A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SARS-CoV-2
, F., Minerva Medica, doi:10.23736/S0026-4806.20.06570-2, Apr 2021
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of the potential use of S. salivarius K12 for COVID-19. Author notes that strain K12 of streptococcus salivarius may reduce occurrence of viral upper respiratory tract infections, possibly due to its ability to stimulate IFN-γ release and to activate natural killer cells without triggering aggressive inflammatory responses.
1.
Chau et al., Effectiveness of probiotics on COVID-19 prevention and treatment against mild COVID-19 in outpatient care: A systematic review, Nutrition and Health, doi:10.1177/02601060251378200.
2.
Bajić et al., Immunity's core reset: Synbiotics and gut microbiota in the COVID-19 era, Innate Immunity, doi:10.1177/17534259251362023.
3.
Bigman et al., A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery, Nutrients, doi:10.3390/nu17111802.
4.
Fazli et al., Possible Link between Gut Microbiota, Diet, and COVID-19 Infection, Journal of Medical Bacteriology, 12:4, jmb.tums.ac.ir/index.php/jmb/article/view/525.
5.
Santa et al., Comparative analysis of COVID-19 responses in Japan and Africa: diet, phytochemicals, vitamin D, and gut microbiota in reducing mortality—A systematic review and meta-analysis, Frontiers in Nutrition, doi:10.3389/fnut.2024.1465324.
6.
Kaushal, A., Nutraceuticals and pharmacological to balance the transitional microbiome to extend immunity during COVID-19 and other viral infections, Journal of Translational Medicine, doi:10.1186/s12967-024-05587-9.
7.
Mu et al., Anti-inflammatory and Nutritional Interventions Against SARS-CoV-2: A Comprehensive Review, Journal of Agriculture and Food Research, doi:10.1016/j.jafr.2024.101422.
8.
Taufer et al., Lactobacilli in COVID-19: A Systematic Review Based on Next-Generation Sequencing Studies, Microorganisms, doi:10.3390/microorganisms12020284.
9.
Righi et al., Gut Microbiome Disruption Following SARS-CoV-2: A Review, Microorganisms, doi:10.3390/microorganisms12010131.
10.
Petrariu et al., Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases, Frontiers in Microbiology, doi:10.3389/fmicb.2023.1296447.
11.
Taufer (B) et al., The Role of Bifidobacterium in COVID-19: A Systematic Review, Life, doi:10.3390/life13091847.
12.
Di Pierro, F., A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SARS-CoV-2, Minerva Medica, doi:10.23736/S0026-4806.20.06570-2.
13.
Kurian et al., Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects, Archives of Medical Research, doi:10.1016/j.arcmed.2021.03.002.
14.
Singh et al., Probiotics: A potential immunomodulator in COVID-19 infection management, Nutrition Research, doi:10.1016/j.nutres.2020.12.014.
15.
Stavropoulou et al., Probiotics as a Weapon in the Fight Against COVID-19, Frontiers in Nutrition, doi:10.3389/fnut.2020.614986.
Di Pierro et al., 7 Apr 2021, peer-reviewed, 1 author.
Abstract: ©
LETTERS TO THE EDITOR
© 2020 EDIZIONI MINERVA MEDICA
Online version at http://www.minervamedica.it
Minerva Medica 2020 June;111(3):281-3
DOI: 10.23736/S0026-4806.20.06570-2
A possible probiotic (S. salivarius K12)
approach to improve oral
and lung microbiotas and raise
defenses against SARS-CoV-2
The coronavirus disease 2019 (COVID-19), a pathology caused by a novel beta-coronavirus named SARSCoV-2, is spreading rapidly and scientists are endeavoring worldwide to develop drugs for efficacious treatments and vaccines to protect human life. SARS-CoV-2
shares 79% sequence identity with SARS-CoV, the virus that caused a major outbreak in 2002-2003. In an
identical manner to SARS-CoV, SARS-CoV-2 utilizes
the ACE-2 receptor to bind to lung cells where it can
cause severe, and possibly fatal, pneumonia. Most cases
of transmission occur via person-to-person respiratory
droplets and from environmental surfaces to the hands
and then to the nose and mouth. Both pathways allow
the virus to reach, as the first step, the upper respiratory tract from where it can spread to the lungs.1 The
oral and the upper respiratory tract microbiotas contain
large populations of the genus Streptococcus, with both
commensal and pathogenic streptococci competing for
several niches using a variety of strategies. For instance,
streptococci have a remarkable ability to metabolize
carbohydrates via fermentation, thereby generating acids as by-products. Excessive acidification of the oral
environment by aciduric species such as Streptococcus
mutans is directly associated with the development of
dental caries. However, less acid-tolerant species such
as Streptococcus salivarius can also produce large
amounts of alkali, thereby playing an important role in
the acid-base physiology of the oral cavity. Streptococcus salivarius is a numerically-prominent foundation
member of the upper respiratory tract microbiota and
some members of this species have been shown to exert
a bacterial interference versus Streptococcus pyogenes,
Streptococcus pneumoniae, Moraxella catarrhalis and
Haemophilus influenzae, pathogens involved in recurrent pharyngitis, tonsillitis and in acute otitis media.2 A
particular strain of Streptococcus salivarius, known as
K12,3 has been clinically demonstrated to play a role
in creating a stable upper respiratory tract microbiota
capable of protecting the host from pathogenic bacte-
Vol. 111 - No. 3
ria, fungi and viruses, thereby reducing the incidence of
streptococcal pharyngo-tonsillitis, acute and secretory
otitis media, halitosis, oral thrush and viral infections
(rhinitis, influenza, pharyngitis, laryngitis, tracheitis
and enteritis). The antibacterial role of strain K12 has
been attributed to the release of bacteriocins (Salivaricin A2 and Salivaricin B) that can create instability in
the membranes of susceptible, pathogenic bacteria. In
contrast, the anti-Candida action seems to be mainly
due to the ability of strain K12 to compete with fungal
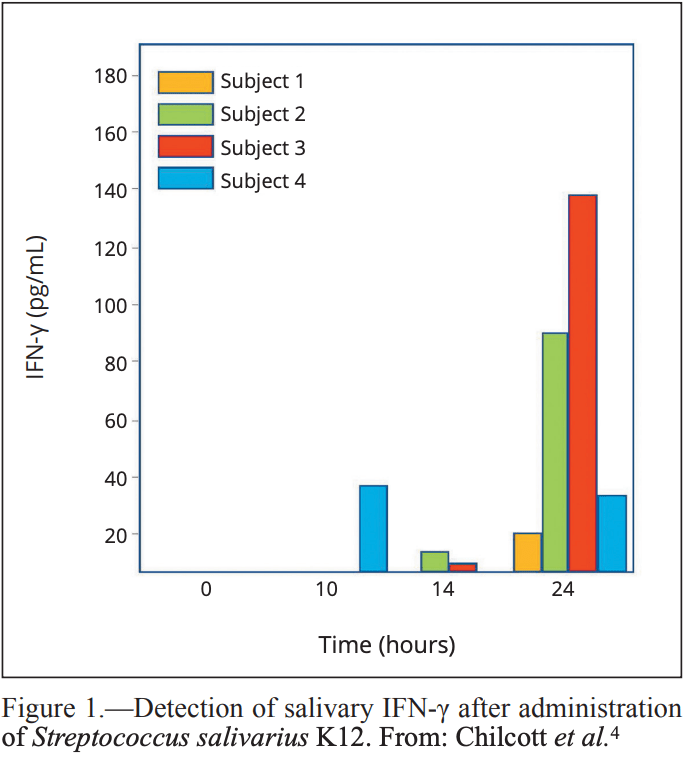
hyphae in adhering to oral mucosa. The proposed antiviral capability of strain K12 has been attributed to the
observed development of an adaptive immune response
as revealed by detection of enhanced levels of IFN-γ
in human saliva 10 hours after oral lozenge administration, with values at 24 hours between 22 and 139 pg/
mL (Figure 1).4 Intrinsic antiviral activities are mediated by interferon-induced proteins and can cause the
induction of nitric oxide synthase, which can directly
impacts upon virus..
DOI record:
{
"DOI": "10.23736/s0026-4806.20.06570-2",
"ISSN": [
"0026-4806",
"1827-1669"
],
"URL": "http://dx.doi.org/10.23736/S0026-4806.20.06570-2",
"author": [
{
"affiliation": [],
"family": "Di Pierro",
"given": "Francesco",
"sequence": "first"
}
],
"container-title": "Minerva Medica",
"container-title-short": "Minerva Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
6,
11
]
],
"date-time": "2020-06-11T14:14:06Z",
"timestamp": 1591884846000
},
"deposited": {
"date-parts": [
[
2020,
6,
11
]
],
"date-time": "2020-06-11T14:14:55Z",
"timestamp": 1591884895000
},
"indexed": {
"date-parts": [
[
2023,
9,
1
]
],
"date-time": "2023-09-01T13:08:43Z",
"timestamp": 1693573723135
},
"is-referenced-by-count": 28,
"issue": "3",
"issued": {
"date-parts": [
[
2020,
6
]
]
},
"journal-issue": {
"issue": "3"
},
"link": [
{
"URL": "https://www.minervamedica.it/pdf.php?cod=R10Y2020N03A0281",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "17149",
"original-title": [],
"prefix": "10.23736",
"published": {
"date-parts": [
[
2020,
6
]
]
},
"published-print": {
"date-parts": [
[
2020,
6
]
]
},
"publisher": "Edizioni Minerva Medica",
"reference": [
{
"DOI": "10.1186/s40779-020-00240-0",
"doi-asserted-by": "publisher",
"key": "10.23736/S0026-4806.20.06570-2_ref001"
},
{
"DOI": "10.1128/microbiolspec.GPP3-0042-2018",
"doi-asserted-by": "publisher",
"key": "10.23736/S0026-4806.20.06570-2_ref002"
},
{
"DOI": "10.23736/S0026-4946.18.05182-4",
"doi-asserted-by": "publisher",
"key": "10.23736/S0026-4806.20.06570-2_ref003"
},
{
"key": "10.23736/S0026-4806.20.06570-2_ref004",
"unstructured": "Chilcott CN, Crowley L, Kulkani V, Jack RW, McLellan AD, Tagg J. Elevated levels of interferon gamma in human saliva following ingestion of Streptococcus salivarius K12. Presented at: Joint New Zealand and Australian Microbiological Societies Annual Meeting. Dunedin, New Zealand, 22-25 November 2005."
},
{
"DOI": "10.1007/s11427-017-9151-1",
"doi-asserted-by": "publisher",
"key": "10.23736/S0026-4806.20.06570-2_ref005"
},
{
"DOI": "10.1093/cid/ciaa203",
"doi-asserted-by": "publisher",
"key": "10.23736/S0026-4806.20.06570-2_ref006"
},
{
"DOI": "10.1089/jir.2012.0116",
"doi-asserted-by": "publisher",
"key": "10.23736/S0026-4806.20.06570-2_ref007"
}
],
"reference-count": 7,
"references-count": 7,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.minervamedica.it/index2.php?show=R10Y2020N03A0281"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SAR S-CoV-2",
"type": "journal-article",
"volume": "111"
}
