Efficacy of a Probiotic Consisting of Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium bifidum PDV 0903, Bifidobacterium longum subsp. infantis PDV 1911, and Bifidobacterium longum subsp. longum PDV 2301 in the Treatment of Hospitalized Patients with COVID-19: a Randomized Controlled Trial
et al., Probiotics Antimicrob Proteins, doi:10.1007/s12602-021-09858-5, NCT04854941, Oct 2021
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
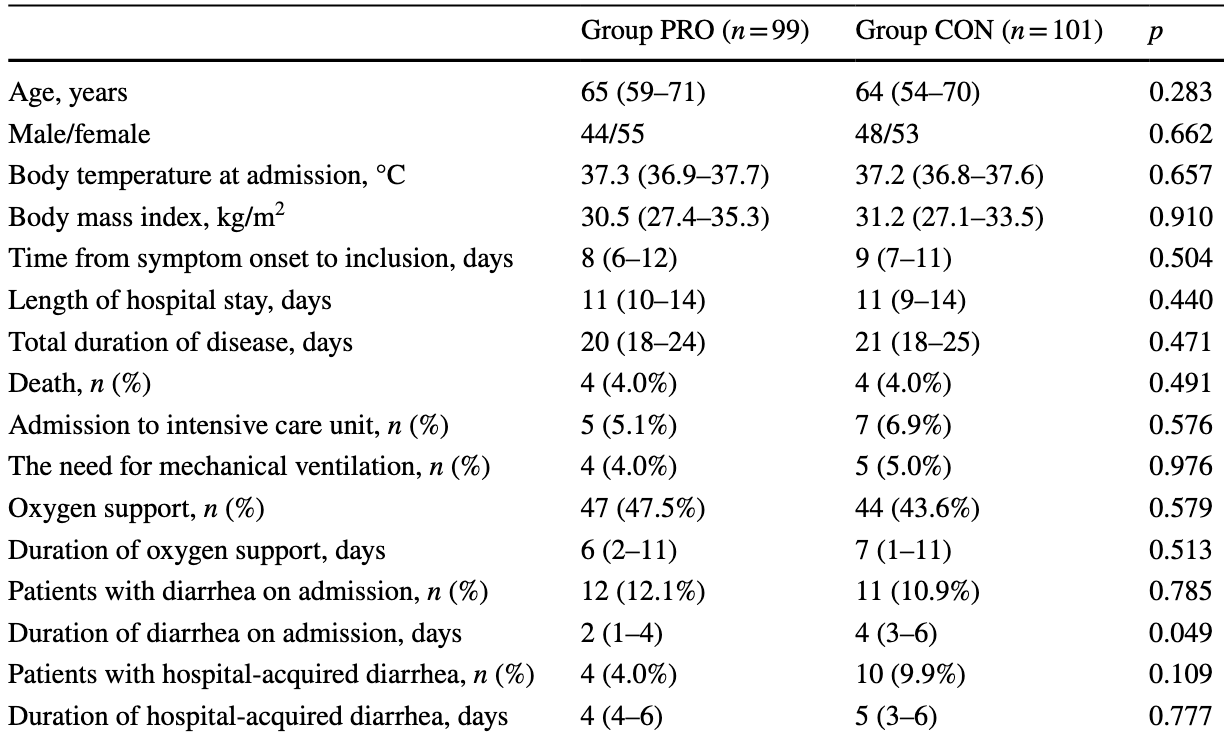
RCT 200 patients, 99 treated with a probiotic (Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium bifidum PDV 0903, Bifidobacterium longum subsp. infantis PDV 1911, and Bifidobacterium longum subsp. longum PDV 2301). There was no significant difference in mortality or recovery time, however benefits were seen for diarrhea. NCT04854941 (history).
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
|
risk of death, 2.0% higher, RR 1.02, p = 1.00, treatment 4 of 99 (4.0%), control 4 of 101 (4.0%).
|
|
risk of mechanical ventilation, 18.4% lower, RR 0.82, p = 1.00, treatment 4 of 99 (4.0%), control 5 of 101 (5.0%), NNT 110.
|
|
risk of ICU admission, 27.1% lower, RR 0.73, p = 0.77, treatment 5 of 99 (5.1%), control 7 of 101 (6.9%), NNT 53.
|
|
recovery time, 4.8% lower, relative time 0.95, p = 0.47, treatment 99, control 101.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ivashkin et al., 13 Oct 2021, Randomized Controlled Trial, Russia, peer-reviewed, 11 authors, study period December 2020 - March 2021, average treatment delay 8.0 days, trial NCT04854941 (history).
Efficacy of a Probiotic Consisting of Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium bifidum PDV 0903, Bifidobacterium longum subsp. infantis PDV 1911, and Bifidobacterium longum subsp. longum PDV 2301 in the Treatment of Hospitalized Patients with COVID-19: a Randomized Controlled Trial
Probiotics and Antimicrobial Proteins, doi:10.1007/s12602-021-09858-5
The treatment of coronavirus disease (COVID-19) and COVID-19-associated diarrhea remains challenging. This study aimed to evaluate the efficacy of a multi-strain probiotic in the treatment of COVID-19. This was a randomized, controlled, singlecenter, open-label trial (NCT04854941). Inpatients with confirmed COVID-19 and pneumonia were randomly assigned to a group that received a multi-strain probiotic (PRO group) or to the control group (CON group). There were 99 and 101 patients in the PRO and CON groups, respectively. No significant differences in mortality, total duration of disease and hospital stay, incidence of intensive care unit admission, need for mechanical ventilation or oxygen support, liver injury development, and changes in inflammatory biomarker levels were observed between the PRO and CON groups among all included patients as well as among subgroups delineated based on age younger or older than 65 years, and subgroups with chronic cardiovascular diseases and diabetes. Diarrhea on admission was observed in 11.5% of patients; it resolved earlier in the PRO group than in the CON group (2 [1-4] vs. 4 [3-6] days; p = 0.049). Hospital-acquired diarrhea developed less frequently in the PRO group than in the CON group among patients who received a single antibiotic (0% vs. 12.5%; p = 0.023) unlike among those who received > 1 antibiotic (10.5% vs. 13.3%; p = 0.696). The studied probiotic had no significant effect on mortality and changes in most biomarkers in COVID-19. However, it was effective in treating diarrhea associated with COVID-19 and in preventing hospital-acquired diarrhea in patients who received a single antibiotic.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s12602-021-09858-5.
Author Contribution The idea and design of the study were developed by Vladimir Ivashkin and Elena Poluektova. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Roman Maslennikov, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations Ethics Approval The study was approved by the local ethics committee (Conclusion №. 34-20 of September 9, 2020) in accordance with the Declaration of Helsinki. Consent to Participate Informed consent was obtained from all individual participants included in the study. Consent for Publication Patients' personal data are not published.
Conflicts of Interest The authors declare no competing interests.
References
Agamennone, Krul, Rijkers, A practical guide for probiotics applied to the case of antibiotic-associated diarrhoea in the Netherlands, BMC Gastroenterol, doi:10.1186/s12876-018-0831-x
Bozkurt, Quigley, The probiotic Bifidobacterium in the management of Coronavirus: a theoretical basis, Int J Immunopathol Pharmacol, doi:10.1177/2058738420961304
Cangemi, Lacy, Management of irritable bowel syndrome with diarrhoea: a review of nonpharmacological and pharmacological interventions, Ther Adv Gastroenterol, doi:10.1177/1756284819878950
Ceccarelli, Borrazzo, Pinacchio, Oral bacteriotherapy in patients with COVID-19: a retrospective cohort study, Front Nutr, doi:10.3389/fnut.2020.613928
D'ettorre, Ceccarelli, Marazzato, Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19, Front Med, doi:10.3389/fmed.2020.00389
Di, Gai, Protective efficacy of probiotics on the treatment of acute rotavirus diarrhea in children: an updated metaanalysis, Eur Rev Med Pharmacol Sci, doi:10.26355/eurrev_202009_23057
Isolauri, Probiotics for infectious diarrhoea, Gut, doi:10.1136/gut.52.3.436
Ivashkin, Fadeeva, Skhirtladze, Intestinal microbiota in the pathogenesis of chronic heart failure, Ital J Med, doi:10.4081/itjm.2020.1185
Kamkin, Interim guidelines for the prevention, diagnosis and treatment of new coronaviral infection
Kawahara, Makizaki, Oikawa, Oral administration of Bifidobacterium bifidum G9-1 alleviates rotavirus gastroenteritis through regulation of intestinal homeostasis by inducing mucosal protective factors, PLoS One, doi:10.1371/journal.pone.0173979
Kumar, Hecht, Priyamvada, Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells, Am J Physiol Cell Physiol, doi:10.1152/ajpcell.00194.2014
Li, Cheng, Xu, The role of probiotics in coronavirus disease-19 infection in Wuhan: a retrospective study of 311 severe patients, Int Immunopharmacol, doi:10.1016/j.intimp.2021
Li, Xu, Ye, Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: a systematic review with meta-analysis, World J Gastroenterol, doi:10.3748/wjg.v25.i33.4999
Li, Yang, Zhao, Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China, Clin Res Cardiol, doi:10.1007/s00392-020-01626-9
Mahooti, Miri, Abdolalipour, Ghaemi, The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment?, Microb Pathog, doi:10.1016/j.micpath.2020.104452
Manna, Chowdhury, Chakraborty, Mandal, Probiotics-derived peptides and their immunomodulatory molecules can play a preventive role against viral diseases including COVID-19, Probiotics Antimicrob Proteins, doi:10.1007/s12602-020-09727-7
Maslennikov, Ivashkin, Efremova, Poluektova, Shirokova, Probiotics in hepatology: an update, World J Hepatol, doi:10.4254/wjh.v13.i9.1154
Maslennikov, Ivashkin, Ufimtseva, Poluektova, A clinical variant of coronavirus disease 2019 with diarrhoea as the initial symptom compared with other variants, doi:10.23736/S2724-5985.21.02827-0
Maslennikov, Poluektova, Ivashkin, Diarrhoea in adults with coronavirus disease-beyond incidence and mortality: a systematic review and meta-analysis, Infect Dis (Lond), doi:10.1080/23744235.2021.1885733
Megyeri, Dernovics, Zii, COVID-19-associated diarrhea, World J Gastroenterol, doi:10.3748/wjg.v27.i23.3208
Mirzaei, Attar, Papizadeh, The emerging role of probiotics as a mitigation strategy against coronavirus disease 2019 (COVID-19), Arch Virol, doi:10.1007/s00705-021-05036-8
Mohamadian, Chiti, Shoghli, COVID-19: virology, biology and novel laboratory diagnosis, J Gene Med, doi:10.1002/jgm.3303
Mullish, Marchesi, Mcdonald, Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: should we be considering probiotics during viral pandemics?, Gut Microbes, doi:10.1080/19490976.2021.1900997
Olaimat, Aolymat, Holy, The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19, NPJ Sci Food, doi:10.1038/s41538-020-00078-9
Patra, Saxena, Sahu, Systematic network and meta-analysis on the antiviral mechanisms of probiotics: a preventive and treatment strategy to mitigate SARS-CoV-2 infection, Probiotics Antimicrob Proteins, doi:10.1007/s12602-021-09748-w
Rasinkangas, Tytgat, Ritari, Characterization of highly mucus-adherent non-GMO derivatives of Lacticaseibacillus rhamnosus GG, Front Bioeng Biotechnol, doi:10.3389/fbioe.2020.01024
Singh, Rao, Probiotics: a potential immunomodulator in COVID-19 infection management, Nutr Res, doi:10.1016/j.nutres.2020.12.014
Szajewska, Kołodziej, Systematic review with metaanalysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults, Aliment Pharmacol Ther, doi:10.1111/apt.13404
Tariq, Saha, Furqan, Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: a systematic review and meta-analysis, Mayo Clin Proc
Trush, Poluektova, Beniashvilli, The evolution of human probiotics: challenges and prospects, Probiotics Antimicrob Proteins, doi:10.1007/s12602-019-09628-4
Umakanthan, Sahu, Ranade, Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19), Postgrad Med, doi:10.1136/postgradmedj-2020-138234
Valdés-Varela, Hernández-Barranco, Ruas-Madiedo, Gueimonde, Effect of Bifidobacterium upon Clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates, Front Microbiol, doi:10.3389/fmicb.2016.00738
Wijarnpreecha, Ungprasert, Panjawatanan, COVID-19 and liver injury: a meta-analysis, Eur J Gastroenterol Hepatol, doi:10.1097/MEG.0000000000001817
Zolnikova, Komkova, Potskherashvili, Application of probiotics for acute respiratory tract infections, Ital J Med, doi:10.4081/itjm.2018.931
DOI record:
{
"DOI": "10.1007/s12602-021-09858-5",
"ISSN": [
"1867-1306",
"1867-1314"
],
"URL": "http://dx.doi.org/10.1007/s12602-021-09858-5",
"alternative-id": [
"9858"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "5 October 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 2,
"value": "13 October 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics Approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was approved by the local ethics committee (Conclusion №. 34-20 of September 9, 2020) in accordance with the Declaration of Helsinki."
},
{
"group": {
"label": "Consent to Participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Informed consent was obtained from all individual participants included in the study."
},
{
"group": {
"label": "Consent for Publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Patients’ personal data are not published."
},
{
"group": {
"label": "Conflicts of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "The authors declare no competing interests."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"affiliation": [],
"family": "Ivashkin",
"given": "Vladimir",
"sequence": "first"
},
{
"affiliation": [],
"family": "Fomin",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moiseev",
"given": "Sergey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brovko",
"given": "Michail",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7513-1636",
"affiliation": [],
"authenticated-orcid": false,
"family": "Maslennikov",
"given": "Roman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ulyanin",
"given": "Anatoly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sholomova",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vasilyeva",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trush",
"given": "Elizaveta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shifrin",
"given": "Oleg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poluektova",
"given": "Elena",
"sequence": "additional"
}
],
"container-title": "Probiotics and Antimicrobial Proteins",
"container-title-short": "Probiotics & Antimicro. Prot.",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
13
]
],
"date-time": "2021-10-13T18:12:56Z",
"timestamp": 1634148776000
},
"deposited": {
"date-parts": [
[
2023,
5,
26
]
],
"date-time": "2023-05-26T09:55:06Z",
"timestamp": 1685094906000
},
"indexed": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T11:06:43Z",
"timestamp": 1711796803762
},
"is-referenced-by-count": 25,
"issue": "3",
"issued": {
"date-parts": [
[
2021,
10,
13
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2023,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
13
]
],
"date-time": "2021-10-13T00:00:00Z",
"timestamp": 1634083200000
}
},
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
13
]
],
"date-time": "2021-10-13T00:00:00Z",
"timestamp": 1634083200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s12602-021-09858-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s12602-021-09858-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s12602-021-09858-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "460-468",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2021,
10,
13
]
]
},
"published-online": {
"date-parts": [
[
2021,
10,
13
]
]
},
"published-print": {
"date-parts": [
[
2023,
6
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1002/jgm.3303",
"author": "M Mohamadian",
"doi-asserted-by": "publisher",
"journal-title": "J Gene Med",
"key": "9858_CR1",
"unstructured": "Mohamadian M, Chiti H, Shoghli A et al (2021) COVID-19: virology, biology and novel laboratory diagnosis. J Gene Med 23:e3303. https://doi.org/10.1002/jgm.3303",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1136/postgradmedj-2020-138234",
"author": "S Umakanthan",
"doi-asserted-by": "publisher",
"first-page": "753",
"journal-title": "Postgrad Med",
"key": "9858_CR2",
"unstructured": "Umakanthan S, Sahu P, Ranade AV et al (2020) Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med 96:753–758. https://doi.org/10.1136/postgradmedj-2020-138234",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1007/s12602-019-09628-4",
"author": "EA Trush",
"doi-asserted-by": "publisher",
"first-page": "1291",
"journal-title": "Probiotics Antimicrob Proteins",
"key": "9858_CR3",
"unstructured": "Trush EA, Poluektova EA, Beniashvilli AG et al (2020) The evolution of human probiotics: challenges and prospects. Probiotics Antimicrob Proteins 12:1291–1299. https://doi.org/10.1007/s12602-019-09628-4",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.4081/itjm.2018.931",
"author": "O Zolnikova",
"doi-asserted-by": "publisher",
"first-page": "32",
"journal-title": "Ital J Med",
"key": "9858_CR4",
"unstructured": "Zolnikova O, Komkova I, Potskherashvili N et al (2018) Application of probiotics for acute respiratory tract infections. Ital J Med 12:32–38. https://doi.org/10.4081/itjm.2018.931",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.1016/j.mayocp.2020.06.003",
"author": "R Tariq",
"doi-asserted-by": "publisher",
"first-page": "1632",
"journal-title": "Mayo Clin Proc",
"key": "9858_CR5",
"unstructured": "Tariq R, Saha S, Furqan F et al (2020) Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: a systematic review and meta-analysis. Mayo Clin Proc 95:1632–1648",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1080/23744235.2021.1885733",
"author": "R Maslennikov",
"doi-asserted-by": "publisher",
"first-page": "348",
"journal-title": "Infect Dis (Lond)",
"key": "9858_CR6",
"unstructured": "Maslennikov R, Poluektova E, Ivashkin V et al (2021) Diarrhoea in adults with coronavirus disease-beyond incidence and mortality: a systematic review and meta-analysis. Infect Dis (Lond) 53:348–360. https://doi.org/10.1080/23744235.2021.1885733",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.23736/S2724-5985.21.02827-0",
"doi-asserted-by": "publisher",
"key": "9858_CR7",
"unstructured": "Maslennikov R, Ivashkin V, Ufimtseva A, Poluektova E (2021) A clinical variant of coronavirus disease 2019 with diarrhoea as the initial symptom compared with other variants. Minerva Gastroenterol (Torino). https://doi.org/10.23736/S2724-5985.21.02827-0"
},
{
"DOI": "10.3748/wjg.v27.i23.3208",
"author": "K Megyeri",
"doi-asserted-by": "publisher",
"first-page": "3208",
"journal-title": "World J Gastroenterol",
"key": "9858_CR8",
"unstructured": "Megyeri K, Dernovics Á, Al-Luhaibi ZII, Rosztóczy A (2021) COVID-19-associated diarrhea. World J Gastroenterol 27:3208–3222. https://doi.org/10.3748/wjg.v27.i23.3208",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1186/s12876-018-0831-x",
"author": "V Agamennone",
"doi-asserted-by": "publisher",
"first-page": "103",
"journal-title": "BMC Gastroenterol",
"key": "9858_CR9",
"unstructured": "Agamennone V, Krul CAM, Rijkers G et al (2018) A practical guide for probiotics applied to the case of antibiotic-associated diarrhoea in the Netherlands. BMC Gastroenterol 18:103. https://doi.org/10.1186/s12876-018-0831-x",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1177/1756284819878950",
"author": "DJ Cangemi",
"doi-asserted-by": "publisher",
"first-page": "1-19",
"journal-title": "Ther Adv Gastroenterol",
"key": "9858_CR10",
"unstructured": "Cangemi DJ, Lacy BE (2019) Management of irritable bowel syndrome with diarrhoea: a review of nonpharmacological and pharmacological interventions. Ther Adv Gastroenterol 12:1-19. https://doi.org/10.1177/1756284819878950",
"volume": "12",
"year": "2019"
},
{
"DOI": "10.1136/gut.52.3.436",
"author": "E Isolauri",
"doi-asserted-by": "publisher",
"first-page": "436",
"journal-title": "Probiotics for infectious diarrhoea Gut",
"key": "9858_CR11",
"unstructured": "Isolauri E (2003) Probiotics for infectious diarrhoea Gut 52:436–437. https://doi.org/10.1136/gut.52.3.436",
"volume": "52",
"year": "2003"
},
{
"DOI": "10.1097/MEG.0000000000001817",
"author": "K Wijarnpreecha",
"doi-asserted-by": "publisher",
"first-page": "990",
"journal-title": "Eur J Gastroenterol Hepatol",
"key": "9858_CR12",
"unstructured": "Wijarnpreecha K, Ungprasert P, Panjawatanan P et al (2021) COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol 33:990–995. https://doi.org/10.1097/MEG.0000000000001817",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.4254/wjh.v13.i9.1154",
"doi-asserted-by": "publisher",
"key": "9858_CR13",
"unstructured": "Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E (2021) Probiotics in hepatology: an update. World J Hepatol 13:1154–1166 https://doi.org/10.4254/wjh.v13.i9.1154"
},
{
"DOI": "10.1007/s00705-021-05036-8",
"author": "R Mirzaei",
"doi-asserted-by": "publisher",
"first-page": "1819",
"journal-title": "Arch Virol",
"key": "9858_CR14",
"unstructured": "Mirzaei R, Attar A, Papizadeh S et al (2021) The emerging role of probiotics as a mitigation strategy against coronavirus disease 2019 (COVID-19). Arch Virol 166:1819–1840. https://doi.org/10.1007/s00705-021-05036-8",
"volume": "166",
"year": "2021"
},
{
"DOI": "10.1016/j.nutres.2020.12.014",
"author": "K Singh",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nutr Res",
"key": "9858_CR15",
"unstructured": "Singh K, Rao A (2021) Probiotics: a potential immunomodulator in COVID-19 infection management. Nutr Res 87:1–12. https://doi.org/10.1016/j.nutres.2020.12.014",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1007/s12602-021-09748-w",
"author": "S Patra",
"doi-asserted-by": "publisher",
"first-page": "1138",
"journal-title": "Probiotics Antimicrob Proteins",
"key": "9858_CR16",
"unstructured": "Patra S, Saxena S, Sahu N et al (2021) Systematic network and meta-analysis on the antiviral mechanisms of probiotics: a preventive and treatment strategy to mitigate SARS-CoV-2 infection. Probiotics Antimicrob Proteins 13:1138–1156. https://doi.org/10.1007/s12602-021-09748-w",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1080/19490976.2021.1900997",
"author": "BH Mullish",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Gut Microbes",
"key": "9858_CR17",
"unstructured": "Mullish BH, Marchesi JR, McDonald JAK et al (2021) Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: should we be considering probiotics during viral pandemics? Gut Microbes 13:1–9. https://doi.org/10.1080/19490976.2021.1900997",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1007/s12602-020-09727-7",
"author": "S Manna",
"doi-asserted-by": "publisher",
"first-page": "611",
"journal-title": "Probiotics Antimicrob Proteins",
"key": "9858_CR18",
"unstructured": "Manna S, Chowdhury T, Chakraborty R, Mandal SM (2021) Probiotics-derived peptides and their immunomodulatory molecules can play a preventive role against viral diseases including COVID-19. Probiotics Antimicrob Proteins 13:611–623. https://doi.org/10.1007/s12602-020-09727-7",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41538-020-00078-9",
"author": "AN Olaimat",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "NPJ Sci Food",
"key": "9858_CR19",
"unstructured": "Olaimat AN, Aolymat I, Al-Holy M et al (2020) The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci Food 4:17. https://doi.org/10.1038/s41538-020-00078-9",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1016/j.micpath.2020.104452",
"author": "M Mahooti",
"doi-asserted-by": "publisher",
"journal-title": "Microb Pathog",
"key": "9858_CR20",
"unstructured": "Mahooti M, Miri SM, Abdolalipour E, Ghaemi A (2020) The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment? Microb Pathog 148:104452. https://doi.org/10.1016/j.micpath.2020.104452",
"volume": "148",
"year": "2020"
},
{
"DOI": "10.1177/2058738420961304",
"author": "HS Bozkurt",
"doi-asserted-by": "publisher",
"first-page": "205873842096130",
"journal-title": "Int J Immunopathol Pharmacol",
"key": "9858_CR21",
"unstructured": "Bozkurt HS, Quigley EM (2020) The probiotic Bifidobacterium in the management of Coronavirus: a theoretical basis. Int J Immunopathol Pharmacol 34:2058738420961304. https://doi.org/10.1177/2058738420961304",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2021",
"author": "Q Li",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "9858_CR22",
"unstructured": "Li Q, Cheng F, Xu Q et al (2021) The role of probiotics in coronavirus disease-19 infection in Wuhan: a retrospective study of 311 severe patients. Int Immunopharmacol 95:107531. https://doi.org/10.1016/j.intimp.2021",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.3389/fnut.2020.613928",
"author": "G Ceccarelli",
"doi-asserted-by": "publisher",
"journal-title": "Front Nutr",
"key": "9858_CR23",
"unstructured": "Ceccarelli G, Borrazzo C, Pinacchio C et al (2021) Oral bacteriotherapy in patients with COVID-19: a retrospective cohort study. Front Nutr 7:613928. https://doi.org/10.3389/fnut.2020.613928",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2020.00389",
"author": "G d’Ettorre",
"doi-asserted-by": "publisher",
"first-page": "389",
"journal-title": "Front Med (Lausanne)",
"key": "9858_CR24",
"unstructured": "d’Ettorre G, Ceccarelli G, Marazzato M et al (2020) Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med (Lausanne) 7:389. https://doi.org/10.3389/fmed.2020.00389",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1007/s00392-020-01626-9",
"author": "B Li",
"doi-asserted-by": "publisher",
"first-page": "531",
"journal-title": "Clin Res Cardiol",
"key": "9858_CR25",
"unstructured": "Li B, Yang J, Zhao F et al (2020) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109:531–538. https://doi.org/10.1007/s00392-020-01626-9",
"volume": "109",
"year": "2020"
},
{
"key": "9858_CR26",
"unstructured": "Kamkin E et al. (2020) Interim guidelines for the prevention, diagnosis and treatment of new coronaviral infection (COVID-19). https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/051/777/original/030902020_COVID-19_v8.pdf. Accessed 03 Sept 2020"
},
{
"DOI": "10.4081/itjm.2020.1185",
"author": "V Ivashkin",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Ital J Med",
"key": "9858_CR27",
"unstructured": "Ivashkin V, Fadeeva M, Skhirtladze M et al (2020) Intestinal microbiota in the pathogenesis of chronic heart failure. Ital J Med 14:1–8. https://doi.org/10.4081/itjm.2020.1185",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1111/apt.13404",
"author": "H Szajewska",
"doi-asserted-by": "publisher",
"first-page": "1149",
"journal-title": "Aliment Pharmacol Ther",
"key": "9858_CR28",
"unstructured": "Szajewska H, Kołodziej M (2015) Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther 42:1149–1157. https://doi.org/10.1111/apt.13404",
"volume": "42",
"year": "2015"
},
{
"DOI": "10.3748/wjg.v25.i33.4999",
"author": "YT Li",
"doi-asserted-by": "publisher",
"first-page": "4999",
"journal-title": "World J Gastroenterol",
"key": "9858_CR29",
"unstructured": "Li YT, Xu H, Ye JZ (2019) Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: a systematic review with meta-analysis. World J Gastroenterol 25:4999–5016. https://doi.org/10.3748/wjg.v25.i33.4999",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.26355/eurrev_202009_23057",
"doi-asserted-by": "publisher",
"key": "9858_CR30",
"unstructured": "Di JB, Gai ZT (2020) Protective efficacy of probiotics on the treatment of acute rotavirus diarrhea in children: an updated meta-analysis. Eur Rev Med Pharmacol Sci 24:9675–9683. https://doi.org/10.26355/eurrev_202009_23057"
},
{
"DOI": "10.3389/fbioe.2020.01024",
"author": "P Rasinkangas",
"doi-asserted-by": "publisher",
"first-page": "1024",
"journal-title": "Front Bioeng Biotechnol",
"key": "9858_CR31",
"unstructured": "Rasinkangas P, Tytgat HLP, Ritari J et al (2020) Characterization of highly mucus-adherent non-GMO derivatives of Lacticaseibacillus rhamnosus GG. Front Bioeng Biotechnol 8:1024. https://doi.org/10.3389/fbioe.2020.01024",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2016.00738",
"author": "L Valdés-Varela",
"doi-asserted-by": "publisher",
"first-page": "738",
"journal-title": "Front Microbiol",
"key": "9858_CR32",
"unstructured": "Valdés-Varela L, Hernández-Barranco AM, Ruas-Madiedo P, Gueimonde M (2016) Effect of Bifidobacterium upon Clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front Microbiol 7:738. https://doi.org/10.3389/fmicb.2016.00738",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0173979",
"author": "T Kawahara",
"doi-asserted-by": "publisher",
"journal-title": "PLoS ONE",
"key": "9858_CR33",
"unstructured": "Kawahara T, Makizaki Y, Oikawa Y et al (2017) Oral administration of Bifidobacterium bifidum G9–1 alleviates rotavirus gastroenteritis through regulation of intestinal homeostasis by inducing mucosal protective factors. PLoS One 12:e0173979. https://doi.org/10.1371/journal.pone.0173979",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1152/ajpcell.00194.2014",
"author": "A Kumar",
"doi-asserted-by": "publisher",
"first-page": "C1084",
"journal-title": "Am J Physiol Cell Physiol",
"key": "9858_CR34",
"unstructured": "Kumar A, Hecht C, Priyamvada S (2014) Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol 307:C1084–C1092. https://doi.org/10.1152/ajpcell.00194.2014",
"volume": "307",
"year": "2014"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s12602-021-09858-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Molecular Biology",
"Molecular Medicine",
"Microbiology"
],
"subtitle": [],
"title": "Efficacy of a Probiotic Consisting of Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium bifidum PDV 0903, Bifidobacterium longum subsp. infantis PDV 1911, and Bifidobacterium longum subsp. longum PDV 2301 in the Treatment of Hospitalized Patients with COVID-19: a Randomized Controlled Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "15"
}
