-container-title": [
"J of Gastro and Hepatol"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Gastroenterology",
"Hepatology"
],
"subtitle": [],
"title": [
"Gut microbiota‐derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID‐19: An open‐label pilot study"
],
"type": "journal-article"
}
