Effect of Lactoferrin on Clinical Outcomes of Hospitalized Patients with COVID-19: The LAC Randomized Clinical Trial
et al., Nutrients, doi:10.3390/nu15051285, LAC, NCT04847791, Mar 2023
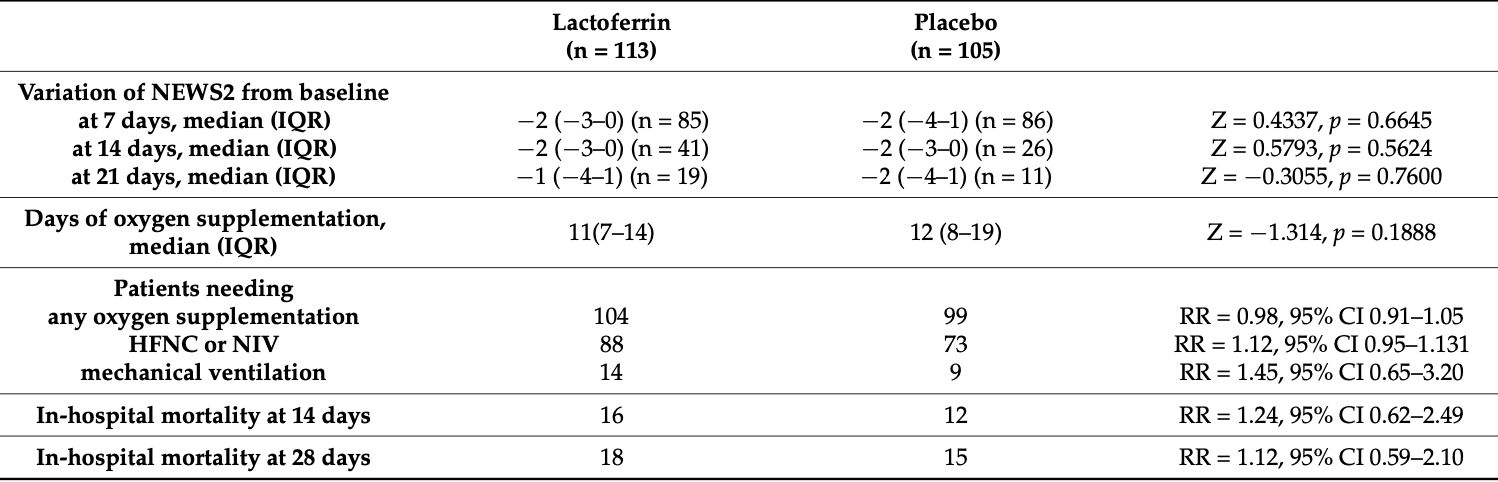
RCT 218 hospitalized patients in Italy, showing no significant differences with lactoferrin treatment. Authors note that in several previous studies showing clinical improvement, lactoferrin was given at an earlier stage of disease. Authors also note that potential benefits with the late treatment in this study could be masked by other SOC medications - corticosteroids may have masked immunomodulatory effects of lactoferrin, and there may be heparin-dependent reduction in lactoferrin antiviral activity. 800mg oral bovine lactoferrin daily.
|
risk of death, 11.5% higher, RR 1.12, p = 0.85, treatment 18 of 113 (15.9%), control 15 of 105 (14.3%), day 28.
|
|
risk of death, 23.9% higher, RR 1.24, p = 0.69, treatment 16 of 113 (14.2%), control 12 of 105 (11.4%), day 14.
|
|
risk of mechanical ventilation, 44.5% higher, RR 1.45, p = 0.39, treatment 14 of 113 (12.4%), control 9 of 105 (8.6%).
|
|
risk of death/ICU, 6.2% higher, RR 1.06, p = 0.87, treatment 24 of 113 (21.2%), control 21 of 105 (20.0%).
|
|
not reaching NEWS2 ≤2 or discharge within 14 days, 33.6% higher, RR 1.34, p = 0.12, treatment 46 of 113 (40.7%), control 32 of 105 (30.5%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Matino et al., 4 Mar 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Italy, peer-reviewed, 53 authors, study period January 2021 - May 2021, average treatment delay 6.0 days, trial NCT04847791 (history) (LAC).
Effect of Lactoferrin on Clinical Outcomes of Hospitalized Patients with COVID-19: The LAC Randomized Clinical Trial
Nutrients, doi:10.3390/nu15051285
As lactoferrin is a nutritional supplement with proven antiviral and immunomodulatory abilities, it may be used to improve the clinical course of COVID-19. The clinical efficacy and safety of bovine lactoferrin were evaluated in the LAC randomized double-blind placebo-controlled trial. A total of 218 hospitalized adult patients with moderate-to-severe COVID-19 were randomized to receive 800 mg/die oral bovine lactoferrin (n = 113) or placebo (n = 105), both given in combination with standard COVID-19 therapy. No differences in lactoferrin vs. placebo were observed in the primary outcomes: the proportion of death or intensive care unit admission (risk ratio of 1.06 (95% CI 0.63-1.79)) or proportion of discharge or National Early Warning Score 2 (NEWS2) ≤ 2 within 14 days from enrollment (RR of 0.85 (95% CI 0.70-1.04)). Lactoferrin showed an excellent safety and Nutrients 2023, 15, 1285. https://doi.org/10.3390/nu15051285 https://www.mdpi.com/journal/nutrients tolerability profile. Even though bovine lactoferrin is safe and tolerable, our results do not support its use in hospitalized patients with moderate-to-severe COVID-19.
Conflicts of Interest: The authors declare no conflict of interest.
References
Al-Alaiyan, Abdulaziz, Alkohlani, Almairi, Al Hazzani et al., Effects of Probiotics and Lactoferrin on Necrotizing Enterocolitis in Preterm Infants, Cureus, doi:10.7759/cureus.18256
Algahtani, Elabbasy, Sanak, Adeboye, Yusuf et al., The prospect of lactoferrin use ad adjunctive agent in management of SARS-CoV-2 patients: A randomized pilot study, Medicina, doi:10.3390/medicina57080842
Alt, median (IQR), U/L
Andersen, Jenssen, Sandvik, Gutteberg, Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface, J. Med. Virol, doi:10.1002/jmv.20171
Ansems, Grundeis, Dahms, Mikolajewska, Thieme et al., Remdesivir for the Treatment of COIVD-19, Cochrane Database of Systematic Reviews, doi:10.1002/14651858.CD014962/full
Arribas, Bhagani, Lobo, Khaertynova, Mateu et al., Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with COVID-19, NEJM
Ashida, Sasaki, Suzuki, Lönnerdal, Cellular internalization of lactoferrin in intestinal epithelial cells, Biometals, doi:10.1023/B:BIOM.0000027710.13543.3f
Ast, median (IQR, U/L
Avula, Nalleballe, Narula, Sapozhnikov, Dandu et al., COVID-19 presenting as stroke, Brain Behav. Immun, doi:10.1016/j.bbi.2020.04.077
Barnes, How corticosteroids control inflammation: Quintiles Prize Lecture 2005, Br. J. Pharmacol, doi:10.1038/sj.bjp.0706736
Barrington, Assaad, Janvier, The Lacuna Trial: A double-blind randomized controlled pilot trial of lactoferrin supplementation in the very preterm infant, J. Perinatol, doi:10.1038/jp.2016.24
Belting, Heparan sulfate proteoglycan as a plasma membrane carrier, Trends Biochem. Sci, doi:10.1016/S0968-0004(03)00031-8
Berlutti, Pantanella, Natalizi, Frioni, Paesano et al., Antiviral properties of lactoferrin-A natural immunity molecule, Molecules, doi:10.3390/molecules16086992
Broglio, Randomization in clinical trials. Permuted blocks and stratification, JAMA, doi:10.1001/jama.2018.6360
Cabaro, D'esposito, Di Matola, Sale, Cennamo et al., Cytokine signature and COVID-19 prediction models in the two waves of pandemics, Sci. Rep, doi:10.1038/s41598-021-00190-0
Cairns, Dulko, Griffiths, Golan, Cohen et al., Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19. A phase 2 randomized clinical trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.44942
Campione, Cosio, Rosa, Lanna, Di Girolamo et al., Lactoferrin as protective natural barrier of respiratory and intestinal mucosa against coronavirus infection and inflammation, Int. J. Mol. Sci
Campione, Lanna, Cosio, Rosa, Conte et al., Lactoferrin against SARS-COV-2: In Vitro and in silico evidences, Front. Pharmacol, doi:10.3389/fphar.2021.666600
Campione, Lanna, Cosio, Rosa, Conte et al., Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph182010985
Campione, Lanna, Cosio, Rosa, Conte et al., Pleiotropic effect of lactoferrin in the prevention and treatment of COIVD-19 infection: In Vivo, doi:10.1101/2020.08.11.244996v2.full.pdf
Carvalho, Sousa, Silva, Oliveira, Gonçalves et al., Inhibition of Mayaro virus infection by bovine lactoferrin, Virology, doi:10.1016/j.virol.2014.01.022
Champely, Ekstrom, Dalgaard, Gill, Weibelzahl et al., Pwr", R Package Version
Chang, Ng, Sun, Lactoferrin as potential preventative and adjunct treatment for COVID-19, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106118
Chen, Liu, Guo, Emerging coronaviruses: Genome structure, replication, and pathogenesis, J. Med. Virol, doi:10.1002/jmv.25681
Cohen, Statistical Power Analysis for the Behavioral Sciences
Core, A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team
Creatinine, None
Crp, None
Dhama, Khan, Tiwari, Sircar, Bhat et al., Coronavirus Disease 2019-COVID-19, Clin. Microbiol. Rev, doi:10.1128/CMR.00028-20
Ding, Zhang, Zhang, Huang, Yang et al., Prognostic Role and Diagnostic Power of Seven Indicators in COVID-19 Patients, Front. Med, doi:10.3389/fmed.2021.733274
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30120-1
Dyer, Covid-19: Unvaccinated face 11 times risk of death from delta variant, CDC data show, BMJ, doi:10.1136/bmj.n2282
Elfin Trial, Group, Enteral lactoferrin supplementation for very preterm infants: A randomized place-bo-controlled trial, Lancet, doi:10.1016/S0140-6736(18)32221-9
Ferritin, median (IQR
Fischer, Debbabi, Blais, Dubarry, Rautureau et al., Uptake of ingested bovine lactoferrin and its accumulation in adult mouse tissues, Int. Immunopharmacol, doi:10.1016/j.intimp.2007.05.019
Gavriatopoulou, Ntanasis-Stathopoulos, Korompoki, Fotiou, Migkou et al., Emerging treatment strategies for COVID-19 infection, Clin. Exp. Med, doi:10.1007/s10238-020-00671-y
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N. Engl. J. Med, doi:10.1056/NEJMoa2116846
Griffiths, Jenkins, Vargova, Bowler, Juszczak et al., Enteral lactoferrin to prevent infection for very preterm infants: The ELFIN RCT, Health Technol. Assess, doi:10.3310/hta22740
Habib, Ibrahim, Zaim, Ibrahim, The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.111228
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)-A metadatadriven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform, doi:10.1016/j.jbi.2008.08.010
He, Cai, Yu, Prophylactic lactoferrin for preventing late-onset sepsis and necrotizing enterocolitis in preterm infants. A PRISMA-complaint systematic review and meta-analysis, Medicine, doi:10.1097/MD.0000000000011976
Hemoglobin, median (IQR
Hoxa, Hodaj, Potential role of lactoferrin and heparin in COIVD-19: A review, Eur. Sci. J. ESJ
Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19, Nat. Rev. Microbiol, doi:10.1038/s41579-020-00459-7
Hu, Meng, Zhang, Xiang, Wang, The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor, Emerg. Microbes Infect, doi:10.1080/22221751.2021.1888660
Izda, Jeffries, Sawalha, COVID-19: A review of therapeutic strategies and vaccine candidates, Clin. Immunol, doi:10.1016/j.clim.2020.108634
Jiang, Lopez, Kelleher, Lönnerdal, Apo-and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in caco-2 cells, J. Cell. Physiol, doi:10.1002/jcp.22650
Kaur, Gatwala, Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neo-nates: A randomized placebo-controlled clinical trial, J. Trop. Pediatr, doi:10.1093/tropej/fmv044
Kawakami, Lonnerdal, Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes, Am. J. Physiol, doi:10.1152/ajpgi.1991.261.5.G841
Kitagawa, Yoshizawa, Yokoyama, Takeuchi, Talukder et al., Persorption of bovine lactoferrin from the intestinal lumen into the systemic circulation via the portal vein and the mesenteric lymphatics in growing pigs, J. Vet.-Med. Sci, doi:10.1292/jvms.65.567
Kochi, Tagliari, Forleo, Fassini, Tondo, Cardiac and arrhythmic complications in patients with COVID-19, J. Cardiovasc. Electrophysiol, doi:10.1111/jce.14479
Lamers, Haagmans, SARS-CoV-2 pathogenesis, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00713-0
Lang, Yang, Deng, Liu, Yang et al., Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans, PLoS ONE, doi:10.1371/journal.pone.0023710
Ldh, None, U/L
Legrand, Elass, Carpentier, Mazurier, Interactions of lactoferrin with cells involved in immune function, Biochem. Cell Biol, doi:10.1139/o06-045
Legrand, Mazurier, A critical review of the roles of host lactoferrin in immunity, Biometals, doi:10.1007/s10534-010-9297-1
Lin, Jiang, Zhang, Huang, Zhang et al., Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection, Gut, doi:10.1136/gutjnl-2020-321013
Lopez, Kelleher, Lönnerdal, Lactoferrin receptor mediates apo-but not hol-lactoferrin internalization via clath-rinmediated endocytosis in trophoblasts, Biochem. J, doi:10.1042/BJ20070393
Manzoni, Meyer, Stolfi, Rinaldi, Cattani et al., Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial, Early Hum. Dev, doi:10.1016/S0378-3782(14)70020-9
Manzoni, Rinaldi, Cattani, Pugni, Romeo et al., Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial, JAMA, doi:10.1001/jama.2009.1403
Miotto, Di Rienzo, Bò, Boffi, Ruocco et al., Molecular mechanisms behind anti SARS-CoV-2 action of lactoferrin, Front. Mol. Biosci, doi:10.3389/fmolb.2021.607443
Mirabelli, Wotring, Zhang, Mccarty, Fursmidt et al., Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2105815118
Ochoa, Loli, Mendoza, Carcamo, Bellomo et al., Effect of bovine lactoferrin on prevention of late-onset sepsis in infants < 1500 g: A pooled analysis of individual patient data from two randomized controlled trials, Biochem. Cell Biol, doi:10.1139/bcb-2020-0046
Ochoa, Zegarra, Bellomo, Carcamo, Cam et al., Randomized controlled trial of bovine lactoferrin for prevention of sepsis and neuro-development impairment in infants weighing less than 2000 grams, J. Pediatr, doi:10.1016/j.jpeds.2019.12.038
Ochoa, Zegarra, Cam, Llanos, Pezo et al., Randomized Controlled Trial of Lactoferrin for Prevention of Sepsis in Peruvian Neonates Less than 2500 g, Pediatr. Infect. Dis. J, doi:10.1097/INF.0000000000000593
Osuchowski, Winkler, Skirecki, Cajander, Shankar-Hari et al., The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00218-6
Presti, Manti, Parisi, Papale, Barbagallo et al., Lactoferrin: Cytokine modulation and application in clinical partice, J. Clin. Med, doi:10.3390/jcm10235482
Redwan, Uversky, El-Fakharany, Al-Mehdar, Potential lactoferrin activity against pathogenic viruses, Comptes Rendus Biol, doi:10.1016/j.crvi.2014.08.003
Ricordi, Pacifici, Lanzoni, Palamara, Garaci et al., Dietary and Protective Factors to Halt or Mitigate Progression of Autoimmunity, COVID-19 and Its Associated Metabolic Diseases, Int. J. Mol. Sci, doi:10.3390/ijms22063134
Rosa, Cutone, Conte, Campione, Bianchi et al., An overview on in vitro and in vivo antiviral activity of lactoferrin: Its efficacy against SARS-CoV-2 infection, BioMetals, doi:10.1007/s10534-022-00427-z
Rosa, Cutone, Lepanto, Paesano, Valenti, Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis, Int. J. Mol. Sci, doi:10.3390/ijms18091985
Rosa, Tripepi, Naldi, Aimati, Santangeli et al., Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study, J. Clin. Med, doi:10.3390/jcm10184276
Rosemberger, Lachin, Randomization and the Clinical Trial
Salaris, Scarpa, Elli, Bertolini, Guglielmetti et al., Protective effects of lactoferrin against SARS-CoV-2 infection in vitro, Nutrients, doi:10.3390/nu13020328
Sapp, Bienkowska-Haba, Viral entry mechanisms: Human papillomavirus and a long journey from extracellular matrix to the nucleus, FEBS J, doi:10.1111/j.1742-4658.2009.07400.x
Serrano, Kochergina, Albors, Diaz, Oroval et al., Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19, Int. J. Res. Health Sci, doi:10.5530/ijrhs.8.1.3
Shafqat, Shafqat, Al Salameh, Kashir, Alkattan et al., Mechanistic Insights into the Immune Pathophysiology of COVID-19
Spear, Herpes simplex virus: Receptors and ligands for cell entry, Cell Microbiol, doi:10.1111/j.1462-5822.2004.00389.x
Suresh, An overview of randomization techniques: An unbiased assessment of outcome in clinical research, J. Hum. Reprod. Sci, doi:10.4103/0974-1208.82352
Suzuki, Lopez, Lönnerdal, Mammalian lactoferrin receptors: Structure and function, Cell. Mol. Life Sci, doi:10.1007/s00018-005-5371-1
Tarnow-Mordi, Abdel-Latif, Martin, Pammi, Robledo et al., The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): A multicentre, double-blind, randomised controlled trial, Lancet Child Adolesc. Health, doi:10.1016/S2352-4642(20)30093-6
Troponin, median (IQR
Valenti, Antonini, Lactoferrin: An important host defence against microbial and viral attack, Cell Mol. Life Sci, doi:10.1007/s00018-005-5372-0
Wakabayashi, Oda, Yamauchi, Abe, Lactoferrin for prevention of common viral infections, J. Infect Chemother, doi:10.1016/j.jiac.2014.08.003
Wang, Wang, Wang, Luo, Wan et al., Lactoferrin for the treatment of COVID-19, Exp. Ther. Med, doi:10.3892/etm.2020.9402
Wang, Zhang, Wu, Niu, Song et al., Structural and functional basis of SARS-CoV-2 entry by using human ACE2, Cell, doi:10.1016/j.cell.2020.03.045
Wen, Chen, Tang, Wang, Zhou et al., Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis, Ann. Med, doi:10.1080/07853890.2022.2034936
Williams, Clinical Pharmacology of Corticosteroids, Respir. Care, doi:10.4187/respcare.06314
Wotring, Fursmidt, Ward, Sexton, Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern, J. Diary Sci, doi:10.3168/jds.2021-21247
Xiao, Tang, Zheng, Liu, Li et al., Evidence for gastrointestinal infection of SARS-CoV-2, Gastroenterology, doi:10.1053/j.gastro.2020.02.055
Yang, Jiang, Chen, Wang, Duan et al., Concentration of lactoferrin in human milk and its variation during lactation in different Chinese populations, Nutrients, doi:10.3390/nu10091235
DOI record:
{
"DOI": "10.3390/nu15051285",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu15051285",
"abstract": "<jats:p>As lactoferrin is a nutritional supplement with proven antiviral and immunomodulatory abilities, it may be used to improve the clinical course of COVID-19. The clinical efficacy and safety of bovine lactoferrin were evaluated in the LAC randomized double-blind placebo-controlled trial. A total of 218 hospitalized adult patients with moderate-to-severe COVID-19 were randomized to receive 800 mg/die oral bovine lactoferrin (n = 113) or placebo (n = 105), both given in combination with standard COVID-19 therapy. No differences in lactoferrin vs. placebo were observed in the primary outcomes: the proportion of death or intensive care unit admission (risk ratio of 1.06 (95% CI 0.63–1.79)) or proportion of discharge or National Early Warning Score 2 (NEWS2) ≤ 2 within 14 days from enrollment (RR of 0.85 (95% CI 0.70–1.04)). Lactoferrin showed an excellent safety and tolerability profile. Even though bovine lactoferrin is safe and tolerable, our results do not support its use in hospitalized patients with moderate-to-severe COVID-19.</jats:p>",
"alternative-id": [
"nu15051285"
],
"author": [
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"family": "Matino",
"given": "Erica",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-3396-0609",
"affiliation": [
{
"name": "Department of Maternal-Infant Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
},
{
"name": "Internal Medicine, Department of Medical Sciences, Azienda Ospedaliero-Universitaria (AOU) Città della Salute e della Scienza, University of Turin School of Medicine, 10126 Turin, Italy"
}
],
"authenticated-orcid": false,
"family": "Tavella",
"given": "Elena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6174-7111",
"affiliation": [
{
"name": "Department of Health Sciences, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Rizzi",
"given": "Manuela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"family": "Avanzi",
"given": "Gian Carlo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8185-5742",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Azzolina",
"given": "Danila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Dermatology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Battaglia",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Oncology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Becco",
"given": "Paolo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1488-8736",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "CAAD, Center for Autoimmune and Allergic Diseases, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Bellan",
"given": "Mattia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Internal Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Bertinieri",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pneumology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Bertoletti",
"given": "Massimo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"family": "Casciaro",
"given": "Giuseppe Francesco",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8248-1976",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Division of Internal Medicine, Azienda Ospedaliera “SS. Antonio e Biagio e Cesare Arrigo”, 15121 Alessandria, Italy"
}
],
"authenticated-orcid": false,
"family": "Castello",
"given": "Luigi Mario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Intensive Care Unit, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Colageo",
"given": "Umberto",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2277-3226",
"affiliation": [
{
"name": "Department of Health Sciences, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Colangelo",
"given": "Donato",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"family": "Comolli",
"given": "Davide",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"family": "Costanzo",
"given": "Martina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"family": "Croce",
"given": "Alessandro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3196-940X",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "D’Onghia",
"given": "Davide",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1736-2318",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Anesthesia and Intensive Care Medicine, AOU “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Della Corte",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Diabetology and Endocrinology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "De Mitri",
"given": "Luigi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine, Department of Medical Sciences, Azienda Ospedaliero-Universitaria (AOU) Città della Salute e della Scienza, University of Turin School of Medicine, 10126 Turin, Italy"
}
],
"family": "Dodaro",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pneumology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Givone",
"given": "Filippo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Emergency Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Gravina",
"given": "Alessia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Emergency Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Grillenzoni",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Neurology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Gusmaroli",
"given": "Graziano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"family": "Landi",
"given": "Raffaella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Lingua",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Dermatology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Manzoni",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Geriatric Care, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Marinoni",
"given": "Vito",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5243-0940",
"affiliation": [
{
"name": "Division of Obstetrics and Gynecology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"authenticated-orcid": false,
"family": "Masturzo",
"given": "Bianca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7548-9731",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Minisini",
"given": "Rosalba",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Emergency Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Morello",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Diabetology and Endocrinology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Nelva",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Geriatric Care, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Ortone",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Emergency Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Paolella",
"given": "Rita",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5404-3968",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Medical Department, Division of Cardiology, AOU “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Patti",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"family": "Pedrinelli",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "CAAD, Center for Autoimmune and Allergic Diseases, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"family": "Pirisi",
"given": "Mario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pneumology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Ravizzi",
"given": "Lidia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9615-2452",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Rizzi",
"given": "Eleonora",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7863-8192",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Sola",
"given": "Daniele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Emergency Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Sola",
"given": "Mariolina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Emergency Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Tonello",
"given": "Nadir",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0836-0890",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "CAAD, Center for Autoimmune and Allergic Diseases, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Tonello",
"given": "Stelvio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Diabetology and Endocrinology, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Topazzo",
"given": "Gigliola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Emergency Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Tua",
"given": "Aldo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Public Health and Infectious Diseases, University of Rome, La Sapienza, 00185 Rome, Italy"
}
],
"family": "Valenti",
"given": "Piera",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4625-4367",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Anesthesia and Intensive Care Medicine, AOU “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Vaschetto",
"given": "Rosanna",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7097-0710",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Vassia",
"given": "Veronica",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2134-6986",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Zecca",
"given": "Erika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Palliative Care, Ospedale degli Infermi, 13875 Ponderano, Italy"
}
],
"family": "Zublena",
"given": "Nicoletta",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1340-3493",
"affiliation": [
{
"name": "Department of Maternal-Infant Medicine, Ospedale degli Infermi, 13875 Ponderano, Italy"
},
{
"name": "Internal Medicine, Department of Medical Sciences, Azienda Ospedaliero-Universitaria (AOU) Città della Salute e della Scienza, University of Turin School of Medicine, 10126 Turin, Italy"
}
],
"authenticated-orcid": false,
"family": "Manzoni",
"given": "Paolo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8322-9158",
"affiliation": [
{
"name": "Department of Translational Medicine, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
},
{
"name": "Department of Internal Medicine and COVID-19 Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "Division of Emergency Medicine and COVID-19 Sub-Intensive Unit, Azienda Ospedaliero-Universitaria (AOU) “Maggiore della Carità”, 28100 Novara, Italy"
},
{
"name": "CAAD, Center for Autoimmune and Allergic Diseases, Università del Piemonte Orientale (UPO), 28100 Novara, Italy"
}
],
"authenticated-orcid": false,
"family": "Sainaghi",
"given": "Pier Paolo",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
3,
6
]
],
"date-time": "2023-03-06T06:35:30Z",
"timestamp": 1678084530000
},
"deposited": {
"date-parts": [
[
2023,
3,
6
]
],
"date-time": "2023-03-06T07:05:53Z",
"timestamp": 1678086353000
},
"indexed": {
"date-parts": [
[
2024,
3,
27
]
],
"date-time": "2024-03-27T18:28:55Z",
"timestamp": 1711564135547
},
"is-referenced-by-count": 7,
"issue": "5",
"issued": {
"date-parts": [
[
2023,
3,
4
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2023,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
4
]
],
"date-time": "2023-03-04T00:00:00Z",
"timestamp": 1677888000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/15/5/1285/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1285",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
3,
4
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
4
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41579-022-00713-0",
"article-title": "SARS-CoV-2 pathogenesis",
"author": "Lamers",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_1",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.835104",
"article-title": "Mechanistic Insights into the Immune Pathophysiology of COVID-19; An In-Depth Review",
"author": "Shafqat",
"doi-asserted-by": "crossref",
"first-page": "835104",
"journal-title": "Front. Immunol.",
"key": "ref_2",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41579-020-00459-7",
"article-title": "Characteristics of SARS-CoV-2 and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_3",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00218-6",
"article-title": "The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity",
"author": "Osuchowski",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "Lancet Respir. Med.",
"key": "ref_4",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-00190-0",
"article-title": "Cytokine signature and COVID-19 prediction models in the two waves of pandemics",
"author": "Cabaro",
"doi-asserted-by": "crossref",
"first-page": "20793",
"journal-title": "Sci. Rep.",
"key": "ref_5",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.733274",
"article-title": "Prognostic Role and Diagnostic Power of Seven Indicators in COVID-19 Patients",
"author": "Ding",
"doi-asserted-by": "crossref",
"first-page": "733274",
"journal-title": "Front. Med.",
"key": "ref_6",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"article-title": "Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis",
"author": "Wen",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Ann. Med.",
"key": "ref_7",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1136/bmj.n2282",
"article-title": "Covid-19: Unvaccinated face 11 times risk of death from delta variant, CDC data show",
"author": "Dyer",
"doi-asserted-by": "crossref",
"first-page": "n2282",
"journal-title": "BMJ",
"key": "ref_8",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1007/s10238-020-00671-y",
"article-title": "Emerging treatment strategies for COVID-19 infection",
"author": "Gavriatopoulou",
"doi-asserted-by": "crossref",
"first-page": "167",
"journal-title": "Clin. Exp. Med.",
"key": "ref_9",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2020.108634",
"article-title": "COVID-19: A review of therapeutic strategies and vaccine candidates",
"author": "Izda",
"doi-asserted-by": "crossref",
"first-page": "108634",
"journal-title": "Clin. Immunol.",
"key": "ref_10",
"volume": "222",
"year": "2021"
},
{
"key": "ref_11",
"unstructured": "(2022, May 25). COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health, Available online: https://www.covid19treatmentguidelines.nih.gov."
},
{
"DOI": "10.1001/jamanetworkopen.2021.44942",
"article-title": "Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19. A phase 2 randomized clinical trial",
"author": "Cairns",
"doi-asserted-by": "crossref",
"first-page": "e2144942",
"journal-title": "JAMA Netw. Open",
"key": "ref_12",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1073/pnas.2105815118",
"article-title": "Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19",
"author": "Mirabelli",
"doi-asserted-by": "crossref",
"first-page": "e2105815118",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_13",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.3390/ijms22063134",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Ricordi, C., Pacifici, F., Lanzoni, G., Palamara, A., Garaci, E., and Della-Morte, D. (2021). Dietary and Protective Factors to Halt or Mitigate Progression of Autoimmunity, COVID-19 and Its Associated Metabolic Diseases. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1016/j.biopha.2021.111228",
"article-title": "The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators",
"author": "Habib",
"doi-asserted-by": "crossref",
"first-page": "111228",
"journal-title": "Biomed. Pharmacother.",
"key": "ref_15",
"volume": "136",
"year": "2021"
},
{
"DOI": "10.3390/jcm10235482",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Presti, S., Manti, S., Parisi, G.F., Papale, M., Barbagallo, I.A., Li Volti, G., and Leonardi, S. (2021). Lactoferrin: Cytokine modulation and application in clinical partice. J. Clin. Med., 10."
},
{
"DOI": "10.1016/j.ijantimicag.2020.106118",
"article-title": "Lactoferrin as potential preventative and adjunct treatment for COVID-19",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "106118",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "ref_17",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0023710",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Lang, J., Yang, N., Deng, J., Liu, K., Yang, P., Zhang, G., and Jiang, C. (2011). Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE, 6."
},
{
"DOI": "10.1007/s10534-022-00427-z",
"doi-asserted-by": "crossref",
"key": "ref_19",
"unstructured": "Rosa, L., Cutone, A., Conte, M.P., Campione, E., Bianchi, L., and Valenti, P. (2022). An overview on in vitro and in vivo antiviral activity of lactoferrin: Its efficacy against SARS-CoV-2 infection. BioMetals, 1–20."
},
{
"DOI": "10.3168/jds.2021-21247",
"article-title": "Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern",
"author": "Wotring",
"doi-asserted-by": "crossref",
"first-page": "2791",
"journal-title": "J. Diary Sci.",
"key": "ref_20",
"volume": "105",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.666600",
"article-title": "Lactoferrin against SARS-COV-2: In Vitro and in silico evidences",
"author": "Campione",
"doi-asserted-by": "crossref",
"first-page": "666600",
"journal-title": "Front. Pharmacol.",
"key": "ref_21",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2021.1888660",
"article-title": "The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "317",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_22",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3390/nu13020328",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Salaris, C., Scarpa, M., Elli, M., Bertolini, A., Guglielmetti, S., Pregliasco, F., Blandizzi, C., Brun, P., and Castagliuolo, I. (2021). Protective effects of lactoferrin against SARS-CoV-2 infection in vitro. Nutrients, 13."
},
{
"DOI": "10.3389/fmolb.2021.607443",
"article-title": "Molecular mechanisms behind anti SARS-CoV-2 action of lactoferrin",
"author": "Miotto",
"doi-asserted-by": "crossref",
"first-page": "607443",
"journal-title": "Front. Mol. Biosci.",
"key": "ref_24",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.3390/jcm10184276",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Rosa, L., Tripepi, G., Naldi, E., Aimati, M., Santangeli, S., Venditto, F., Caldarelli, M., and Valenti, P. (2021). Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med., 10."
},
{
"article-title": "Effects of Probiotics and Lactoferrin on Necrotizing Enterocolitis in Preterm Infants",
"author": "Abdulaziz",
"first-page": "e18256",
"journal-title": "Cureus",
"key": "ref_26",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1139/bcb-2020-0046",
"article-title": "Effect of bovine lactoferrin on prevention of late-onset sepsis in infants < 1500 g: A pooled analysis of individual patient data from two randomized controlled trials",
"author": "Ochoa",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Biochem. Cell Biol.",
"key": "ref_27",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.1016/j.jpeds.2019.12.038",
"article-title": "Randomized controlled trial of bovine lactoferrin for prevention of sepsis and neuro-development impairment in infants weighing less than 2000 grams",
"author": "Ochoa",
"doi-asserted-by": "crossref",
"first-page": "118",
"journal-title": "J. Pediatr.",
"key": "ref_28",
"volume": "219",
"year": "2020"
},
{
"DOI": "10.1016/S2352-4642(20)30093-6",
"article-title": "The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): A multicentre, double-blind, randomised controlled trial",
"author": "Martin",
"doi-asserted-by": "crossref",
"first-page": "444",
"journal-title": "Lancet Child Adolesc. Health",
"key": "ref_29",
"volume": "4",
"year": "2020"
},
{
"key": "ref_30",
"unstructured": "ELFIN Trial Investigators Group (2019). Enteral lactoferrin supplementation for very preterm infants: A randomized place-bo-controlled trial. Lancet, 393, 423–433."
},
{
"DOI": "10.3310/hta22740",
"article-title": "Enteral lactoferrin to prevent infection for very preterm infants: The ELFIN RCT",
"author": "Griffiths",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Health Technol. Assess.",
"key": "ref_31",
"volume": "22",
"year": "2018"
},
{
"DOI": "10.1097/MD.0000000000011976",
"article-title": "Prophylactic lactoferrin for preventing late-onset sepsis and necrotizing enterocolitis in preterm infants. A PRISMA-complaint systematic review and meta-analysis",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "e11976",
"journal-title": "Medicine",
"key": "ref_32",
"volume": "97",
"year": "2018"
},
{
"DOI": "10.1038/jp.2016.24",
"article-title": "The Lacuna Trial: A double-blind randomized controlled pilot trial of lactoferrin supplementation in the very preterm infant",
"author": "Barrington",
"doi-asserted-by": "crossref",
"first-page": "666",
"journal-title": "J. Perinatol.",
"key": "ref_33",
"volume": "36",
"year": "2016"
},
{
"DOI": "10.1093/tropej/fmv044",
"article-title": "Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neo-nates: A randomized placebo-controlled clinical trial",
"author": "Kaur",
"doi-asserted-by": "crossref",
"first-page": "370",
"journal-title": "J. Trop. Pediatr.",
"key": "ref_34",
"volume": "61",
"year": "2015"
},
{
"DOI": "10.1097/INF.0000000000000593",
"article-title": "Randomized Controlled Trial of Lactoferrin for Prevention of Sepsis in Peruvian Neonates Less than 2500 g",
"author": "Ochoa",
"doi-asserted-by": "crossref",
"first-page": "571",
"journal-title": "Pediatr. Infect. Dis. J.",
"key": "ref_35",
"volume": "34",
"year": "2015"
},
{
"DOI": "10.1016/S0378-3782(14)70020-9",
"article-title": "Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial",
"author": "Manzoni",
"doi-asserted-by": "crossref",
"first-page": "S60",
"journal-title": "Early Hum. Dev.",
"key": "ref_36",
"volume": "90",
"year": "2014"
},
{
"DOI": "10.1001/jama.2009.1403",
"article-title": "Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial",
"author": "Manzoni",
"doi-asserted-by": "crossref",
"first-page": "1421",
"journal-title": "JAMA",
"key": "ref_37",
"volume": "302",
"year": "2009"
},
{
"DOI": "10.3390/ijerph182010985",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Campione, E., Lanna, C., Cosio, T., Rosa, L., Conte, M.P., Iacovelli, F., Romeo, A., Falconi, M., Del Vecchio, C., and Franchin, E. (2021). Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Health, 18."
},
{
"article-title": "Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19",
"author": "Serrano",
"first-page": "08",
"journal-title": "Int. J. Res. Health Sci.",
"key": "ref_39",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.4103/0974-1208.82352",
"article-title": "An overview of randomization techniques: An unbiased assessment of outcome in clinical research",
"author": "Suresh",
"doi-asserted-by": "crossref",
"first-page": "8",
"journal-title": "J. Hum. Reprod. Sci.",
"key": "ref_40",
"volume": "4",
"year": "2011"
},
{
"DOI": "10.1001/jama.2018.6360",
"article-title": "Randomization in clinical trials. Permuted blocks and stratification",
"author": "Broglio",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "JAMA",
"key": "ref_41",
"volume": "319",
"year": "2018"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"article-title": "Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support",
"author": "Harris",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "J. Biomed. Inform.",
"key": "ref_42",
"volume": "42",
"year": "2009"
},
{
"key": "ref_43",
"unstructured": "R Core Team (2015). A Language and Environment for Statistical Computing, R Core Team. R Foundation for Statistical Computing."
},
{
"key": "ref_44",
"unstructured": "Champely, S., Ekstrom, C., Dalgaard, P., Gill, J., Weibelzahl, S., Anandkumar, A., Ford, C., Volcic, R., and De Rosario, H. (2020, December 15). Package “Pwr”, R Package Version. Available online: https://CRAN.R-project.org/package=pwr."
},
{
"DOI": "10.3390/medicina57080842",
"doi-asserted-by": "crossref",
"key": "ref_45",
"unstructured": "Algahtani, F.D., ELabbasy, M.T., Sanak, M.A., Adeboye, A.A., Yusuf, R.A., and Ghoniem, M.E. (2021). The prospect of lactoferrin use ad adjunctive agent in management of SARS-CoV-2 patients: A randomized pilot study. Medicina, 57."
},
{
"DOI": "10.1016/j.intimp.2007.05.019",
"article-title": "Uptake of ingested bovine lactoferrin and its accumulation in adult mouse tissues",
"author": "Fischer",
"doi-asserted-by": "crossref",
"first-page": "1387",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_46",
"volume": "7",
"year": "2007"
},
{
"DOI": "10.1292/jvms.65.567",
"article-title": "Persorption of bovine lactoferrin from the intestinal lumen into the systemic circulation via the portal vein and the mesenteric lymphatics in growing pigs",
"author": "Kitagawa",
"doi-asserted-by": "crossref",
"first-page": "567",
"journal-title": "J. Vet.-Med. Sci.",
"key": "ref_47",
"volume": "65",
"year": "2003"
},
{
"DOI": "10.1023/B:BIOM.0000027710.13543.3f",
"article-title": "Cellular internalization of lactoferrin in intestinal epithelial cells",
"author": "Ashida",
"doi-asserted-by": "crossref",
"first-page": "311",
"journal-title": "Biometals",
"key": "ref_48",
"volume": "17",
"year": "2004"
},
{
"article-title": "Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes",
"author": "Kawakami",
"first-page": "G841",
"journal-title": "Am. J. Physiol.",
"key": "ref_49",
"volume": "261",
"year": "1991"
},
{
"DOI": "10.1002/jcp.22650",
"article-title": "Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in caco-2 cells",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "3022",
"journal-title": "J. Cell. Physiol.",
"key": "ref_50",
"volume": "226",
"year": "2011"
},
{
"DOI": "10.1042/BJ20070393",
"article-title": "Lactoferrin receptor mediates apo- but not hol-lactoferrin internalization via clath-rin-mediated endocytosis in trophoblasts",
"author": "Lopez",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Biochem. J.",
"key": "ref_51",
"volume": "411",
"year": "2008"
},
{
"DOI": "10.1007/s00018-005-5371-1",
"article-title": "Mammalian lactoferrin receptors: Structure and function",
"author": "Suzuki",
"doi-asserted-by": "crossref",
"first-page": "2560",
"journal-title": "Cell. Mol. Life Sci.",
"key": "ref_52",
"volume": "62",
"year": "2005"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "ref_53",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1002/14651858.CD014962",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "Ansems, K., Grundeis, F., Dahms, K., Mikolajewska, A., Thieme, V., Piechotta, V., Metzendorf, M.I., Stegemann, M., Benstoem, C., and Fichtner, F. (2022, August 08). Remdesivir for the Treatment of COIVD-19. Cochrane Database of Systematic Reviews. Art. N:CD014962. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014962/full."
},
{
"article-title": "Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with COVID-19",
"author": "Arribas",
"first-page": "EVIDoa2100044",
"journal-title": "NEJM Évid.",
"key": "ref_55",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.1007/s10534-010-9297-1",
"article-title": "A critical review of the roles of host lactoferrin in immunity",
"author": "Legrand",
"doi-asserted-by": "crossref",
"first-page": "365",
"journal-title": "Biometals",
"key": "ref_56",
"volume": "23",
"year": "2010"
},
{
"DOI": "10.4187/respcare.06314",
"article-title": "Clinical Pharmacology of Corticosteroids",
"author": "Williams",
"doi-asserted-by": "crossref",
"first-page": "655",
"journal-title": "Respir. Care",
"key": "ref_57",
"volume": "63",
"year": "2018"
},
{
"DOI": "10.1038/sj.bjp.0706736",
"article-title": "How corticosteroids control inflammation: Quintiles Prize Lecture 2005",
"author": "Barnes",
"doi-asserted-by": "crossref",
"first-page": "245",
"journal-title": "Br. J. Pharmacol.",
"key": "ref_58",
"volume": "148",
"year": "2006"
},
{
"DOI": "10.1016/j.crvi.2014.08.003",
"article-title": "Potential lactoferrin activity against pathogenic viruses",
"author": "Redwan",
"doi-asserted-by": "crossref",
"first-page": "581",
"journal-title": "Comptes Rendus Biol.",
"key": "ref_59",
"volume": "337",
"year": "2014"
},
{
"article-title": "Potential role of lactoferrin and heparin in COIVD-19: A review",
"author": "Hoxa",
"first-page": "14",
"journal-title": "Eur. Sci. J. ESJ",
"key": "ref_60",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1002/jmv.20171",
"article-title": "Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface",
"author": "Andersen",
"doi-asserted-by": "crossref",
"first-page": "262",
"journal-title": "J. Med. Virol.",
"key": "ref_61",
"volume": "74",
"year": "2004"
},
{
"DOI": "10.1016/j.bbi.2020.04.077",
"article-title": "COVID-19 presenting as stroke",
"author": "Avula",
"doi-asserted-by": "crossref",
"first-page": "115",
"journal-title": "Brain Behav. Immun.",
"key": "ref_62",
"volume": "87",
"year": "2020"
},
{
"DOI": "10.1016/S0968-0004(03)00031-8",
"article-title": "Heparan sulfate proteoglycan as a plasma membrane carrier",
"author": "Belting",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "Trends Biochem. Sci.",
"key": "ref_63",
"volume": "28",
"year": "2003"
},
{
"DOI": "10.3390/molecules16086992",
"article-title": "Antiviral properties of lactoferrin—A natural immunity molecule",
"author": "Berlutti",
"doi-asserted-by": "crossref",
"first-page": "6992",
"journal-title": "Molecules",
"key": "ref_64",
"volume": "16",
"year": "2011"
},
{
"DOI": "10.3390/ijms21144903",
"doi-asserted-by": "crossref",
"key": "ref_65",
"unstructured": "Campione, E., Cosio, T., Rosa, L., Lanna, C., Di Girolamo, S., Gaziano, R., Valenti, P., and Bianchi, L. (2020). Lactoferrin as protective natural barrier of respiratory and intestinal mucosa against coronavirus infection and inflammation. Int. J. Mol. Sci., 21."
},
{
"key": "ref_66",
"unstructured": "Campione, E., Lanna, C., Cosio, T., Rosa, L., Conte, M.P., Iacovelli, F., Romeo, A., Falconi, M., Del Vecchio, C., and Franchin, E. (2020). Pleiotropic effect of lactoferrin in the prevention and treatment of COIVD-19 infection: In Vivo, in silico and in vitro preliminary evidences. BioRxiv, Available online: https://www.biorxiv.org/content/10.1101/2020.08.11.244996v2.full.pdf."
},
{
"DOI": "10.1016/j.virol.2014.01.022",
"article-title": "Inhibition of Mayaro virus infection by bovine lactoferrin",
"author": "Carvalho",
"doi-asserted-by": "crossref",
"first-page": "297",
"journal-title": "Virology",
"key": "ref_67",
"volume": "452-453",
"year": "2014"
},
{
"DOI": "10.1002/jmv.25681",
"article-title": "Emerging coronaviruses: Genome structure, replication, and pathogenesis",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "418",
"journal-title": "J. Med. Virol.",
"key": "ref_68",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1128/CMR.00028-20",
"article-title": "Coronavirus Disease 2019-COVID-19",
"author": "Dhama",
"doi-asserted-by": "crossref",
"first-page": "e00028-20",
"journal-title": "Clin. Microbiol. Rev.",
"key": "ref_69",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"article-title": "An interactive web-based dashboard to track COVID-19 in real time",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "533",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_70",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1111/jce.14479",
"article-title": "Cardiac and arrhythmic complications in patients with COVID-19",
"author": "Kochi",
"doi-asserted-by": "crossref",
"first-page": "1003",
"journal-title": "J. Cardiovasc. Electrophysiol.",
"key": "ref_71",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1139/o06-045",
"article-title": "Interactions of lactoferrin with cells involved in immune function",
"author": "Legrand",
"doi-asserted-by": "crossref",
"first-page": "282",
"journal-title": "Biochem. Cell Biol.",
"key": "ref_72",
"volume": "84",
"year": "2006"
},
{
"DOI": "10.1136/gutjnl-2020-321013",
"article-title": "Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "997",
"journal-title": "Gut",
"key": "ref_73",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.3390/ijms18091985",
"doi-asserted-by": "crossref",
"key": "ref_74",
"unstructured": "Rosa, L., Cutone, A., Lepanto, M.S., Paesano, R., and Valenti, P. (2017). Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci., 18."
},
{
"DOI": "10.1111/j.1742-4658.2009.07400.x",
"article-title": "Viral entry mechanisms: Human papillomavirus and a long journey from extracellular matrix to the nucleus",
"author": "Sapp",
"doi-asserted-by": "crossref",
"first-page": "7206",
"journal-title": "FEBS J.",
"key": "ref_75",
"volume": "276",
"year": "2009"
},
{
"DOI": "10.1111/j.1462-5822.2004.00389.x",
"article-title": "Herpes simplex virus: Receptors and ligands for cell entry",
"author": "Spear",
"doi-asserted-by": "crossref",
"first-page": "401",
"journal-title": "Cell Microbiol.",
"key": "ref_76",
"volume": "6",
"year": "2004"
},
{
"DOI": "10.1007/s00018-005-5372-0",
"article-title": "Lactoferrin: An important host defence against microbial and viral attack",
"author": "Valenti",
"doi-asserted-by": "crossref",
"first-page": "2576",
"journal-title": "Cell Mol. Life Sci.",
"key": "ref_77",
"volume": "62",
"year": "2005"
},
{
"DOI": "10.1016/j.jiac.2014.08.003",
"article-title": "Lactoferrin for prevention of common viral infections",
"author": "Wakabayashi",
"doi-asserted-by": "crossref",
"first-page": "666",
"journal-title": "J. Infect Chemother.",
"key": "ref_78",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1016/j.cell.2020.03.045",
"article-title": "Structural and functional basis of SARS-CoV-2 entry by using human ACE2",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "894",
"journal-title": "Cell",
"key": "ref_79",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.3892/etm.2020.9402",
"article-title": "Lactoferrin for the treatment of COVID-19",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "272",
"journal-title": "Exp. Ther. Med.",
"key": "ref_80",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1053/j.gastro.2020.02.055",
"article-title": "Evidence for gastrointestinal infection of SARS-CoV-2",
"author": "Xiao",
"doi-asserted-by": "crossref",
"first-page": "1831",
"journal-title": "Gastroenterology",
"key": "ref_81",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.3390/nu10091235",
"doi-asserted-by": "crossref",
"key": "ref_82",
"unstructured": "Yang, Z., Jiang, R., Chen, Q., Wang, J., Duan, Y., Pang, X., Jiang, S., Bi, Y., Zhang, H., and Lönnerdal, B. (2018). Concentration of lactoferrin in human milk and its variation during lactation in different Chinese populations. Nutrients, 10."
},
{
"key": "ref_83",
"unstructured": "Rosemberger, W.F., and Lachin, J.M. (2002). Wiley Online Library."
},
{
"key": "ref_84",
"unstructured": "Cohen, J. (1977). Academic Press."
}
],
"reference-count": 84,
"references-count": 84,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/15/5/1285"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Effect of Lactoferrin on Clinical Outcomes of Hospitalized Patients with COVID-19: The LAC Randomized Clinical Trial",
"type": "journal-article",
"volume": "15"
}