Oral booster probiotic bifidobacteria in SARS-COV-2 patients
et al., International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211059677, Nov 2021
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small retrospective 44 hospitalized patients in Turkey, showing improved outcomes with probiotic bifidobacterium, however minimal group details are provided (for example, the age of the control patients is unknown), and no adjustments were made.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
This study is excluded in meta-analysis:
unadjusted results with key group information missing.
Bozkurt et al., 25 Nov 2021, retrospective, Turkey, peer-reviewed, 2 authors.
Oral booster probiotic bifidobacteria in SARS-COV-2 patients
International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211059677
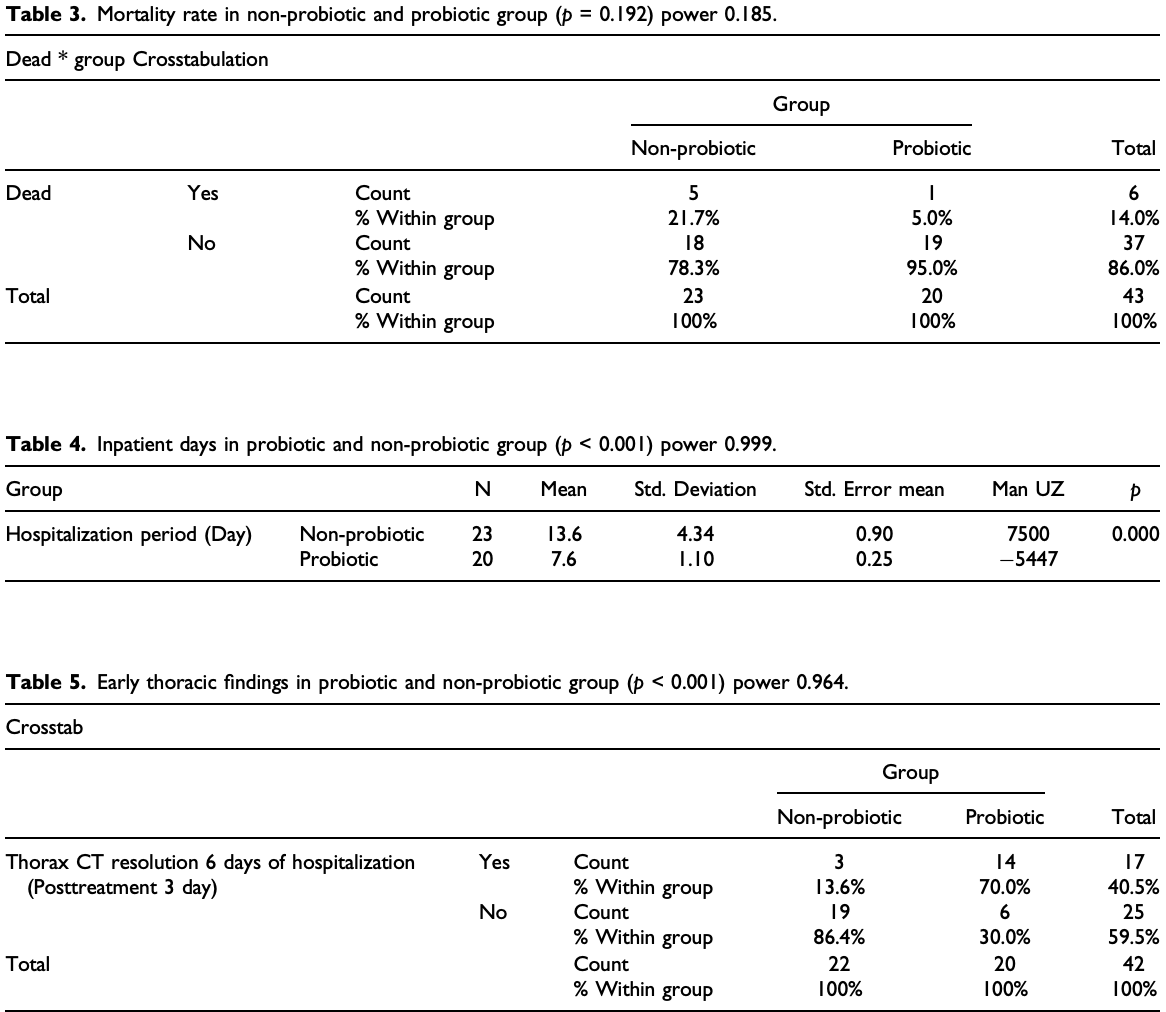
Oral booster-single strain probiotic bifidobacteria could be a potential strategy for SARS-CoV-2. This study aims to evaluate the role of oral probiotic Bifidobacterium on moderate/severe SARS-CoV-2 inpatients. In this single-center study, we analyzed data of 44 moderate/severe inpatients with diagnosed COVID-19 in Istanbul Maltepe University Medical Faculty Hospital, 2020 from 1 November 2020 to 15 December 2020. Clinical and medication features were compared and analyzed between patients with or without probiotic. In result, 19 of the 44 patients (43.18%) who were administrated with oral booster-single strain probiotic were discharged with the median inpatient day of 7.6 days which were significantly shorter than those of patients without probiotic. There were significant differences in inpatient days, radiological improvement at day 6 and week 3, and reduction in interleukin-6 levels in those receiving oral probiotic therapy. Although the mortality rate was 5% in the probiotic group, it was 25% in the non-probiotic group. Booster-single strain probiotic bifidobacteria could be an effective treatment strategy for moderate/severe SARS-CoV-2 inpatients to reduce the mortality and length of stay in hospital.
Conclusion Oral booster dose Bifidobacterium animalis subsp. Lactis administration could provide lower mortality, shortening the length of stay in hospital, early radiologic improvement and decrease plasma IL-6 level in moderate/severe SARS-CoV-2 patients. It is still necessary to develop diagnostic strategies to determine the patients to whom this method would be the most applicable.
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs Hüseyin S Bozkurt https://orcid.org/0000-0003-2097-2950 Ömer Bilen https://orcid.org/0000-0001-7198-8421
References
Baindara, Chakraborty, Holliday, Oral probiotics in coronavirus disease 2019: connecting the gut-lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials, New Microbes and New Infections
Bozkurt, Denktas, Ozdemir, Charge transport in bifidobacterium animalis subsp.lactis BB-12 under various atmospheres, Open Journal of Applied Sciences
Bozkurt, Kara, A new treatment for ulcerative colitis: intracolonic Bifidobacterium and xyloglucan application, European Journal of Inflammation
Bozkurt, Quigley, The probiotic Bifidobacterium in the management of Coronavirus: A theoretical basis, International Journal of Immunopathology and Pharmacology
Cavalli, Dagna, The right place for IL-1 inhibition in COVID-19, The Lancet Respiratory Medicine
Fung, Liu, The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus, Virology
Grifoni, Valoriani, Cei, Interleukin-6 as prognosticator in patients with COVID-19, The Journal of Infection
Hazan, Stollman, Bozkurt, The missing microbes: bifidobacterium and faecalibacterium depletion and loss of microbiome diversity as potential susceptibility markers for SARS-CoV-2 infection and severity, British Medical Journal
Hou, Jin, Kang, Kim, Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function, Journal of Virology
Jungersen, Wind, Johansen, The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12 ®, Microorganisms
Kong, Cheng, Yu, Induction of autophagy and interleukin 6 secretion in bystander cells: metabolic cooperation for radiation-induced rescue effect?, Journal of Radiation Research
Larsen, Nielsen, Kaestel, Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults, European Journal of Clinical Nutrition
Li, Cheng, Xu, The role of probiotics in coronavirus disease-19 infection in Wuhan: A retrospective study of 311 severe patients, International Immunopharmacology
Lu, Drug treatment options for the 2019-new coronavirus (2019-nCoV), BioScience Trends
Mahooti, Miri, Abdolalipour, The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment?, Microbial Pathogenesis
Rosas, Bräu, Waters, Tocilizumab in hospitalized patients with severe Covid-19 pneumonia, The New England Journal of Medicine
Rozga, Cheng, Handu, Effects of probiotics in conditions or infections similar to COVID-19 on health outcomes: an evidence analysis center scoping review, Journal of the Academy Nutrition and Dietetics
Santacroce, Inchingolo, Topi, Potential beneficial role of probiotics on the outcome of COVID-19 patients: an evolving perspective, Diabetes & Metabolic Syndrome: Clinical Research & Reviews
Sundararaman, Ray, Ravindra, Role of probiotics to combat viral infections with emphasis on COVID-19, Applied Microbiology and Biotechnology
Wong, Lui, Jj, Covid-19 and the digestive system, J ournal of Gastroenterology and Hepatology
Xu, Shi, Wang, Pathological findings of COVID-19 associated with acute respiratory distress syndrome, The Lancet Respiratory Medicine
Zhang, Li, Zhan, Analysis of serum cytokines in patients with severe acute respiratory syndrome, Infection and Immunity
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, Lancet
DOI record:
{
"DOI": "10.1177/20587384211059677",
"ISSN": [
"2058-7384",
"2058-7384"
],
"URL": "http://dx.doi.org/10.1177/20587384211059677",
"abstract": "<jats:p> Oral booster-single strain probiotic bifidobacteria could be a potential strategy for SARS-CoV-2. This study aims to evaluate the role of oral probiotic Bifidobacterium on moderate/severe SARS-CoV-2 inpatients. In this single-center study, we analyzed data of 44 moderate/severe inpatients with diagnosed COVID-19 in Istanbul Maltepe University Medical Faculty Hospital, 2020 from 1 November 2020 to 15 December 2020. Clinical and medication features were compared and analyzed between patients with or without probiotic. In result, 19 of the 44 patients (43.18%) who were administrated with oral booster-single strain probiotic were discharged with the median inpatient day of 7.6 days which were significantly shorter than those of patients without probiotic. There were significant differences in inpatient days, radiological improvement at day 6 and week 3, and reduction in interleukin-6 levels in those receiving oral probiotic therapy. Although the mortality rate was 5% in the probiotic group, it was 25% in the non-probiotic group. Booster-single strain probiotic bifidobacteria could be an effective treatment strategy for moderate/severe SARS-CoV-2 inpatients to reduce the mortality and length of stay in hospital. </jats:p>",
"alternative-id": [
"10.1177/20587384211059677"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2097-2950",
"affiliation": [
{
"name": "Faculty of Medical, Clinic of Gastroenterology, Istanbul Maltepe University, Istanbul, Turkey"
}
],
"authenticated-orcid": false,
"family": "Bozkurt",
"given": "Hüseyin S",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7198-8421",
"affiliation": [
{
"name": "Faculty of Design, Bursa Technical University, Bursa, Turkey"
}
],
"authenticated-orcid": false,
"family": "Bilen",
"given": "Ömer",
"sequence": "additional"
}
],
"container-title": [
"International Journal of Immunopathology and Pharmacology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
11,
25
]
],
"date-time": "2021-11-25T08:14:22Z",
"timestamp": 1637828062000
},
"deposited": {
"date-parts": [
[
2021,
11,
25
]
],
"date-time": "2021-11-25T08:14:25Z",
"timestamp": 1637828065000
},
"indexed": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T03:58:24Z",
"timestamp": 1639540704367
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2058-7384"
},
{
"type": "electronic",
"value": "2058-7384"
}
],
"issued": {
"date-parts": [
[
2021,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/20587384211059677",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/20587384211059677",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/20587384211059677",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "205873842110596",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2021,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
25
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1111/jgh.15047",
"doi-asserted-by": "publisher",
"key": "bibr1-20587384211059677"
},
{
"DOI": "10.1177/2058738420961304",
"doi-asserted-by": "publisher",
"key": "bibr2-20587384211059677"
},
{
"author": "Republic Of Turkey Ministry Of Health",
"key": "bibr3-20587384211059677",
"volume-title": "COVID-19 (SARS-CoV-2 infection) guide",
"year": "2020"
},
{
"author": "Bozkurt HS",
"first-page": "S57",
"issue": "3",
"journal-title": "European Journal of Inflammation",
"key": "bibr4-20587384211059677",
"volume": "158",
"year": "2020"
},
{
"author": "Hazan S",
"first-page": "21262832",
"journal-title": "British Medical Journal",
"key": "bibr5-20587384211059677",
"year": "2021"
},
{
"DOI": "10.5582/bst.2020.01020",
"doi-asserted-by": "publisher",
"key": "bibr6-20587384211059677"
},
{
"DOI": "10.1016/j.jand.2020.07.016",
"doi-asserted-by": "publisher",
"key": "bibr7-20587384211059677"
},
{
"DOI": "10.1016/j.micpath.2020.104452",
"doi-asserted-by": "publisher",
"key": "bibr8-20587384211059677"
},
{
"DOI": "10.1007/s00253-020-10832-4",
"doi-asserted-by": "publisher",
"key": "bibr9-20587384211059677"
},
{
"DOI": "10.1016/j.virol.2019.05.002",
"doi-asserted-by": "publisher",
"key": "bibr10-20587384211059677"
},
{
"DOI": "10.1093/jrr/rrx101",
"doi-asserted-by": "publisher",
"key": "bibr11-20587384211059677"
},
{
"DOI": "10.1128/JVI.00724-14",
"doi-asserted-by": "publisher",
"key": "bibr12-20587384211059677"
},
{
"DOI": "10.1128/IAI.72.8.4410-4415.2004",
"doi-asserted-by": "publisher",
"key": "bibr13-20587384211059677"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "bibr14-20587384211059677"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"doi-asserted-by": "publisher",
"key": "bibr15-20587384211059677"
},
{
"DOI": "10.1016/j.jinf.2020.06.008",
"doi-asserted-by": "publisher",
"key": "bibr16-20587384211059677"
},
{
"DOI": "10.1056/NEJMoa2028700",
"doi-asserted-by": "publisher",
"key": "bibr17-20587384211059677"
},
{
"DOI": "10.1016/S2213-2600(21)00035-7",
"doi-asserted-by": "publisher",
"key": "bibr18-20587384211059677"
},
{
"DOI": "10.1016/j.intimp.2021.107531",
"doi-asserted-by": "publisher",
"key": "bibr19-20587384211059677"
},
{
"DOI": "10.1016/j.dsx.2020.12.040",
"doi-asserted-by": "publisher",
"key": "bibr20-20587384211059677"
},
{
"DOI": "10.3390/microorganisms2020092",
"doi-asserted-by": "publisher",
"key": "bibr21-20587384211059677"
},
{
"DOI": "10.4236/ojapps.2019.96040",
"doi-asserted-by": "publisher",
"key": "bibr22-20587384211059677"
},
{
"DOI": "10.1038/sj.ejcn.1602450",
"doi-asserted-by": "publisher",
"key": "bibr23-20587384211059677"
},
{
"DOI": "10.1016/j.nmni.2021.100837",
"doi-asserted-by": "publisher",
"key": "bibr24-20587384211059677"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"score": 1,
"short-container-title": [
"Int J Immunopathol Pharmacol"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": [
"Oral booster probiotic bifidobacteria in SARS-COV-2 patients"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "35"
}
