Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19
et al., Therapeutic Advances in Gastroenterology, doi:10.1177/17562848211035670
, Aug 2021
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 375 patients in China, 179 treated with probiotics (Bifidobacterium, Lactobacillus, and Enterococcus), showing improved clinical outcomes with treatment.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments2.
|
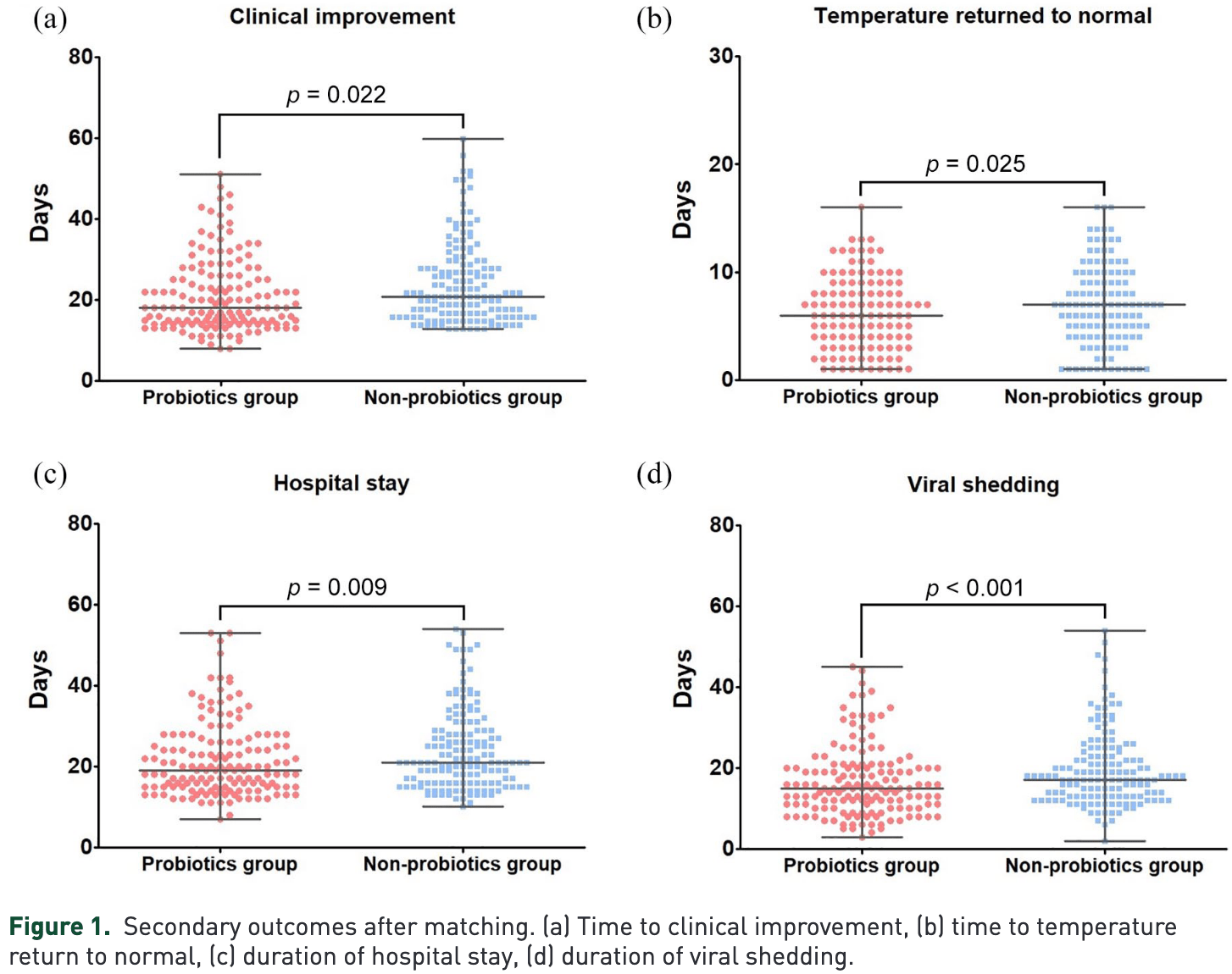
hospitalization time, 13.6% lower, relative time 0.86, p = 0.009, treatment 150, control 150, PSM.
|
|
time to clinical improvement, 14.3% lower, relative time 0.86, p = 0.02, treatment 150, control 150, PSM.
|
|
time to viral-, 16.7% lower, relative time 0.83, p < 0.001, treatment 150, control 150, PSM.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zhang et al., 4 Aug 2021, retrospective, China, peer-reviewed, 14 authors.
Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19
Therapeutic Advances in Gastroenterology, doi:10.1177/17562848211035670
Background and aims: Currently, there are no definitive therapies for coronavirus disease 2019 (COVID-19). Gut microbial dysbiosis has been proved to be associated with COVID-19 severity and probiotics is an adjunctive therapy for COIVD-19. However, the potential benefit of probiotics in COVID-19 has not been studied. We aimed to assess the relationship of probiotics use with clinical outcomes in patients with COVID-19. Methods: We conducted a propensity-score matched retrospective cohort study of adult patients with COVID-19. Eligible patients received either probiotics plus standard care (probiotics group) or standard care alone (non-probiotics group). The primary outcome was the clinical improvement rate, which was compared among propensity-score matched groups and in the unmatched cohort. Secondary outcomes included the duration of viral shedding, fever, and hospital stay. Results: Among the propensity-score matched groups, probiotics use was related to clinical improvement rates (log-rank p = 0.028). This relationship was driven primarily by a shorter (days) time to clinical improvement [difference, −3 (−4 to −1), p = 0.022], reduction in duration of fever [−1.0 (−2.0 to 0.0), p = 0.025], viral shedding [−3 (−6 to −1), p < 0.001], and hospital stay [−3 (−5 to −1), p = 0.009]. Using the Cox model with time-varying exposure, use of probiotics remained independently related to better clinical improvement rate in the unmatched cohort. Conclusion: Our study suggested that probiotics use was related to improved clinical outcomes in patients with COVID-19. Further studies are required to validate the effect of probiotics in combating the COVID-19 pandemic.
Author contributions Contributors: LNZ, HQH, XL, and CZC served as co-first authors. TWL, JYW, and ZQW served as co-corresponding authors. TWL and ZQW designed the study, interpreted the results, the accuracy of the data analysis, and drafted the manuscript. LNZ, JYW, XL, and HQH contributed to data collection, data analysis, and data interpretation. XBX, CLW, SCY, QH, FW, FH, and GMS contributed to literature search and data collection. All authors approved the final version of the manuscript.
Conflict of interest statement The authors declare that there is no conflict of interest.
Data availability statement The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Funding
ORCID iD Tianwen Lai https://orcid.org/0000-0001-9921-3425
Supplemental material Supplemental material for this article is available online.
References
Alberca, Oliveira, Branco, Obesity as a risk factor for COVID-19: an overview, Crit Rev Food Sci Nutr
Argenziano, Bruce, Slater, Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series, BMJ
Austin, An introduction to propensity score methods for reducing the effects of confounding in observational studies, Multivariate Behav Res
Austin, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples, Stat Med
Biosearch, Evaluation of the probiotic lactobacillus coryniformis K8 on COVID-19 prevention in healthcare
Cao, Wang, Wen, A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Cheung, Hung, Chan, Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from Hong Kong Cohort and systematic review and meta-analysis, Gastroenterology
Dhar, Mohanty, Gut microbiota and Covid-19-possible link and implications, Virus Res
Dickson, The microbiome and critical illness, Lancet Respir Med
Dumas, Bernard, Poquet, The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases, Cell Microbiol
Eder, Łodyga, Dobrowolska, Addressing multiple gastroenterological aspects of coronavirus disease 2019, Pol Arch Intern Med
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Hatakka, Savilahti, Pönkä, Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial, Br Med J
Jin, Lian, Hu, Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2018 (COVID-19) with gastrointestinal symptoms, Gut
Leyer, Li, Mubasher, Probiotic effects on cold and influenza-like symptom incidence and duration in children, Pediatrics
Negi, Das, Pahari, Potential role of gut microbiota in induction and regulation of innate immune memory, Front Immunol
Nobel, Phipps, Zucker, Gastrointestinal symptoms and COVID-19: case-control study from the United States, Gastroenterology
Pazgan-Simon, Rorat, Buczyńska, Gastrointestinal symptoms as the first, atypical indication of SARS-CoV-2 infection, Pol Arch Intern Med
Poscia, Oxygen-ozone as adjuvant treatment in early control of COVID-19 progression and modulation of the gut microbial flora (PROBIOZOVID)
Rautava, Salminen, Isolauri, Specific probiotics in reducing the risk of acute infections in infancy-a randomised, double-blind, placebocontrolled study, Br J Nutr
Sanders, Guarner, Guerrant, An update on the use and investigation of probiotics in health and disease, Gut
Su, Shen, Zhu, Involvement of digestive system in COVID-19: manifestations, pathology, management and challenges, Therap Adv Gastroenterol
Wan, Li, Shen, Enteric involvement in hospitalised patients with COVID-19 outside Wuhan, Lancet Gastroenterol Hepatol
Xu, Cai, Shen, Management of COVID-19: the Zhejiang experience (in Chinese), Zhejiang Da Xue Xue Bao Yi Xue Ban
DOI record:
{
"DOI": "10.1177/17562848211035670",
"ISSN": [
"1756-2848",
"1756-2848"
],
"URL": "http://dx.doi.org/10.1177/17562848211035670",
"abstract": "<jats:sec><jats:title>Background and aims:</jats:title><jats:p> Currently, there are no definitive therapies for coronavirus disease 2019 (COVID-19). Gut microbial dysbiosis has been proved to be associated with COVID-19 severity and probiotics is an adjunctive therapy for COIVD-19. However, the potential benefit of probiotics in COVID-19 has not been studied. We aimed to assess the relationship of probiotics use with clinical outcomes in patients with COVID-19. </jats:p></jats:sec><jats:sec><jats:title>Methods:</jats:title><jats:p> We conducted a propensity-score matched retrospective cohort study of adult patients with COVID-19. Eligible patients received either probiotics plus standard care (probiotics group) or standard care alone (non-probiotics group). The primary outcome was the clinical improvement rate, which was compared among propensity-score matched groups and in the unmatched cohort. Secondary outcomes included the duration of viral shedding, fever, and hospital stay. </jats:p></jats:sec><jats:sec><jats:title>Results:</jats:title><jats:p> Among the propensity-score matched groups, probiotics use was related to clinical improvement rates (log-rank p = 0.028). This relationship was driven primarily by a shorter (days) time to clinical improvement [difference, −3 (−4 to −1), p = 0.022], reduction in duration of fever [−1.0 (−2.0 to 0.0), p = 0.025], viral shedding [−3 (−6 to −1), p < 0.001], and hospital stay [−3 (−5 to −1), p = 0.009]. Using the Cox model with time-varying exposure, use of probiotics remained independently related to better clinical improvement rate in the unmatched cohort. </jats:p></jats:sec><jats:sec><jats:title>Conclusion:</jats:title><jats:p> Our study suggested that probiotics use was related to improved clinical outcomes in patients with COVID-19. Further studies are required to validate the effect of probiotics in combating the COVID-19 pandemic. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/17562848211035670"
],
"author": [
{
"affiliation": [
{
"name": "Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China"
}
],
"family": "Zhang",
"given": "Lina",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Infectious Diseases Center, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China"
}
],
"family": "Han",
"given": "Huanqin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China"
}
],
"family": "Li",
"given": "Xuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Breast Surgery, the Second Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China"
}
],
"family": "Chen",
"given": "Caozhen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China"
}
],
"family": "Xie",
"given": "Xiaobing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China"
}
],
"family": "Su",
"given": "Guomei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China"
}
],
"family": "Ye",
"given": "Shicai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China"
}
],
"family": "Wang",
"given": "Cuili",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China"
}
],
"family": "He",
"given": "Qing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China"
}
],
"family": "Wang",
"given": "Fang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, China"
}
],
"family": "Huang",
"given": "Fang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Liver Diseases, National Clinical Research Center for Infectious Disease, Shenzhen Third People’s Hospital, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen 518000, Guangdong, China"
}
],
"family": "Wang",
"given": "Zhaoqin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China"
}
],
"family": "Wu",
"given": "Jiayuan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9921-3425",
"affiliation": [
{
"name": "Department of Respiratory and Critical Care Medicine, Affiliated Hospital, Institute of Respiratory Diseases, Guangdong Medical University, Zhanjiang, Guangdong, China"
}
],
"authenticated-orcid": false,
"family": "Lai",
"given": "Tianwen",
"sequence": "additional"
}
],
"container-title": "Therapeutic Advances in Gastroenterology",
"container-title-short": "Therap Adv Gastroenterol",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
4
]
],
"date-time": "2021-08-04T10:00:35Z",
"timestamp": 1628071235000
},
"deposited": {
"date-parts": [
[
2021,
8,
4
]
],
"date-time": "2021-08-04T10:00:49Z",
"timestamp": 1628071249000
},
"funder": [
{
"award": [
"2018KQNCX095"
],
"name": "Project of Young Innovative Talents in Colleges and Universities in Guangdong Province"
},
{
"award": [
"2020B1515020004, 2021A1515011056, 2018A0303130269"
],
"name": "the Guangdong Basic and Applied Basic Research Foundation"
},
{
"award": [
"B2021206, A2018162"
],
"name": "Guangdong Medical Science and Technology Research Fund"
},
{
"award": [
"SZSM201612014"
],
"name": "Shenzhen Key Medical Discipline Construction Fund"
},
{
"DOI": "10.13039/501100001809",
"award": [
"81873404"
],
"doi-asserted-by": "publisher",
"name": "national natural science foundation of china"
},
{
"DOI": "10.13039/501100012151",
"award": [
"SZSM201612014"
],
"doi-asserted-by": "crossref",
"name": "Sanming Project of Medicine in Shenzhen"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
1
]
],
"date-time": "2024-04-01T15:20:12Z",
"timestamp": 1711984812345
},
"is-referenced-by-count": 30,
"issued": {
"date-parts": [
[
2021,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/17562848211035670",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/17562848211035670",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/17562848211035670",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "175628482110356",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2021,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
4
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1136/bmj.m1996",
"doi-asserted-by": "publisher",
"key": "bibr1-17562848211035670"
},
{
"DOI": "10.1053/j.gastro.2020.04.017",
"author": "Nobel YR",
"doi-asserted-by": "crossref",
"first-page": "e2",
"journal-title": "Gastroenterology",
"key": "bibr2-17562848211035670",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "bibr3-17562848211035670"
},
{
"DOI": "10.1016/j.virusres.2020.198018",
"doi-asserted-by": "publisher",
"key": "bibr4-17562848211035670"
},
{
"author": "Xu K",
"first-page": "147",
"journal-title": "Zhejiang Da Xue Xue Bao Yi Xue Ban",
"key": "bibr5-17562848211035670",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1177/1756284820934626",
"doi-asserted-by": "publisher",
"key": "bibr7-17562848211035670"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "bibr8-17562848211035670"
},
{
"DOI": "10.1080/00273171.2011.568786",
"doi-asserted-by": "publisher",
"key": "bibr9-17562848211035670"
},
{
"DOI": "10.1002/sim.3697",
"doi-asserted-by": "publisher",
"key": "bibr10-17562848211035670"
},
{
"author": "Eder P",
"first-page": "420",
"journal-title": "Pol Arch Intern Med",
"key": "bibr11-17562848211035670",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1016/S2468-1253(20)30118-7",
"doi-asserted-by": "publisher",
"key": "bibr12-17562848211035670"
},
{
"DOI": "10.1136/gutjnl-2020-320926",
"doi-asserted-by": "publisher",
"key": "bibr13-17562848211035670"
},
{
"DOI": "10.1053/j.gastro.2020.03.065",
"doi-asserted-by": "publisher",
"key": "bibr14-17562848211035670"
},
{
"author": "Pazgan-Simon M",
"first-page": "338",
"journal-title": "Pol Arch Intern Med",
"key": "bibr15-17562848211035670",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2019.02441",
"doi-asserted-by": "publisher",
"key": "bibr16-17562848211035670"
},
{
"DOI": "10.1111/cmi.12966",
"doi-asserted-by": "publisher",
"key": "bibr17-17562848211035670"
},
{
"DOI": "10.1016/S2213-2600(15)00427-0",
"doi-asserted-by": "publisher",
"key": "bibr18-17562848211035670"
},
{
"DOI": "10.1080/10408398.2020.1775546",
"doi-asserted-by": "publisher",
"key": "bibr19-17562848211035670"
},
{
"DOI": "10.1542/peds.2008-2666",
"doi-asserted-by": "publisher",
"key": "bibr20-17562848211035670"
},
{
"DOI": "10.1017/S0007114508116282",
"doi-asserted-by": "publisher",
"key": "bibr21-17562848211035670"
},
{
"DOI": "10.1136/bmj.322.7298.1327",
"doi-asserted-by": "publisher",
"key": "bibr22-17562848211035670"
},
{
"DOI": "10.1136/gutjnl-2012-302504",
"doi-asserted-by": "publisher",
"key": "bibr23-17562848211035670"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/17562848211035670"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Gastroenterology"
],
"subtitle": [],
"title": "Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "14"
}
