Lactoferrin Supplementation in Preventing and Protecting from SARS-CoV-2 Infection: Is There Any Role in General and Special Populations? An Updated Review of Literature
et al., International Journal of Molecular Sciences, doi:10.3390/ijms251910248, Sep 2024
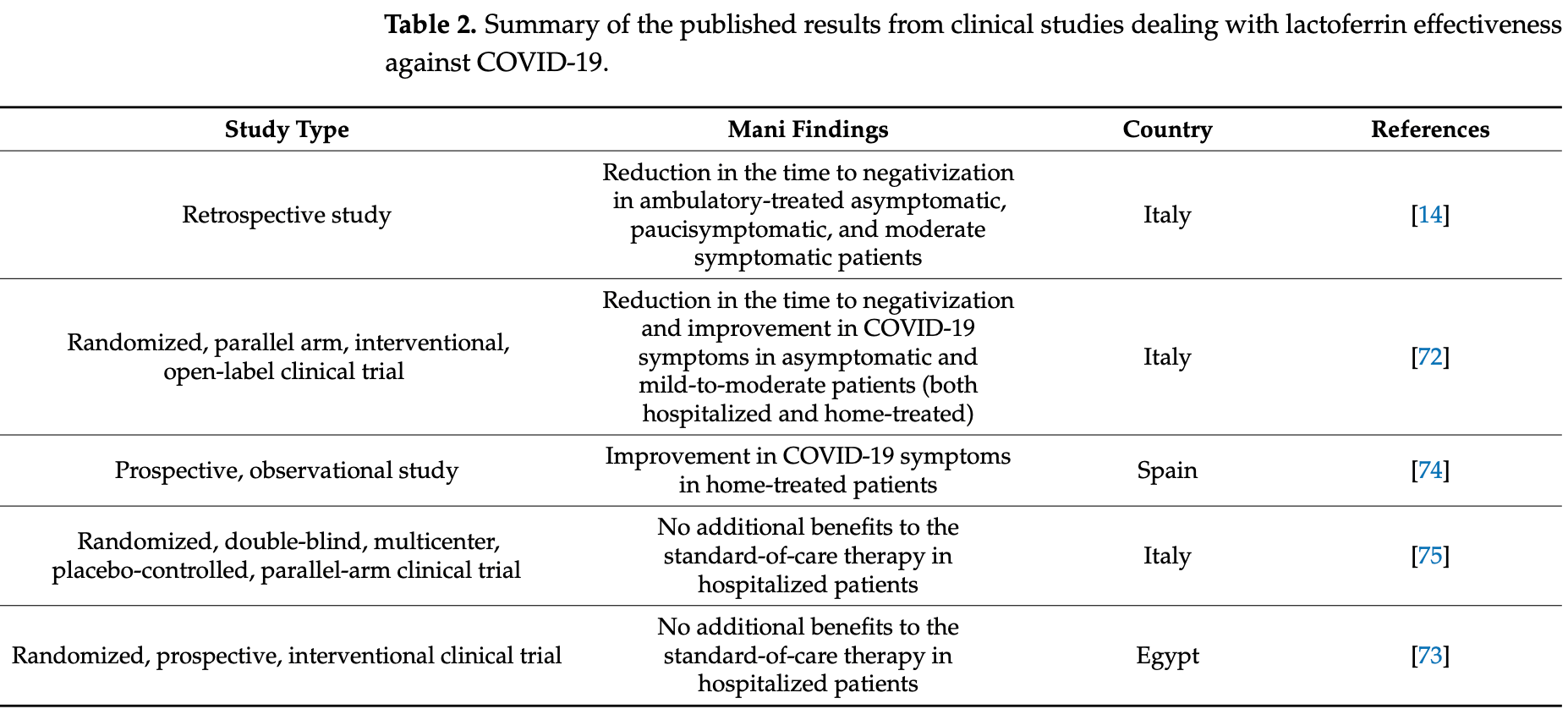
Review of lactoferrin supplementation for prevention and treatment of SARS-CoV-2. Authors summarize in vitro evidence showing lactoferrin can inhibit viral entry and replication through multiple mechanisms. Clinical trials in adults have shown mixed results, with some finding reduced time to viral clearance while others found no benefit when lactoferrin was added to standard of care treatment in hospitalized patients.
1.
Palacios-Rosas et al., COVID-19 and Lactoferrin: A Systematic Review and Meta-Analysis, COVID, doi:10.3390/covid5100176.
2.
Eker et al., The potential of lactoferrin as antiviral and immune-modulating agent in viral infectious diseases, Frontiers in Immunology, doi:10.3389/fimmu.2024.1402135.
3.
Manzoni et al., Lactoferrin Supplementation in Preventing and Protecting from SARS-CoV-2 Infection: Is There Any Role in General and Special Populations? An Updated Review of Literature, International Journal of Molecular Sciences, doi:10.3390/ijms251910248.
4.
Rosa et al., An overview on in vitro and in vivo antiviral activity of lactoferrin: its efficacy against SARS-CoV-2 infection, BioMetals, doi:10.1007/s10534-022-00427-z.
5.
Mattar et al., Natural resources to control COVID-19: could lactoferrin amend SARS-CoV-2 infectivity?, PeerJ, doi:10.7717/peerj.11303.
Manzoni et al., 24 Sep 2024, peer-reviewed, 8 authors.
Contact: pierpaolo.sainaghi@med.uniupo.it (corresponding author), bianca.masturzo@aslbi.piemonte.it, alessandro.messina@hotmail.it.
Lactoferrin Supplementation in Preventing and Protecting from SARS-CoV-2 Infection: Is There Any Role in General and Special Populations? An Updated Review of Literature
International Journal of Molecular Sciences, doi:10.3390/ijms251910248
At the beginning of the pandemic, SARS-CoV-2 infection represented a great medical burden worldwide, as targeted and effective therapeutic options were lacking. This resulted in the revival of existing molecules and the increasing popularity of over-the-counter nutritional supplements. Among the latter, lactoferrin has been investigated as an adjuvant in COVID-19 therapy with conflicting results, mainly depending on different study designs. Considering that lactoferrin is one of the main components of human breast milk with anti-microbial and anti-inflammatory activity, it is conceivable that such bioactive molecule could be effective in supporting anti-SARS-CoV-2 infection therapy, especially in infants and pregnant women, two subpopulations that have been poorly evaluated in the existing clinical trials. This narrative review is intended to offer insight into the existing literature on lactoferrin's biological functions and protective effects against COVID-19, with a special focus on pregnant women and their infants.
Author Contributions: All authors contributed to the literature review and manuscript drafting. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
Abu-Raya, Michalski, Sadarangani, Lavoie, Maternal immunological adaptation during normal pregnancy, Front. Immunol, doi:10.3389/fimmu.2020.575197
Actor, Hwang, Kruzel, Lactoferrin as a natural immune modulator, Curr. Pharm. Des, doi:10.2174/138161209788453202
Akin, Atasay, Dogu, Okulu, Arsan et al., Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells, Am. J. Perinatol, doi:10.1055/s-0034-1371704
Akiyama, Oshima, Kuhara, Shin, Abe et al., A lactoferrin-receptor, intelectin 1, affects uptake, subcellular localization and release of immunochemically detectable lactoferrin by intestinal epithelial Caco-2 cells, J. Biochem, doi:10.1093/jb/mvt073
Algahtani, Elabbasy, Samak, Adeboye, Yusuf et al., The prospect of lactoferrin use as adjunctive agent in management of SARS-CoV-2 patients: A randomized pilot study, Medicina, doi:10.3390/medicina57080842
Ali, Hasan, Kow, Merchant, Lactoferrin reduces the risk of respiratory tract infections: A meta-analysis of randomized controlled trials, Clin. Nutr. ESPEN, doi:10.1016/j.clnesp.2021.08.019
Ansems, Grundeis, Dahms, Mikolajewska, Thieme et al., Remdesivir for the treatment of COVID-19, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD014962
Artym, Zimecki, Antimicrobial and prebiotic activity of lactoferrin in the female reproductive tract: A comprehensive review, Biomedicines, doi:10.3390/biomedicines9121940
Artym, Zimecki, Kruzel, Lactoferrin for prevention and treatment of anemia and inflammation in pregnant women: A comprehensive review, Biomedicines, doi:10.3390/biomedicines9080898
Ashida, Sasaki, Suzuki, Lönnerdal, Cellular internalization of lactoferrin in intestinal epithelial cells, Biometals, doi:10.1023/B:BIOM.0000027710.13543.3f
Ashraf, Zubair, Bashir, Alagawany, Ahmed et al., Nutraceutical and health-promoting potential of lactoferrin, an iron-binding protein in human and animal: Current knowledge, Biol. Trace Elem. Res, doi:10.1007/s12011-023-03658-4
Badr, Mattern, Carlin, Cordier, Maillart et al., Are clinical outcomes worse for pregnant women at ≥20 weeks' gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching, Am. J. Obstet. Gynecol, doi:10.1016/j.ajog.2020.07.045
Barnes, How corticosteroids control inflammation: Quintiles Prize Lecture, Br. J. Pharmacol, doi:10.1038/sj.bjp.0706736
Berlutti, Pantanella, Natalizi, Frioni, Paesano et al., Antiviral properties of lactoferrin-A natural immunity molecule, Molecules, doi:10.3390/molecules16086992
Berthon, Williams, Williams, Wood, Effect of lactoferrin supplementation on inflammation, immune function, and prevention of respiratory tract infections in humans: A systematic review and meta-analysis, Adv. Nutr, doi:10.1093/advances/nmac047
Bolat, Eker, Kaplan, Duman, Arslan et al., Lactoferrin for COVID-19 prevention, treatment, and recovery, Front. Nutr, doi:10.3389/fnut.2022.992733
Brady, Gurijala, Huang, Hussain, Lingan et al., A guide to COVID-19 antiviral therapeutics: A summary and perspective of the antiviral weapons against SARS-CoV-2 infection, FEBS J, doi:10.1111/febs.16662
Briana, Papadopoulou, Syridou, Marchisio, Kapsabeli et al., Early human milk lactoferrin during SARS-CoV-2 infection, J. Matern.-Fetal Neonatal Med, doi:10.1080/14767058.2021.1920010
Burki, WHO ends the COVID-19 public health emergency, Lancet Respir. Med, doi:10.1016/S2213-2600(23)00217-5
Cairns, Dulko, Griffiths, Golan, Cohen et al., Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: A phase 2 randomized clinical trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.44942
Campione, Lanna, Cosio, Rosa, Conte et al., Lactoferrin against SARS-CoV-2: In vitro and in silico evidence, Front. Pharmacol, doi:10.3389/fphar.2021.666600
Campione, Lanna, Cosio, Rosa, Conte et al., Lactoferrin as antiviral treatment in COVID-19 management: Preliminary evidence, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph182010985
Cao, Ren, Lu, Wang, Wu et al., Lactoferrin: A glycoprotein that plays an active role in human health, Front. Nutr, doi:10.3389/fnut.2022.1018336
Caputo, Libera, Sisti, Giuliani, Diotti et al., The initial interplay between HIV and mucosal innate immunity, Front. Immunol, doi:10.3389/fimmu.2023.1104423
Carrillo-Lozano, Sebastián-Valles, Knott-Torcal, Circulating microRNAs in breast milk and their potential impact on the infant, Nutrients, doi:10.3390/nu12103066
Chambers, Krogstad, Bertrand, Contreras, Tobin et al., Evaluation for SARS-CoV-2 in breast milk from 18 infected women, JAMA, doi:10.1001/jama.2020.15580
Chang, Ng, Sun, Lactoferrin as potential preventative and adjunct treatment for COVID-19, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106118
Conesa, Bellés, Grasa, Sánchez, The role of lactoferrin in intestinal health, Pharmaceutics, doi:10.3390/pharmaceutics15061569
Corradini, Ventura, Ageno, Cogliati, Muiesan et al., Clinical factors associated with death in 3044 COVID-19 patients managed in internal medicine wards in Italy: Results from the SIMI-COVID-19 study of the Italian Society of Internal Medicine (SIMI), Intern Emerg. Med, doi:10.1007/s11739-021-02742-8
Cutone, Ianiro, Lepanto, Rosa, Valenti et al., Lactoferrin in the prevention and treatment of intestinal inflammatory pathologies associated with colorectal cancer development, Cancers, doi:10.3390/cancers12123806
Cutone, Rosa, Bonaccorsi Di Patti, Iacovelli, Conte et al., Lactoferrin binding to SARS-CoV-2 spike glycoprotein blocks pseudoviral entry and relieves iron protein dysregulation in several in vitro models, Pharmaceutics, doi:10.3390/pharmaceutics14102111
Czosnykowska-Łukacka, Orczyk-Pawiłowicz, Broers, Królak-Olejnik, Lactoferrin in human milk of prolonged lactation, Nutrients, doi:10.3390/nu11102350
De Luca, Vauloup-Fellous, Benachi, Vivanti, Transmission of SARS-CoV-2 from mother to fetus or neonate: What to know and what to do?, Semin. Fetal Neonatal Med, doi:10.1016/j.siny.2023.101429
Di Girolamo, Khalil, Alameddine, D'angelo, Galliani et al., Placental histopathology after SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis, Am. J. Obstet. Gynecol. MFM, doi:10.1016/j.ajogmf.2021.100468
Dix, Wright, Bioavailability of a novel form of microencapsulated bovine lactoferrin and its effect on inflammatory markers and the gut microbiome: A pilot study, Nutrients, doi:10.3390/nu10081115
Drago-Serrano, Campos-Rodríguez, Carrero, De La, Garza, Lactoferrin: Balancing ups and downs of inflammation due to microbial infections, Int. J. Mol. Sci, doi:10.3390/ijms18030501
Einerhand, Van Loo-Bouwman, Weiss, Wang, Ba et al., Can lactoferrin, a natural mammalian milk protein, assist in the battle against COVID-19?, Nutrients, doi:10.3390/nu14245274
Galderisi, Lista, Cavigioli, Trevisanuto, Clinical features of neonatal COVID-19, Semin. Fetal Neonatal Med, doi:10.1016/j.siny.2023.101430
García-Montoya, Cendón, Arévalo-Gallegos, Rascón-Cruz, Lactoferrin a multiple bioactive protein: An overview, Biochim. Biophys. Acta (BBA) Gen. Subj, doi:10.1016/j.bbagen.2011.06.018
Gawel, Krolak-Olejnik, Lactoferrin supplementation during pregnancy-A review of the literature and current recommendations, Ginekol. Pol, doi:10.5603/GP.a2023.0020
Gaweł, Łukianowski, Kościelska-Kasprzak, Bartoszek, Krajewska et al., Colostrum lactoferrin following active and recovered SARS-CoV-2 infections during pregnancy, Biomedicines, doi:10.3390/biomedicines12051120
Giansanti, Panella, Leboffe, Antonini, Lactoferrin from milk: Nutraceutical and pharmacological properties, Pharmaceuticals, doi:10.3390/ph9040061
Giunta, Giuffrida, Mangano, Fagone, Cianci, Influence of lactoferrin in preventing preterm delivery: A pilot study, Mol. Med. Rep, doi:10.3892/mmr.2011.584
Golan, Ilala, Li, Gay, Hunagund et al., Milk antibody response after 3rd COVID-19 vaccine and SARS-CoV-2 infection and implications for infant protection, iScience, doi:10.1016/j.isci.2023.107767
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe COVID-19 in outpatients, N. Engl. J. Med, doi:10.1056/NEJMoa2116846
Gruden, Poklar Ulrih, Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrinderived peptides, Int. J. Mol. Sci, doi:10.3390/ijms222011264
Gulbis, Jauniaux, Decuyper, Thiry, Jurkovic et al., Distribution of iron and iron-binding proteins in first-trimester human pregnancies, Obstet. Gynecol
Habib, Ibrahim, Zaim, Ibrahim, The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.111228
Hatmal, Al-Hatamleh, Olaimat, Alshaer, Hasan et al., Immunomodulatory properties of human breast milk: microRNA contents and potential epigenetic effects, Biomedicines, doi:10.3390/biomedicines10061219
He, Liu, Hu, Li, Wu et al., Breastfeeding vs. breast milk transmission during COVID-19 pandemic, which is more important? Front, Pediatr, doi:10.3389/fped.2023.1253333
Heller, Greig, Heine, Amniotic-fluid lactoferrin: A marker for subclinical intraamniotic infection prior to 32 weeks gestation, Infect. Dis. Obstet. Gynecol, doi:10.1155/S1064744995000573
Hu, Meng, Zhang, Xiang, Wang, The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor, Emerg. Microbes Infect, doi:10.1080/22221751.2021.1888660
Ishikado, Imanaka, Kotani, Fujita, Mitsuishi et al., Liposomal lactoferrin induced significant increase of the interferon-alpha (IFN-α) producibility in healthy volunteers, BioFactors, doi:10.1002/biof.552210113
Jiang, Lopez, Kelleher, Lönnerdal, Apo-and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells, J. Cell Physiol, doi:10.1002/jcp.22650
Kaur, Gathwala, Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neonates: A randomized placebo-controlled clinical trial, J. Trop. Pediatr, doi:10.1093/tropej/fmv044
Kell, Heyden, Pretorius, The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria, Front. Immunol, doi:10.3389/fimmu.2020.01221
King, Cummings, Guo, Trivedi, Readmond et al., A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants, J. Pediatr. Gastroenterol. Nutr, doi:10.1097/01.mpg.0000243435.54958.68
Kourtis, Read, Jamieson, Pregnancy and infection, N. Engl. J. Med, doi:10.1056/NEJMra1213566
Kowalczyk, Kaczy Ńska, Kleczkowska, Bukowska-Ośko, Kramkowski et al., The lactoferrin phenomenon-A miracle molecule, Molecules, doi:10.3390/molecules27092941
Kruzel, Actor, Zimecki, Wise, Płoszaj et al., Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression, J. Biotechnol, doi:10.1016/j.jbiotec.2013.09.011
Laguila Altoé, Marques Mambriz, Cardozo, Valentini Zacarias, Laguila et al., Vaccine protection through placenta and breastfeeding: The unmet topic in COVID-19 pandemic, Front. Immunol, doi:10.3389/fimmu.2022.910138
Lai, Yu, Xian, Ye, Ju et al., Identified human breast milk compositions effectively inhibit SARS-CoV-2 and variants infection and replication, iScience
Lamers, Haagmans, SARS-CoV-2 pathogenesis, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00713-0
Lang, Yang, Deng, Liu, Yang et al., Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans, PLoS ONE, doi:10.1371/journal.pone.0023710
Latorre, Berlutti, Valenti, Gessani, Puddu, LF immunomodulatory strategies: Mastering bacterial endotoxin, Biochem. Cell Biol, doi:10.1139/o11-059
Legrand, Elass, Carpentier, Mazurier, Lactoferrin: A modulator of immune and inflammatory responses, Cell. Mol. Life Sci, doi:10.1007/s00018-005-5370-2
Legrand, Overview of lactoferrin as a natural immune modulator, J. Ped, doi:10.1016/j.jpeds.2016.02.071
Lepanto, Rosa, Cutone, Conte, Paesano et al., Efficacy of lactoferrin oral administration in the treatment of anemia and anemia of inflammation in pregnant and non-pregnant women: An interventional study, Front. Immunol, doi:10.3389/fimmu.2018.02123
Lepanto, Rosa, Paesano, Valenti, Cutone, Lactoferrin in aseptic and septic inflammation, Molecules, doi:10.3390/molecules24071323
Li, Liu, Lin, Xue, Lu et al., The application of lactoferrin in infant formula: The past, present and future, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2022.2157792
Li, Zhang, Liu, Zheng, Han et al., The effect of lactoferrin in aging: Role and potential, Food Funct, doi:10.1039/D1FO02750F
Liu, Chen, Featured prebiotic agent: The roles and mechanisms of direct and indirect prebiotic activities of lactoferrin and its application in disease control, Nutrients, doi:10.3390/nu15122759
Liu, Feng, Zhang, Hu, Sun et al., The functional role of lactoferrin in intestine mucosal immune system and inflammatory bowel disease, Front. Nutr, doi:10.3389/fnut.2021.759507
Lu, Francis, Doster, Haley, Craft et al., Lactoferrin: A critical mediator of both host immune response and antimicrobial activity in response to streptococcal infections, ACS Infect. Dis, doi:10.1021/acsinfecdis.0c00050
Lönnerdal, Bioactive proteins in human milk: Health, nutrition, and implications for infant formulas, J. Ped, doi:10.1016/j.jpeds.2016.02.070
Lönnerdal, Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas, Am. J. Clin. Nutr, doi:10.3945/ajcn.113.071993
Maneva, Taleva, Maneva, Lactoferrin-protector against oxidative stress and regulator of glycolysis in human erythrocytes, Z. Naturforschung C, doi:10.1515/znc-2003-3-420
Manzoni, Meyer, Stolfi, Rinaldi, Cattani et al., Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial, Early Hum. Dev, doi:10.1016/S0378-3782(14)70020-9
Manzoni, Rinaldi, Cattani, Pugni, Romeo et al., Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates. A randomized trial, JAMA, doi:10.1001/jama.2009.1403
Martins-Filho, Santos, Santos, To breastfeed or not to breastfeed? Lack of evidence on the presence of SARS-CoV-2 in breastmilk of pregnant women with COVID-19, Rev. Panamer. Salud Públ, doi:10.26633/RPSP.2020.59
Matino, Tavella, Rizzi, Avanzi, Azzolina et al., Effect of lactoferrin on clinical outcomes of hospitalized patients with COVID-19: The LAC randomized clinical trial, Nutrients, doi:10.3390/nu15051285
Mayeur, Spahis, Pouliot, Levy, Lactoferrin, a pleiotropic protein in health and disease, Antioxid. Redox Signal, doi:10.1089/ars.2015.6458
Miotto, Di Rienzo, Bò, Boffi, Ruocco et al., Molecular mechanisms behind anti SARS-CoV-2 action of lactoferrin, Front. Mol. Biosci, doi:10.3389/fmolb.2021.607443
Mirabelli, Wotring, Zhang, Mccarty, Fursmidt et al., Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2105815118
Mirbeyk, Saghazadeh, Rezaei, A systematic review of pregnant women with COVID-19 and their neonates, Arch. Gynecol. Obstet, doi:10.1007/s00404-021-06049-z
Moza, Duica, Antoniadis, Bernad, Lungeanu et al., Outcome of newborns with confirmed or possible SARS-CoV-2 vertical infection-A scoping review, Diagnostics, doi:10.3390/diagnostics13020245
Mrityunjaya, Pavithra, Neelam, Janhavi, Halami et al., Immune-boosting, antioxidant and antiinflammatory food supplements targeting pathogenesis of COVID-19, Front. Immunol, doi:10.3389/fimmu.2020.570122
Naidu, Clemens, Pressman, Zaigham, Davies et al., COVID-19 during pregnancy and postpartum, J. Diet. Suppl, doi:10.1080/19390211.2020.1834047
Nobrega, Stoll, Casarini, Bertagnolli, Role of ACE2 in pregnancy and potential implications for COVID-19 susceptibility, Clin. Sci, doi:10.1042/CS20210284
Ochoa, Chea-Woo, Campos, Pecho, Prada et al., Impact of lactoferrin supplementation on growth and prevalence of Giardia colonization in children, Clin. Infect. Dis, doi:10.1086/588476
Ochoa, Loli, Mendoza, Carcamo, Bellomo et al., Effect of bovine lactoferrin on prevention of late-onset sepsis in infants <1500 g: A pooled analysis of individual patient data from two randomized controlled trials, Biochem. Cell Biol, doi:10.1139/bcb-2020-0046
Oda, Wakabayashi, Tanaka, Yamauchi, Sugita et al., Effects of lactoferrin on infectious diseases in Japanese summer: A randomized, double-blinded, placebo-controlled trial, J. Microbiol. Immunol. Infect, doi:10.1016/j.jmii.2020.02.010
Omar, Assem, Ahmed, Abd Elmaksoud, Lactoferrin versus iron hydroxide polymaltose complex for the treatment of iron deficiency anemia in children with cerebral palsy: A randomized controlled trial, Eur. J. Pediatr, doi:10.1007/s00431-021-04125-9
Oshima, Seki, Shibuya, Naka, Yokoyama et al., Soluble human intestinal lactoferrin receptor: Ca 2+ -dependent binding to sepharose-based matrices, Biol. Pharm. Bull, doi:10.1248/bpb.b15-00643
Otsuki, Nishi, Kondo, Okubo, Review, role of lactoferrin in preventing preterm delivery, Biometals, doi:10.1007/s10534-022-00471-9
Otsuki, Yoda, Saito, Mitsuhashi, Toma et al., Amniotic fluid lactoferrin in intrauterine infection, Placenta, doi:10.1053/plac.1998.0368
Piacentini, Centi, Miotto, Milanetti, Di Rienzo et al., Lactoferrin inhibition of the complex formation between ACE2 receptor and SARS CoV-2 recognition binding domain, Int. J. Mol. Sci, doi:10.3390/ijms23105436
Pino, Giunta, Randazzo, Caruso, Caggia et al., Bacterial biota of women with bacterial vaginosis treated with lactoferrin: An open prospective randomized trial, Microb. Ecol. Health Dis, doi:10.1080/16512235.2017.1357417
Pomorski, Trzeszcz, Matera-Witkiewicz, Krupi Ńska, Fuchs et al., The immunological role of the placenta in SARS-CoV-2 infection-viral transmission, immune regulation, and lactoferrin activity, Int. J. Mol. Sci, doi:10.3390/ijms22115799
Presti, Manti, Parisi, Papale, Barbagallo et al., Lactoferrin: Cytokine modulation and application in clinical practice, J. Clin. Med, doi:10.3390/jcm10235482
Puddu, Valenti, Gessani, Immunomodulatory effects of lactoferrin on antigen presenting cells, Biochimie, doi:10.1016/j.biochi.2008.05.005
Raic, Adelman, Zhuang, Rai, Boettcher et al., Longitudinal changes in lactoferrin concentrations in human milk: A global systematic review, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2011.642422
Rascón-Cruz, Espinoza-Sánchez, Siqueiros-Cendón, Nakamura-Bencomo, Arévalo-Gallegos et al., Lactoferrin: A glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes, Molecules, doi:10.3390/molecules26010205
Rebutini, Zanchettin, Stonoga, Prá, De Oliveira et al., Association between COVID-19 pregnant women symptoms severity and placental morphologic features, Front. Immunol, doi:10.3389/fimmu.2021.685919
Redwan, Uversky, El-Fakharany, Al-Mehdar, Potential lactoferrin activity against pathogenic viruses, Comptes Rendus Biol, doi:10.1016/j.crvi.2014.08.003
Ricordi, Pacifici, Lanzoni, Palamara, Garaci et al., Dietary and protective factors to halt or mitigate progression of autoimmunity, COVID-19 and its associated metabolic diseases, Int. J. Mol. Sci, doi:10.3390/ijms22063134
Rizzi, Patrucco, Trevisan, Faolotto, Mercandino et al., Baseline plasma SARS-CoV-2 RNA detection predicts an adverse COVID-19 evolution in moderate to severe hospitalized patients, Panminerva Med, doi:10.23736/S0031-0808.22.04705-X
Rosa, Cutone, Conte, Campione, Bianchi et al., An overview on in vitro and in vivo antiviral activity of lactoferrin: Its efficacy against SARS-CoV-2 infection, Biometals, doi:10.1007/s10534-022-00427-z
Rosa, Lepanto, Cutone, Siciliano, Paesano et al., Influence of oral administration mode on the efficacy of commercial bovine lactoferrin against iron and inflammatory homeostasis disorders, Biometals, doi:10.1007/s10534-020-00236-2
Rosa, Tripepi, Naldi, Aimati, Santangeli et al., Ambulatory COVID-19 patients treated with lactoferrin as a supplementary antiviral agent: A preliminary study, J. Clin. Med, doi:10.3390/jcm10184276
Ryan, Plötz, Hoogen, Latour, Degtyareva et al., Neonates and COVID-19: State of the art: Neonatal sepsis series, Pediatr. Res, doi:10.1038/s41390-021-01875-y
Salaris, Scarpa, Elli, Bertolini, Guglielmetti et al., Protective effects of lactoferrin against SARS-CoV-2 infection in vitro, Nutrients, doi:10.3390/nu13020328
Sankar, Sinha, Chowdhury, Bhandari, Taneja et al., Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis, Acta Paediatr, doi:10.1111/apa.13147
Santacroce, Palmirotta, Bottalico, Charitos, Colella et al., Crosstalk between the resident microbiota and the immune cells regulates female genital tract health, Life, doi:10.3390/life13071531
Serrano, Kochergina, Albors, Diaz, Oroval et al., Liposomal lactoferrin as potential preventative and cure for COVID-19, Int. J. Res. Health Sci, doi:10.5530/ijrhs.8.1.3
Shanes, Mithal, Otero, Azad, Miller et al., Placental pathology in COVID-19, Am. J. Clin. Pathol, doi:10.1093/ajcp/aqaa089
Sharma, Ramya, Saccharide binding by intelectins, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2017.11.007
Shini, Udayarajan, Nisha, A comprehensive review on lactoferrin: A natural multifunctional glycoprotein, Food Funct, doi:10.1039/D2FO02371G
Sinopoli, Isonne, Santoro, Baccolini, The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review, Rev. Med. Virol, doi:10.1002/rmv.2261
Siqueiros-Cendón, Arévalo-Gallegos, Iglesias-Figueroa, García-Montoya, Salazar-Martínez et al., Immunomodulatory effects of lactoferrin, Acta Pharmacol. Sin, doi:10.1038/aps.2013.200
Spatz, Davanzo, Müller, Powell, Rigourd et al., Promoting and protecting human milk and breastfeeding in a COVID-19 world, Front. Pediatr, doi:10.3389/fped.2020.633700
Suzuki, Lopez, Lönnerdal, Mammalian lactoferrin receptors: Structure and function, Cell Mol. Life Sci, doi:10.1007/s00018-005-5371-1
Takeuchi, Kitagawa, Harada, Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats, Exp. Physiol, doi:10.1113/expphysiol.2003.026633
Tarnow-Mordi, Abdel-Latif, Martin, Pammi, Robledo et al., The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): A multicentre, double-blind, randomised controlled trial, Lancet Child Adolesc. Health, doi:10.1016/S2352-4642(20)30093-6
Triggle, Bansal, Ding, Islam, Farag et al., A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic, Front. Immunol, doi:10.3389/fimmu.2021.631139
Valenti, Antonini, Lactoferrin: An important host defence against microbial and viral attack, Cell Mol. Life Sci, doi:10.1007/s00018-005-5372-0
Valenti, Rosa, Capobianco, Lepanto, Schiavi et al., Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense, Front. Immunol, doi:10.3389/fimmu.2018.00376
Van Der Strate, Beljaars, Molema, Harmsen, Meijer, Antiviral activities of lactoferrin, Antivir. Res, doi:10.1016/S0166-3542(01)00195-4
Vassilopoulou, Feketea, Koumbi, Mesiari, Berghea et al., Breastfeeding and COVID-19: From nutrition to immunity, Front. Immunol, doi:10.3389/fimmu.2021.661806
Vega-Bautista, De La Garza, Carrero, Campos-Rodríguez, Godínez-Victoria et al., The impact of lactoferrin on the growth of intestinal inhabitant bacteria, Int. J. Mol. Sci, doi:10.3390/ijms20194707
Wai, Wood, Hornaday, Slater, Potential molecular and cellular mechanisms for adverse placental outcomes in pregnancies complicated by SARS-CoV-2 infection-A scoping review, PLoS ONE, doi:10.1371/journal.pone.0283453
Wakabayashi, Oda, Yamauchi, Abe, Lactoferrin for prevention of common viral infections, J. Infect. Chemother, doi:10.1016/j.jiac.2014.08.003
Walker, Breast milk as the gold standard for protective nutrients, J. Ped, doi:10.1016/j.jpeds.2009.11.021
Wang, Gu, Lewis, Gu, Brown et al., Cell-type specific distribution and activation of type I IFN pathway molecules at the placental maternal-fetal interface in response to COVID-19 infection, Front. Endocrinol, doi:10.3389/fendo.2022.951388
Wang, Timilsena, Blanch, Adhikari, Lactoferrin: Structure, function, denaturation and digestion, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2017.1381583
Wesołowska, Orczyk-Pawiłowicz, Bzikowska-Jura, Gawro Ńska, Walczak, Protecting breastfeeding during the COVID-19 pandemic: A scoping review of perinatal care recommendations in the context of maternal and child well-being, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph19063347
Williams, Clinical pharmacology of corticosteroids, Respir. Care, doi:10.4187/respcare.06314
Wong, Tan, Khong, SARS-CoV-2 transplacental transmission: A rare occurrence? An overview of the protective role of the placenta, Int. J. Mol. Sci, doi:10.3390/ijms24054550
Woodman, Strunk, Patole, Hartmann, Simmer et al., Effects of lactoferrin on neonatal pathogens and bifidobacterium breve in human breast milk, PLoS ONE, doi:10.1371/journal.pone.0201819
Wotring, Fursmidt, Ward, Sexton, Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern, J. Dairy Sci, doi:10.3168/jds.2021-21247
Wrackmeyer, Hansen, Seya, Danielsen, Intelectin: A novel lipid raft-associated protein in the enterocyte brush border, Biochemistry, doi:10.1021/bi060570x
Zambrano, Ellington, Strid, Galang, Oduyebo et al., Update: Characteristics of symptomatic women of reproductive age with laboratoryconfirmed SARS-CoV-2 infection by pregnancy status-United States, January 22-October 3, MMWR Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm6944e3
Zarzosa-Moreno, Avalos-Gómez, Ramírez-Texcalco, Torres-López, Ramírez-Mondragón et al., Lactoferrin and its derived peptides: An alternative for combating virulence mechanisms developed by pathogens, Molecules, doi:10.3390/molecules25245763
Zhao, Zhang, Xu, Luo, Luo et al., Comparative effects between oral lactoferrin and ferrous sulfate supplementation on iron-deficiency anemia: A comprehensive review and meta-analysis of clinical trials, Nutrients, doi:10.3390/nu14030543
DOI record:
{
"DOI": "10.3390/ijms251910248",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms251910248",
"abstract": "<jats:p>At the beginning of the pandemic, SARS-CoV-2 infection represented a great medical burden worldwide, as targeted and effective therapeutic options were lacking. This resulted in the revival of existing molecules and the increasing popularity of over-the-counter nutritional supplements. Among the latter, lactoferrin has been investigated as an adjuvant in COVID-19 therapy with conflicting results, mainly depending on different study designs. Considering that lactoferrin is one of the main components of human breast milk with anti-microbial and anti-inflammatory activity, it is conceivable that such bioactive molecule could be effective in supporting anti-SARS-CoV-2 infection therapy, especially in infants and pregnant women, two subpopulations that have been poorly evaluated in the existing clinical trials. This narrative review is intended to offer insight into the existing literature on lactoferrin’s biological functions and protective effects against COVID-19, with a special focus on pregnant women and their infants.</jats:p>",
"alternative-id": [
"ijms251910248"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1340-3493",
"affiliation": [
{
"name": "Department of Maternal, Neonatal and Infant Medicine, University Hospital “Degli Infermi”, 13875 Ponderano, Italy"
},
{
"name": "School of Medicine, University of Turin, 10124 Turin, Italy"
}
],
"authenticated-orcid": false,
"family": "Manzoni",
"given": "Paolo",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-6868-036X",
"affiliation": [
{
"name": "School of Medicine, University of Turin, 10124 Turin, Italy"
},
{
"name": "Sant’Anna Hospital, Department of Surgical Sciences, University of Turin, 10126 Turin, Italy"
}
],
"authenticated-orcid": false,
"family": "Messina",
"given": "Alessandro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6951-3803",
"affiliation": [
{
"name": "Department of Maternal, Neonatal and Infant Medicine, University Hospital “Degli Infermi”, 13875 Ponderano, Italy"
},
{
"name": "School of Medicine, University of Turin, 10124 Turin, Italy"
}
],
"authenticated-orcid": false,
"family": "Germano",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Neonatology and Neonatal Intensive Care Unit, Policlinico Casilino, 00169 Rome, Italy"
}
],
"family": "Picone",
"given": "Simonetta",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5243-0940",
"affiliation": [
{
"name": "Department of Maternal, Neonatal and Infant Medicine, University Hospital “Degli Infermi”, 13875 Ponderano, Italy"
},
{
"name": "School of Medicine, University of Turin, 10124 Turin, Italy"
}
],
"authenticated-orcid": false,
"family": "Masturzo",
"given": "Bianca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8322-9158",
"affiliation": [
{
"name": "Department of Translational Medicine (DiMeT), Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "IRCAD (Interdisciplinary Research Center of Autoimmune Diseases), Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Sainaghi",
"given": "Pier Paolo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7863-8192",
"affiliation": [
{
"name": "Department of Translational Medicine (DiMeT), Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Laboratory of Metabolic Research, IRCCS Istituto Auxologico Italiano, S. Giuseppe Hospital, 28824 Piancavallo, Italy"
}
],
"authenticated-orcid": false,
"family": "Sola",
"given": "Daniele",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6174-7111",
"affiliation": [
{
"name": "IRCAD (Interdisciplinary Research Center of Autoimmune Diseases), Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Health Sciences (DiSS), Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Rizzi",
"given": "Manuela",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Sciences",
"container-title-short": "IJMS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
9,
24
]
],
"date-time": "2024-09-24T12:54:17Z",
"timestamp": 1727182457000
},
"deposited": {
"date-parts": [
[
2024,
9,
24
]
],
"date-time": "2024-09-24T13:12:50Z",
"timestamp": 1727183570000
},
"indexed": {
"date-parts": [
[
2024,
9,
25
]
],
"date-time": "2024-09-25T04:28:58Z",
"timestamp": 1727238538899
},
"is-referenced-by-count": 0,
"issue": "19",
"issued": {
"date-parts": [
[
2024,
9,
24
]
]
},
"journal-issue": {
"issue": "19",
"published-online": {
"date-parts": [
[
2024,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
24
]
],
"date-time": "2024-09-24T00:00:00Z",
"timestamp": 1727136000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/25/19/10248/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "10248",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
9,
24
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
24
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3389/fnut.2022.992733",
"doi-asserted-by": "crossref",
"key": "ref_1",
"unstructured": "Bolat, E., Eker, F., Kaplan, M., Duman, H., Arslan, A., Saritaş, S., Şahutoğlu, A.S., and Karav, S. (2022). Lactoferrin for COVID-19 prevention, treatment, and recovery. Front. Nutr., 9."
},
{
"DOI": "10.1038/s41579-022-00713-0",
"article-title": "SARS-CoV-2 pathogenesis",
"author": "Lamers",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_2",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-1605740/v1",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Cutone, A., Rosa, L., Bonaccorsi Di Patti, M.C., Iacovelli, F., Conte, M.P., Ianiro, G., Romeo, A., Campione, E., Bianchi, L., and Valenti, P. (2022). Lactoferrin binding to SARS-CoV-2 spike glycoprotein blocks pseudoviral entry and relieves iron protein dysregulation in several in vitro models. Pharmaceutics, 14."
},
{
"DOI": "10.3389/fphar.2021.666600",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Campione, E., Lanna, C., Cosio, T., Rosa, L., Conte, M.P., Iacovelli, F., Romeo, A., Falconi, M., Del Vecchio, C., and Franchin, E. (2021). Lactoferrin against SARS-CoV-2: In vitro and in silico evidence. Front. Pharmacol., 12."
},
{
"DOI": "10.3389/fimmu.2021.631139",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Triggle, C.R., Bansal, D., Ding, H., Islam, M.M., Farag, E.A.B.A., Hadi, H.A., and Sultan, A.A. (2021). A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic. Front. Immunol., 12."
},
{
"DOI": "10.1007/s11739-021-02742-8",
"article-title": "Clinical factors associated with death in 3044 COVID-19 patients managed in internal medicine wards in Italy: Results from the SIMI-COVID-19 study of the Italian Society of Internal Medicine (SIMI)",
"author": "Corradini",
"doi-asserted-by": "crossref",
"first-page": "1005",
"journal-title": "Intern Emerg. Med.",
"key": "ref_6",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(23)00217-5",
"article-title": "WHO ends the COVID-19 public health emergency",
"author": "Burki",
"doi-asserted-by": "crossref",
"first-page": "588",
"journal-title": "Lancet Respir. Med.",
"key": "ref_7",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1111/febs.16662",
"article-title": "A guide to COVID-19 antiviral therapeutics: A summary and perspective of the antiviral weapons against SARS-CoV-2 infection",
"author": "Brady",
"doi-asserted-by": "crossref",
"first-page": "1632",
"journal-title": "FEBS J.",
"key": "ref_8",
"volume": "291",
"year": "2024"
},
{
"DOI": "10.1001/jamanetworkopen.2021.44942",
"article-title": "Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: A phase 2 randomized clinical trial",
"author": "Cairns",
"doi-asserted-by": "crossref",
"first-page": "e2144942",
"journal-title": "JAMA Netw. Open",
"key": "ref_9",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1073/pnas.2105815118",
"article-title": "Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19",
"author": "Mirabelli",
"doi-asserted-by": "crossref",
"first-page": "e2105815118",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_10",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.3390/ijms22063134",
"doi-asserted-by": "crossref",
"key": "ref_11",
"unstructured": "Ricordi, C., Pacifici, F., Lanzoni, G., Palamara, A.T., Garaci, E., and Della-Morte, D. (2021). Dietary and protective factors to halt or mitigate progression of autoimmunity, COVID-19 and its associated metabolic diseases. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.3389/fimmu.2020.570122",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Mrityunjaya, M., Pavithra, V., Neelam, R., Janhavi, P., Halami, P.M., and Ravindra, P.V. (2020). Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol., 11."
},
{
"DOI": "10.1093/advances/nmac047",
"article-title": "Effect of lactoferrin supplementation on inflammation, immune function, and prevention of respiratory tract infections in humans: A systematic review and meta-analysis",
"author": "Berthon",
"doi-asserted-by": "crossref",
"first-page": "1799",
"journal-title": "Adv. Nutr.",
"key": "ref_13",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/jcm10184276",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Rosa, L., Tripepi, G., Naldi, E., Aimati, M., Santangeli, S., Venditto, F., Caldarelli, M., and Valenti, P. (2021). Ambulatory COVID-19 patients treated with lactoferrin as a supplementary antiviral agent: A preliminary study. J. Clin. Med., 10."
},
{
"DOI": "10.1016/j.ijantimicag.2020.106118",
"article-title": "Lactoferrin as potential preventative and adjunct treatment for COVID-19",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "106118",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "ref_15",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1007/s12011-023-03658-4",
"article-title": "Nutraceutical and health-promoting potential of lactoferrin, an iron-binding protein in human and animal: Current knowledge",
"author": "Ashraf",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Biol. Trace Elem. Res.",
"key": "ref_16",
"volume": "202",
"year": "2024"
},
{
"DOI": "10.3390/pharmaceutics15061569",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Conesa, C., Bellés, A., Grasa, L., and Sánchez, L. (2023). The role of lactoferrin in intestinal health. Pharmaceutics, 15."
},
{
"DOI": "10.1139/bcb-2020-0046",
"article-title": "Effect of bovine lactoferrin on prevention of late-onset sepsis in infants <1500 g: A pooled analysis of individual patient data from two randomized controlled trials",
"author": "Ochoa",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Biochem. Cell Biol.",
"key": "ref_18",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.1016/S2352-4642(20)30093-6",
"article-title": "The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): A multicentre, double-blind, randomised controlled trial",
"author": "Martin",
"doi-asserted-by": "crossref",
"first-page": "444",
"journal-title": "Lancet Child Adolesc. Health",
"key": "ref_19",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1093/tropej/fmv044",
"article-title": "Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neonates: A randomized placebo-controlled clinical trial",
"author": "Kaur",
"doi-asserted-by": "crossref",
"first-page": "370",
"journal-title": "J. Trop. Pediatr.",
"key": "ref_20",
"volume": "61",
"year": "2015"
},
{
"DOI": "10.1016/S0378-3782(14)70020-9",
"article-title": "Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial",
"author": "Manzoni",
"doi-asserted-by": "crossref",
"first-page": "S60",
"journal-title": "Early Hum. Dev.",
"key": "ref_21",
"volume": "90",
"year": "2014"
},
{
"DOI": "10.1001/jama.2009.1403",
"article-title": "Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates. A randomized trial",
"author": "Manzoni",
"doi-asserted-by": "crossref",
"first-page": "1421",
"journal-title": "JAMA",
"key": "ref_22",
"volume": "302",
"year": "2009"
},
{
"DOI": "10.3389/fnut.2021.759507",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Liu, N., Feng, G., Zhang, X., Hu, Q., Sun, S., Sun, J., Sun, Y., Wang, R., Zhang, Y., and Wang, P. (2021). The functional role of lactoferrin in intestine mucosal immune system and inflammatory bowel disease. Front. Nutr., 8."
},
{
"DOI": "10.3390/jcm10235482",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Presti, S., Manti, S., Parisi, G.F., Papale, M., Barbagallo, I.A., Li Volti, G., and Leonardi, S. (2021). Lactoferrin: Cytokine modulation and application in clinical practice. J. Clin. Med., 10."
},
{
"DOI": "10.3390/cancers12123806",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Cutone, A., Ianiro, G., Lepanto, M.S., Rosa, L., Valenti, P., Bonaccorsi Di Patti, M.C., and Musci, G. (2020). Lactoferrin in the prevention and treatment of intestinal inflammatory pathologies associated with colorectal cancer development. Cancers, 12."
},
{
"DOI": "10.1016/j.clnesp.2021.08.019",
"article-title": "Lactoferrin reduces the risk of respiratory tract infections: A meta-analysis of randomized controlled trials",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "Clin. Nutr. ESPEN",
"key": "ref_26",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1016/j.jmii.2020.02.010",
"article-title": "Effects of lactoferrin on infectious diseases in Japanese summer: A randomized, double-blinded, placebo-controlled trial",
"author": "Oda",
"doi-asserted-by": "crossref",
"first-page": "566",
"journal-title": "J. Microbiol. Immunol. Infect.",
"key": "ref_27",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.3390/nu14030543",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Zhao, X., Zhang, X., Xu, T., Luo, J., Luo, Y., and An, P. (2022). Comparative effects between oral lactoferrin and ferrous sulfate supplementation on iron-deficiency anemia: A comprehensive review and meta-analysis of clinical trials. Nutrients, 14."
},
{
"DOI": "10.1007/s00431-021-04125-9",
"article-title": "Lactoferrin versus iron hydroxide polymaltose complex for the treatment of iron deficiency anemia in children with cerebral palsy: A randomized controlled trial",
"author": "Omar",
"doi-asserted-by": "crossref",
"first-page": "2609",
"journal-title": "Eur. J. Pediatr.",
"key": "ref_29",
"volume": "180",
"year": "2021"
},
{
"DOI": "10.3390/molecules24071323",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Lepanto, M.S., Rosa, L., Paesano, R., Valenti, P., and Cutone, A. (2019). Lactoferrin in aseptic and septic inflammation. Molecules, 24."
},
{
"DOI": "10.3389/fimmu.2018.02123",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Lepanto, M.S., Rosa, L., Cutone, A., Conte, M.P., Paesano, R., and Valenti, P. (2018). Efficacy of lactoferrin oral administration in the treatment of anemia and anemia of inflammation in pregnant and non-pregnant women: An interventional study. Front. Immunol., 9."
},
{
"DOI": "10.3389/fnut.2022.1018336",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Cao, X., Ren, Y., Lu, Q., Wang, K., Wu, Y., Wang, Y., Zhang, Y., Cui, X., Yang, Z., and Chen, Z. (2023). Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr., 9."
},
{
"DOI": "10.1016/j.bbagen.2011.06.018",
"article-title": "Lactoferrin a multiple bioactive protein: An overview",
"doi-asserted-by": "crossref",
"first-page": "226",
"journal-title": "Biochim. Biophys. Acta (BBA) Gen. Subj.",
"key": "ref_33",
"volume": "1820",
"year": "2012"
},
{
"DOI": "10.1038/aps.2013.200",
"article-title": "Immunomodulatory effects of lactoferrin",
"doi-asserted-by": "crossref",
"first-page": "557",
"journal-title": "Acta Pharmacol. Sin.",
"key": "ref_34",
"volume": "35",
"year": "2014"
},
{
"DOI": "10.1089/ars.2015.6458",
"article-title": "Lactoferrin, a pleiotropic protein in health and disease",
"author": "Mayeur",
"doi-asserted-by": "crossref",
"first-page": "813",
"journal-title": "Antioxid. Redox Signal.",
"key": "ref_35",
"volume": "24",
"year": "2016"
},
{
"DOI": "10.2174/138161209788453202",
"article-title": "Lactoferrin as a natural immune modulator",
"author": "Actor",
"doi-asserted-by": "crossref",
"first-page": "1956",
"journal-title": "Curr. Pharm. Des.",
"key": "ref_36",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.3390/molecules26010205",
"doi-asserted-by": "crossref",
"key": "ref_37",
"unstructured": "Rascón-Cruz, Q., Espinoza-Sánchez, E.A., Siqueiros-Cendón, T.S., Nakamura-Bencomo, S.I., Arévalo-Gallegos, S., and Iglesias-Figueroa, B.F. (2021). Lactoferrin: A glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes. Molecules, 26."
},
{
"DOI": "10.1007/s00018-005-5370-2",
"article-title": "Lactoferrin: A modulator of immune and inflammatory responses",
"author": "Legrand",
"doi-asserted-by": "crossref",
"first-page": "2549",
"journal-title": "Cell. Mol. Life Sci.",
"key": "ref_38",
"volume": "62",
"year": "2005"
},
{
"DOI": "10.3389/fimmu.2020.01221",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Kell, D.B., Heyden, E.L., and Pretorius, E. (2020). The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol., 11."
},
{
"DOI": "10.1021/acsinfecdis.0c00050",
"article-title": "Lactoferrin: A critical mediator of both host immune response and antimicrobial activity in response to streptococcal infections",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "1615",
"journal-title": "ACS Infect. Dis.",
"key": "ref_40",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.3390/molecules25245763",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Zarzosa-Moreno, D., Avalos-Gómez, C., Ramírez-Texcalco, L.S., Torres-López, E., Ramírez-Mondragón, R., Hernández-Ramírez, J.O., Serrano-Luna, J., and De La Garza, M. (2020). Lactoferrin and its derived peptides: An alternative for combating virulence mechanisms developed by pathogens. Molecules, 25."
},
{
"DOI": "10.1139/o11-059",
"article-title": "LF immunomodulatory strategies: Mastering bacterial endotoxin",
"author": "Latorre",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Biochem. Cell Biol.",
"key": "ref_42",
"volume": "90",
"year": "2012"
},
{
"DOI": "10.1016/j.jpeds.2016.02.071",
"article-title": "Overview of lactoferrin as a natural immune modulator",
"author": "Legrand",
"doi-asserted-by": "crossref",
"first-page": "S10",
"journal-title": "J. Ped.",
"key": "ref_43",
"volume": "173",
"year": "2016"
},
{
"DOI": "10.3390/biomedicines9080898",
"doi-asserted-by": "crossref",
"key": "ref_44",
"unstructured": "Artym, J., Zimecki, M., and Kruzel, M.L. (2021). Lactoferrin for prevention and treatment of anemia and inflammation in pregnant women: A comprehensive review. Biomedicines, 9."
},
{
"DOI": "10.1016/j.jbiotec.2013.09.011",
"article-title": "Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression",
"author": "Kruzel",
"doi-asserted-by": "crossref",
"first-page": "666",
"journal-title": "J. Biotechnol.",
"key": "ref_45",
"volume": "168",
"year": "2013"
},
{
"DOI": "10.3390/molecules16086992",
"article-title": "Antiviral properties of lactoferrin—A natural immunity molecule",
"author": "Berlutti",
"doi-asserted-by": "crossref",
"first-page": "6992",
"journal-title": "Molecules",
"key": "ref_46",
"volume": "16",
"year": "2011"
},
{
"DOI": "10.1515/znc-2003-3-420",
"article-title": "Lactoferrin-protector against oxidative stress and regulator of glycolysis in human erythrocytes",
"author": "Maneva",
"doi-asserted-by": "crossref",
"first-page": "256",
"journal-title": "Z. Naturforschung C",
"key": "ref_47",
"volume": "58",
"year": "2003"
},
{
"DOI": "10.1039/D2FO02371G",
"article-title": "A comprehensive review on lactoferrin: A natural multifunctional glycoprotein",
"author": "Shini",
"doi-asserted-by": "crossref",
"first-page": "11954",
"journal-title": "Food Funct.",
"key": "ref_48",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1039/D1FO02750F",
"article-title": "The effect of lactoferrin in aging: Role and potential",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "501",
"journal-title": "Food Funct.",
"key": "ref_49",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/ph9040061",
"doi-asserted-by": "crossref",
"key": "ref_50",
"unstructured": "Giansanti, F., Panella, G., Leboffe, L., and Antonini, G. (2016). Lactoferrin from milk: Nutraceutical and pharmacological properties. Pharmaceuticals, 9."
},
{
"DOI": "10.3389/fimmu.2023.1104423",
"doi-asserted-by": "crossref",
"key": "ref_51",
"unstructured": "Caputo, V., Libera, M., Sisti, S., Giuliani, B., Diotti, R.A., and Criscuolo, E. (2023). The initial interplay between HIV and mucosal innate immunity. Front. Immunol., 14."
},
{
"DOI": "10.1016/j.crvi.2014.08.003",
"article-title": "Potential lactoferrin activity against pathogenic viruses",
"author": "Redwan",
"doi-asserted-by": "crossref",
"first-page": "581",
"journal-title": "Comptes Rendus Biol.",
"key": "ref_52",
"volume": "337",
"year": "2014"
},
{
"DOI": "10.1371/journal.pone.0023710",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Lang, J., Yang, N., Deng, J., Liu, K., Yang, P., Zhang, G., and Jiang, C. (2011). Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE, 6."
},
{
"DOI": "10.1007/s00018-005-5372-0",
"article-title": "Lactoferrin: An important host defence against microbial and viral attack",
"author": "Valenti",
"doi-asserted-by": "crossref",
"first-page": "2576",
"journal-title": "Cell Mol. Life Sci.",
"key": "ref_54",
"volume": "62",
"year": "2005"
},
{
"DOI": "10.1016/S0166-3542(01)00195-4",
"article-title": "Antiviral activities of lactoferrin",
"author": "Beljaars",
"doi-asserted-by": "crossref",
"first-page": "225",
"journal-title": "Antivir. Res.",
"key": "ref_55",
"volume": "52",
"year": "2001"
},
{
"DOI": "10.1002/rmv.2261",
"article-title": "The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review",
"author": "Sinopoli",
"doi-asserted-by": "crossref",
"first-page": "e2261",
"journal-title": "Rev. Med. Virol.",
"key": "ref_56",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.3390/nu13020328",
"doi-asserted-by": "crossref",
"key": "ref_57",
"unstructured": "Salaris, C., Scarpa, M., Elli, M., Bertolini, A., Guglielmetti, S., Pregliasco, F., Blandizzi, C., Brun, P., and Castagliuolo, I. (2021). Protective effects of lactoferrin against SARS-CoV-2 infection in vitro. Nutrients, 13."
},
{
"DOI": "10.3390/ijms18030501",
"doi-asserted-by": "crossref",
"key": "ref_58",
"unstructured": "Drago-Serrano, M., Campos-Rodríguez, R., Carrero, J., and De La Garza, M. (2017). Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci., 18."
},
{
"DOI": "10.1016/j.jiac.2014.08.003",
"article-title": "Lactoferrin for prevention of common viral infections",
"author": "Wakabayashi",
"doi-asserted-by": "crossref",
"first-page": "666",
"journal-title": "J. Infect. Chemother.",
"key": "ref_59",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1016/j.biochi.2008.05.005",
"article-title": "Immunomodulatory effects of lactoferrin on antigen presenting cells",
"author": "Puddu",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "Biochimie",
"key": "ref_60",
"volume": "91",
"year": "2009"
},
{
"DOI": "10.3390/nu15122759",
"doi-asserted-by": "crossref",
"key": "ref_61",
"unstructured": "Liu, Z.S., and Chen, P.W. (2023). Featured prebiotic agent: The roles and mechanisms of direct and indirect prebiotic activities of lactoferrin and its application in disease control. Nutrients, 15."
},
{
"DOI": "10.3389/fimmu.2018.00376",
"doi-asserted-by": "crossref",
"key": "ref_62",
"unstructured": "Valenti, P., Rosa, L., Capobianco, D., Lepanto, M.S., Schiavi, E., Cutone, A., Paesano, R., and Mastromarino, P. (2018). Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front. Immunol., 9."
},
{
"DOI": "10.3390/life13071531",
"doi-asserted-by": "crossref",
"key": "ref_63",
"unstructured": "Santacroce, L., Palmirotta, R., Bottalico, L., Charitos, I.A., Colella, M., Topi, S., and Jirillo, E. (2023). Crosstalk between the resident microbiota and the immune cells regulates female genital tract health. Life, 13."
},
{
"DOI": "10.3390/ijms20194707",
"doi-asserted-by": "crossref",
"key": "ref_64",
"unstructured": "Vega-Bautista, A., de la Garza, M., Carrero, J.C., Campos-Rodríguez, R., Godínez-Victoria, M., and Drago-Serrano, M.E. (2019). The impact of lactoferrin on the growth of intestinal inhabitant bacteria. Int. J. Mol. Sci., 20."
},
{
"DOI": "10.3168/jds.2021-21247",
"article-title": "Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern",
"author": "Wotring",
"doi-asserted-by": "crossref",
"first-page": "2791",
"journal-title": "J. Dairy Sci.",
"key": "ref_65",
"volume": "105",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2021.1888660",
"article-title": "The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "317",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_66",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3389/fmolb.2021.607443",
"doi-asserted-by": "crossref",
"key": "ref_67",
"unstructured": "Miotto, M., Di Rienzo, L., Bò, L., Boffi, A., Ruocco, G., and Milanetti, E. (2021). Molecular mechanisms behind anti SARS-CoV-2 action of lactoferrin. Front. Mol. Biosci., 8."
},
{
"DOI": "10.1007/s10534-022-00427-z",
"article-title": "An overview on in vitro and in vivo antiviral activity of lactoferrin: Its efficacy against SARS-CoV-2 infection",
"author": "Rosa",
"doi-asserted-by": "crossref",
"first-page": "417",
"journal-title": "Biometals",
"key": "ref_68",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.3390/ijms23105436",
"doi-asserted-by": "crossref",
"key": "ref_69",
"unstructured": "Piacentini, R., Centi, L., Miotto, M., Milanetti, E., Di Rienzo, L., Pitea, M., Piazza, P., Ruocco, G., Boffi, A., and Parisi, G. (2022). Lactoferrin inhibition of the complex formation between ACE2 receptor and SARS CoV-2 recognition binding domain. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.3390/nu14245274",
"doi-asserted-by": "crossref",
"key": "ref_70",
"unstructured": "Einerhand, A.W.C., Van Loo-Bouwman, C.A., Weiss, G.A., Wang, C., Ba, G., Fan, Q., He, B., and Smit, G. (2022). Can lactoferrin, a natural mammalian milk protein, assist in the battle against COVID-19?. Nutrients, 14."
},
{
"DOI": "10.1016/j.biopha.2021.111228",
"doi-asserted-by": "crossref",
"key": "ref_71",
"unstructured": "Habib, H.M., Ibrahim, S., Zaim, A., and Ibrahim, W.H. (2021). The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother., 136."
},
{
"DOI": "10.3390/ijerph182010985",
"doi-asserted-by": "crossref",
"key": "ref_72",
"unstructured": "Campione, E., Lanna, C., Cosio, T., Rosa, L., Conte, M.P., Iacovelli, F., Romeo, A., Falconi, M., Del Vecchio, C., and Franchin, E. (2021). Lactoferrin as antiviral treatment in COVID-19 management: Preliminary evidence. Int. J. Environ. Res. Public Health, 18."
},
{
"article-title": "Liposomal lactoferrin as potential preventative and cure for COVID-19",
"author": "Serrano",
"first-page": "8",
"journal-title": "Int. J. Res. Health Sci.",
"key": "ref_73",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/nu15051285",
"doi-asserted-by": "crossref",
"key": "ref_74",
"unstructured": "Matino, E., Tavella, E., Rizzi, M., Avanzi, G.C., Azzolina, D., Battaglia, A., Becco, P., Bellan, M., Bertinieri, G., and Bertoletti, M. (2023). Effect of lactoferrin on clinical outcomes of hospitalized patients with COVID-19: The LAC randomized clinical trial. Nutrients, 15."
},
{
"DOI": "10.3390/medicina57080842",
"doi-asserted-by": "crossref",
"key": "ref_75",
"unstructured": "Algahtani, F.D., Elabbasy, M.T., Samak, M.A., Adeboye, A.A., Yusuf, R.A., and Ghoniem, M.E. (2021). The prospect of lactoferrin use as adjunctive agent in management of SARS-CoV-2 patients: A randomized pilot study. Medicina, 57."
},
{
"DOI": "10.1007/s10534-020-00236-2",
"article-title": "Influence of oral administration mode on the efficacy of commercial bovine lactoferrin against iron and inflammatory homeostasis disorders",
"author": "Rosa",
"doi-asserted-by": "crossref",
"first-page": "159",
"journal-title": "Biometals",
"key": "ref_76",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1080/10408398.2017.1381583",
"article-title": "Lactoferrin: Structure, function, denaturation and digestion",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "580",
"journal-title": "Crit. Rev. Food Sci. Nutr.",
"key": "ref_77",
"volume": "59",
"year": "2019"
},
{
"DOI": "10.3390/nu10081115",
"doi-asserted-by": "crossref",
"key": "ref_78",
"unstructured": "Dix, C., and Wright, O. (2018). Bioavailability of a novel form of microencapsulated bovine lactoferrin and its effect on inflammatory markers and the gut microbiome: A pilot study. Nutrients, 10."
},
{
"DOI": "10.1002/biof.552210113",
"article-title": "Liposomal lactoferrin induced significant increase of the interferon-alpha (IFN-α) producibility in healthy volunteers",
"author": "Ishikado",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "BioFactors",
"key": "ref_79",
"volume": "21",
"year": "2004"
},
{
"DOI": "10.1002/jcp.22650",
"article-title": "Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "3022",
"journal-title": "J. Cell Physiol.",
"key": "ref_80",
"volume": "226",
"year": "2011"
},
{
"DOI": "10.1021/bi060570x",
"article-title": "Intelectin: A novel lipid raft-associated protein in the enterocyte brush border",
"author": "Wrackmeyer",
"doi-asserted-by": "crossref",
"first-page": "9188",
"journal-title": "Biochemistry",
"key": "ref_81",
"volume": "45",
"year": "2006"
},
{
"DOI": "10.1023/B:BIOM.0000027710.13543.3f",
"article-title": "Cellular internalization of lactoferrin in intestinal epithelial cells",
"author": "Ashida",
"doi-asserted-by": "crossref",
"first-page": "311",
"journal-title": "Biometals",
"key": "ref_82",
"volume": "17",
"year": "2004"
},
{
"DOI": "10.1016/j.ijbiomac.2017.11.007",
"article-title": "Saccharide binding by intelectins",
"author": "Sharma",
"doi-asserted-by": "crossref",
"first-page": "1010",
"journal-title": "Int. J. Biol. Macromol.",
"key": "ref_83",
"volume": "108",
"year": "2018"
},
{
"DOI": "10.1248/bpb.b15-00643",
"article-title": "Soluble human intestinal lactoferrin receptor: Ca2+-dependent binding to sepharose-based matrices",
"author": "Oshima",
"doi-asserted-by": "crossref",
"first-page": "435",
"journal-title": "Biol. Pharm. Bull.",
"key": "ref_84",
"volume": "39",
"year": "2016"
},
{
"DOI": "10.1093/jb/mvt073",
"article-title": "A lactoferrin-receptor, intelectin 1, affects uptake, subcellular localization and release of immunochemically detectable lactoferrin by intestinal epithelial Caco-2 cells",
"author": "Akiyama",
"doi-asserted-by": "crossref",
"first-page": "437",
"journal-title": "J. Biochem.",
"key": "ref_85",
"volume": "154",
"year": "2013"
},
{
"DOI": "10.3945/ajcn.113.071993",
"article-title": "Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas",
"doi-asserted-by": "crossref",
"first-page": "712S",
"journal-title": "Am. J. Clin. Nutr.",
"key": "ref_86",
"volume": "99",
"year": "2014"
},
{
"DOI": "10.1007/s00018-005-5371-1",
"article-title": "Mammalian lactoferrin receptors: Structure and function",
"author": "Suzuki",
"doi-asserted-by": "crossref",
"first-page": "2560",
"journal-title": "Cell Mol. Life Sci.",
"key": "ref_87",
"volume": "62",
"year": "2005"
},
{
"DOI": "10.1113/expphysiol.2003.026633",
"article-title": "Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats",
"author": "Takeuchi",
"doi-asserted-by": "crossref",
"first-page": "263",
"journal-title": "Exp. Physiol.",
"key": "ref_88",
"volume": "89",
"year": "2004"
},
{
"article-title": "Remdesivir for the treatment of COVID-19",
"author": "Ansems",
"first-page": "CD014962",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref_89",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe COVID-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "ref_90",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.4187/respcare.06314",
"article-title": "Clinical pharmacology of corticosteroids",
"author": "Williams",
"doi-asserted-by": "crossref",
"first-page": "655",
"journal-title": "Respir. Care",
"key": "ref_91",
"volume": "63",
"year": "2018"
},
{
"DOI": "10.1038/sj.bjp.0706736",
"article-title": "How corticosteroids control inflammation: Quintiles Prize Lecture",
"author": "Barnes",
"doi-asserted-by": "crossref",
"first-page": "245",
"journal-title": "Br. J. Pharmacol.",
"key": "ref_92",
"volume": "148",
"year": "2006"
},
{
"DOI": "10.3389/fimmu.2020.575197",
"doi-asserted-by": "crossref",
"key": "ref_93",
"unstructured": "Abu-Raya, B., Michalski, C., Sadarangani, M., and Lavoie, P.M. (2020). Maternal immunological adaptation during normal pregnancy. Front. Immunol., 11."
},
{
"DOI": "10.1056/NEJMra1213566",
"article-title": "Pregnancy and infection",
"author": "Kourtis",
"doi-asserted-by": "crossref",
"first-page": "2211",
"journal-title": "N. Engl. J. Med.",
"key": "ref_94",
"volume": "370",
"year": "2014"
},
{
"DOI": "10.3390/ijms24054550",
"doi-asserted-by": "crossref",
"key": "ref_95",
"unstructured": "Wong, Y.P., Tan, G.C., and Khong, T.Y. (2023). SARS-CoV-2 transplacental transmission: A rare occurrence? An overview of the protective role of the placenta. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.1371/journal.pone.0283453",
"doi-asserted-by": "crossref",
"key": "ref_96",
"unstructured": "Wai, J.Y., Wood, E.M., Hornaday, K.K., and Slater, D.M. (2023). Potential molecular and cellular mechanisms for adverse placental outcomes in pregnancies complicated by SARS-CoV-2 infection—A scoping review. PLoS ONE, 18."
},
{
"article-title": "Influence of lactoferrin in preventing preterm delivery: A pilot study",
"author": "Giunta",
"first-page": "162",
"journal-title": "Mol. Med. Rep.",
"key": "ref_97",
"volume": "5",
"year": "2012"
},
{
"article-title": "Bacterial biota of women with bacterial vaginosis treated with lactoferrin: An open prospective randomized trial",
"author": "Pino",
"first-page": "1357417",
"journal-title": "Microb. Ecol. Health Dis.",
"key": "ref_98",
"volume": "28",
"year": "2017"
},
{
"DOI": "10.3390/v14092043",
"doi-asserted-by": "crossref",
"key": "ref_99",
"unstructured": "Pomorski, M., Trzeszcz, M., Matera-Witkiewicz, A., Krupińska, M., Fuchs, T., Zimmer, M., Zimmer-Stelmach, A., Rosner-Tenerowicz, A., Budny-Wińska, J., and Tarczyńska-Podraza, A. (2022). SARS-CoV-2 infection and pregnancy: Maternal and neonatal outcomes and placental pathology correlations. Viruses, 14."
},
{
"DOI": "10.3390/ijms22115799",
"doi-asserted-by": "crossref",
"key": "ref_100",
"unstructured": "Bukowska-Ośko, I., Popiel, M., and Kowalczyk, P. (2021). The immunological role of the placenta in SARS-CoV-2 infection-viral transmission, immune regulation, and lactoferrin activity. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1080/19390211.2020.1834047",
"article-title": "COVID-19 during pregnancy and postpartum",
"author": "Naidu",
"doi-asserted-by": "crossref",
"first-page": "78",
"journal-title": "J. Diet. Suppl.",
"key": "ref_101",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1016/j.ajogmf.2021.100468",
"article-title": "Placental histopathology after SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis",
"author": "Khalil",
"doi-asserted-by": "crossref",
"first-page": "100468",
"journal-title": "Am. J. Obstet. Gynecol. MFM",
"key": "ref_102",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1042/CS20210284",
"article-title": "Role of ACE2 in pregnancy and potential implications for COVID-19 susceptibility",
"author": "Stoll",
"doi-asserted-by": "crossref",
"first-page": "1805",
"journal-title": "Clin. Sci.",
"key": "ref_103",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.3389/fendo.2022.951388",
"doi-asserted-by": "crossref",
"key": "ref_104",
"unstructured": "Wang, Y., Gu, Y., Lewis, D.F., Gu, X., Brown, K., Lachute, C., Hankins, M., Scott, R.S., Busada, C., and Cooper, D.B. (2023). Cell-type specific distribution and activation of type I IFN pathway molecules at the placental maternal-fetal interface in response to COVID-19 infection. Front. Endocrinol., 13."
},
{
"DOI": "10.3389/fimmu.2021.685919",
"doi-asserted-by": "crossref",
"key": "ref_105",
"unstructured": "Rebutini, P.Z., Zanchettin, A.C., Stonoga, E.T.S., Prá, D.M.M., de Oliveira, A.L.P., da Silva Dezidério, F., Fonseca, A.S., Dagostini, J.C.H., Hlatchuk, E.C., and Furuie, I.N. (2021). Association between COVID-19 pregnant women symptoms severity and placental morphologic features. Front. Immunol., 12."
},
{
"DOI": "10.1093/ajcp/aqaa089",
"article-title": "Placental pathology in COVID-19",
"author": "Shanes",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Am. J. Clin. Pathol.",
"key": "ref_106",
"volume": "154",
"year": "2020"
},
{
"DOI": "10.1016/j.siny.2023.101429",
"article-title": "Transmission of SARS-CoV-2 from mother to fetus or neonate: What to know and what to do?",
"author": "Benachi",
"doi-asserted-by": "crossref",
"first-page": "101429",
"journal-title": "Semin. Fetal Neonatal Med.",
"key": "ref_107",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.3390/diagnostics13020245",
"doi-asserted-by": "crossref",
"key": "ref_108",
"unstructured": "Moza, A., Duica, F., Antoniadis, P., Bernad, E.S., Lungeanu, D., Craina, M., Bernad, B.C., Paul, C., Muresan, C., and Nitu, R. (2023). Outcome of newborns with confirmed or possible SARS-CoV-2 vertical infection—A scoping review. Diagnostics, 13."
},
{
"DOI": "10.23736/S0031-0808.22.04705-X",
"article-title": "Baseline plasma SARS-CoV-2 RNA detection predicts an adverse COVID-19 evolution in moderate to severe hospitalized patients",
"author": "Rizzi",
"doi-asserted-by": "crossref",
"first-page": "465",
"journal-title": "Panminerva Med.",
"key": "ref_109",
"volume": "64",
"year": "2022"
},
{
"DOI": "10.1007/s00404-021-06049-z",
"article-title": "A systematic review of pregnant women with COVID-19 and their neonates",
"author": "Mirbeyk",
"doi-asserted-by": "crossref",
"first-page": "5",
"journal-title": "Arch. Gynecol. Obstet.",
"key": "ref_110",
"volume": "304",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm6944e3",
"article-title": "Update: Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3",
"author": "Zambrano",
"doi-asserted-by": "crossref",
"first-page": "1641",
"journal-title": "MMWR Morb. Mortal. Wkly. Rep.",
"key": "ref_111",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1016/j.ajog.2020.07.045",
"article-title": "Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching",
"author": "Badr",
"doi-asserted-by": "crossref",
"first-page": "764",
"journal-title": "Am. J. Obstet. Gynecol.",
"key": "ref_112",
"volume": "223",
"year": "2020"
},
{
"DOI": "10.1007/s10534-022-00471-9",
"article-title": "Review, role of lactoferrin in preventing preterm delivery",
"author": "Otsuki",
"doi-asserted-by": "crossref",
"first-page": "521",
"journal-title": "Biometals",
"key": "ref_113",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.3390/biomedicines9121940",
"doi-asserted-by": "crossref",
"key": "ref_114",
"unstructured": "Artym, J., and Zimecki, M. (2021). Antimicrobial and prebiotic activity of lactoferrin in the female reproductive tract: A comprehensive review. Biomedicines, 9."
},
{
"DOI": "10.1053/plac.1998.0368",
"article-title": "Amniotic fluid lactoferrin in intrauterine infection",
"author": "Otsuki",
"doi-asserted-by": "crossref",
"first-page": "175",
"journal-title": "Placenta",
"key": "ref_115",
"volume": "20",
"year": "1999"
},
{
"DOI": "10.1155/S1064744995000573",
"article-title": "Amniotic-fluid lactoferrin: A marker for subclinical intraamniotic infection prior to 32 weeks gestation",
"author": "Heller",
"doi-asserted-by": "crossref",
"first-page": "179",
"journal-title": "Infect. Dis. Obstet. Gynecol.",
"key": "ref_116",
"volume": "3",
"year": "1995"
},
{
"article-title": "Distribution of iron and iron-binding proteins in first-trimester human pregnancies",
"author": "Gulbis",
"first-page": "289",
"journal-title": "Obstet. Gynecol.",
"key": "ref_117",
"volume": "84",
"year": "1994"
},
{
"article-title": "Lactoferrin supplementation during pregnancy—A review of the literature and current recommendations",
"author": "Gawel",
"first-page": "570",
"journal-title": "Ginekol. Pol.",
"key": "ref_118",
"volume": "94",
"year": "2023"
},
{
"DOI": "10.1016/j.siny.2023.101430",
"article-title": "Clinical features of neonatal COVID-19",
"author": "Galderisi",
"doi-asserted-by": "crossref",
"first-page": "101430",
"journal-title": "Semin. Fetal Neonatal Med.",
"key": "ref_119",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.1038/s41390-021-01875-y",
"article-title": "Neonates and COVID-19: State of the art: Neonatal sepsis series",
"author": "Ryan",
"doi-asserted-by": "crossref",
"first-page": "432",
"journal-title": "Pediatr. Res.",
"key": "ref_120",
"volume": "91",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.15580",
"article-title": "Evaluation for SARS-CoV-2 in breast milk from 18 infected women",
"author": "Chambers",
"doi-asserted-by": "crossref",
"first-page": "1347",
"journal-title": "JAMA",
"key": "ref_121",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.3390/ijerph19063347",
"doi-asserted-by": "crossref",
"key": "ref_122",
"unstructured": "Wesołowska, A., Orczyk-Pawiłowicz, M., Bzikowska-Jura, A., Gawrońska, M., and Walczak, B. (2022). Protecting breastfeeding during the COVID-19 pandemic: A scoping review of perinatal care recommendations in the context of maternal and child well-being. Int. J. Environ. Res. Public Health, 19."
},
{
"DOI": "10.3389/fped.2020.633700",
"doi-asserted-by": "crossref",
"key": "ref_123",
"unstructured": "Spatz, D.L., Davanzo, R., Müller, J.A., Powell, R., Rigourd, V., Yates, A., Geddes, D.T., Van Goudoever, J.B., and Bode, L. (2021). Promoting and protecting human milk and breastfeeding in a COVID-19 world. Front. Pediatr., 8."
},
{
"DOI": "10.1111/apa.13147",
"article-title": "Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis",
"author": "Sankar",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Acta Paediatr.",
"key": "ref_124",
"volume": "104",
"year": "2015"
},
{
"DOI": "10.1016/j.jpeds.2009.11.021",
"article-title": "Breast milk as the gold standard for protective nutrients",
"author": "Walker",
"doi-asserted-by": "crossref",
"first-page": "S3",
"journal-title": "J. Ped.",
"key": "ref_125",
"volume": "156",
"year": "2010"
},
{
"DOI": "10.1080/14767058.2021.1920010",
"article-title": "Early human milk lactoferrin during SARS-CoV-2 infection",
"author": "Briana",
"doi-asserted-by": "crossref",
"first-page": "6704",
"journal-title": "J. Matern.-Fetal Neonatal Med.",
"key": "ref_126",
"volume": "35",
"year": "2022"
},
{
"article-title": "To breastfeed or not to breastfeed? Lack of evidence on the presence of SARS-CoV-2 in breastmilk of pregnant women with COVID-19",
"author": "Santos",
"first-page": "e59",
"journal-title": "Rev. Panamer. Salud Públ.",
"key": "ref_127",
"volume": "44",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2022.910138",
"doi-asserted-by": "crossref",
"key": "ref_128",
"unstructured": "Laguila Altoé, A., Marques Mambriz, A.P., Cardozo, D.M., Valentini Zacarias, J.M., Laguila Visentainer, J.E., and Bahls-Pinto, L.D. (2022). Vaccine protection through placenta and breastfeeding: The unmet topic in COVID-19 pandemic. Front. Immunol., 13."
},
{
"DOI": "10.3389/fimmu.2021.661806",
"doi-asserted-by": "crossref",
"key": "ref_129",
"unstructured": "Vassilopoulou, E., Feketea, G., Koumbi, L., Mesiari, C., Berghea, E.C., and Konstantinou, G.N. (2021). Breastfeeding and COVID-19: From nutrition to immunity. Front. Immunol., 12."
},
{
"DOI": "10.3389/fped.2023.1253333",
"doi-asserted-by": "crossref",
"key": "ref_130",
"unstructured": "He, Y.F., Liu, J.Q., Hu, X.D., Li, H.M., Wu, N., Wang, J., and Jiang, Z.G. (2023). Breastfeeding vs. breast milk transmission during COVID-19 pandemic, which is more important?. Front. Pediatr., 11."
},
{
"DOI": "10.1016/j.isci.2023.107767",
"article-title": "Milk antibody response after 3rd COVID-19 vaccine and SARS-CoV-2 infection and implications for infant protection",
"author": "Golan",
"doi-asserted-by": "crossref",
"first-page": "107767",
"journal-title": "iScience",
"key": "ref_131",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.3390/biomedicines10061219",
"doi-asserted-by": "crossref",
"key": "ref_132",
"unstructured": "Hatmal, M.M., Al-Hatamleh, M.A.I., Olaimat, A.N., Alshaer, W., Hasan, H., Albakri, K.A., Alkhafaji, E., Issa, N.N., Al-Holy, M.A., and Abderrahman, S.M. (2022). Immunomodulatory properties of human breast milk: microRNA contents and potential epigenetic effects. Biomedicines, 10."
},
{
"DOI": "10.3390/nu12103066",
"doi-asserted-by": "crossref",
"key": "ref_133",
"unstructured": "Carrillo-Lozano, E., Sebastián-Valles, F., and Knott-Torcal, C. (2020). Circulating microRNAs in breast milk and their potential impact on the infant. Nutrients, 12."
},
{
"DOI": "10.3390/molecules27092941",
"doi-asserted-by": "crossref",
"key": "ref_134",
"unstructured": "Kowalczyk, P., Kaczyńska, K., Kleczkowska, P., Bukowska-Ośko, I., Kramkowski, K., and Sulejczak, D. (2022). The lactoferrin phenomenon—A miracle molecule. Molecules, 27."
},
{
"DOI": "10.3390/ijms222011264",
"doi-asserted-by": "crossref",
"key": "ref_135",
"unstructured": "Gruden, Š., and Poklar Ulrih, N. (2021). Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.3390/nu11102350",
"doi-asserted-by": "crossref",
"key": "ref_136",
"unstructured": "Czosnykowska-Łukacka, M., Orczyk-Pawiłowicz, M., Broers, B., and Królak-Olejnik, B. (2019). Lactoferrin in human milk of prolonged lactation. Nutrients, 11."
},
{
"DOI": "10.1080/10408398.2011.642422",
"article-title": "Longitudinal changes in lactoferrin concentrations in human milk: A global systematic review",
"author": "Raic",
"doi-asserted-by": "crossref",
"first-page": "1539",
"journal-title": "Crit. Rev. Food Sci. Nutr.",
"key": "ref_137",
"volume": "54",
"year": "2014"
},
{
"DOI": "10.1371/journal.pone.0201819",
"doi-asserted-by": "crossref",
"key": "ref_138",
"unstructured": "Woodman, T., Strunk, T., Patole, S., Hartmann, B., Simmer, K., and Currie, A. (2018). Effects of lactoferrin on neonatal pathogens and bifidobacterium breve in human breast milk. PLoS ONE, 13."
},
{
"DOI": "10.1016/j.jpeds.2016.02.070",
"article-title": "Bioactive proteins in human milk: Health, nutrition, and implications for infant formulas",
"doi-asserted-by": "crossref",
"first-page": "S4",
"journal-title": "J. Ped.",
"key": "ref_139",
"volume": "173",
"year": "2016"
},
{
"DOI": "10.1086/588476",
"article-title": "Impact of lactoferrin supplementation on growth and prevalence of Giardia colonization in children",
"author": "Ochoa",
"doi-asserted-by": "crossref",
"first-page": "1881",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_140",
"volume": "46",
"year": "2008"
},
{
"DOI": "10.1097/01.mpg.0000243435.54958.68",
"article-title": "A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants",
"author": "King",
"doi-asserted-by": "crossref",
"first-page": "245",
"journal-title": "J. Pediatr. Gastroenterol. Nutr.",
"key": "ref_141",
"volume": "44",
"year": "2007"
},
{
"DOI": "10.1055/s-0034-1371704",
"article-title": "Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells",
"author": "Akin",
"doi-asserted-by": "crossref",
"first-page": "1111",
"journal-title": "Am. J. Perinatol.",
"key": "ref_142",
"volume": "31",
"year": "2014"
},
{
"DOI": "10.1080/10408398.2022.2157792",
"article-title": "The application of lactoferrin in infant formula: The past, present and future",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "5748",
"journal-title": "Crit. Rev. Food Sci. Nutr.",
"key": "ref_143",
"volume": "64",
"year": "2024"
},
{
"DOI": "10.3390/biomedicines12051120",
"doi-asserted-by": "crossref",
"key": "ref_144",
"unstructured": "Gaweł, P., Łukianowski, B., Kościelska-Kasprzak, K., Bartoszek, D., Krajewska, M., and Królak-Olejnik, B. (2024). Colostrum lactoferrin following active and recovered SARS-CoV-2 infections during pregnancy. Biomedicines, 12."
},
{
"DOI": "10.1016/j.isci.2022.104136",
"article-title": "Identified human breast milk compositions effectively inhibit SARS-CoV-2 and variants infection and replication",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "104136",
"journal-title": "iScience",
"key": "ref_145",
"volume": "25",
"year": "2022"
}
],
"reference-count": 145,
"references-count": 145,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1422-0067/25/19/10248"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Lactoferrin Supplementation in Preventing and Protecting from SARS-CoV-2 Infection: Is There Any Role in General and Special Populations? An Updated Review of Literature",
"type": "journal-article",
"volume": "25"
}