The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study
et al., Medicina, doi:10.3390/medicina57080842, Aug 2021
RCT 54 hospitalized patients in Egypt, showing no significant differences in recovery with lactoferrin treatment. 200mg lactoferrin orally once daily (group 1) or 200mg lactoferrin orally twice daily (group 2).
|
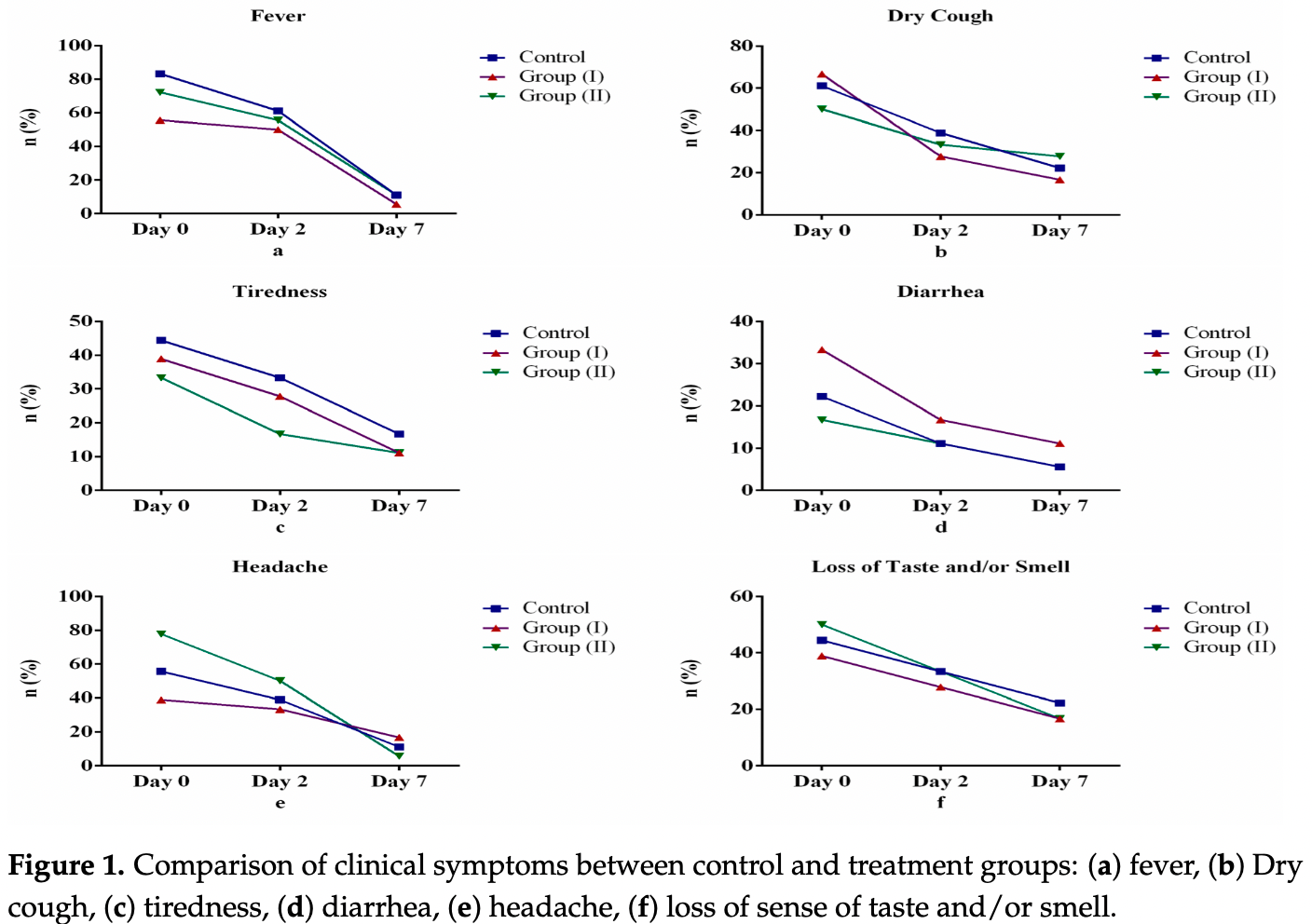
risk of unresolved fever, 25.0% lower, RR 0.75, p = 1.00, treatment 3 of 36 (8.3%), control 2 of 18 (11.1%), NNT 36, day 7.
|
|
risk of unresolved fatigue, 33.3% lower, RR 0.67, p = 0.67, treatment 4 of 36 (11.1%), control 3 of 18 (16.7%), NNT 18, day 7.
|
|
risk of unresolved cough, no change, RR 1.00, p = 1.00, treatment 8 of 36 (22.2%), control 4 of 18 (22.2%), day 7.
|
|
risk of unresolved headache, no change, RR 1.00, p = 1.00, treatment 4 of 36 (11.1%), control 2 of 18 (11.1%), day 7.
|
|
risk of unresolved loss of smell/taste, 25.0% lower, RR 0.75, p = 0.72, treatment 6 of 36 (16.7%), control 4 of 18 (22.2%), NNT 18, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Algahtani et al., 19 Aug 2021, Randomized Controlled Trial, Egypt, peer-reviewed, 6 authors, study period 8 July, 2020 - 18 September, 2020.
The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study
Medicina, doi:10.3390/medicina57080842
Background and Objectives: Preventive, adjunctive and curative properties of lactoferrin have been evaluated since the first wave of severe acute respiratory syndrome coronavirus (SARS-CoV), a viral respiratory disease, emerged 18 years ago. Despite the discovery of new vaccine candidates, there is currently no widely approved treatment for SARS-CoV-2 (COVID-19). Strict adherence to infection prevention and control procedures, as well as vaccines, can, however, prevent the spread of SARS-CoV-2. This study aimed to evaluate the efficacy of lactoferrin treatment in improving clinical symptoms and laboratory indices among individuals with mild to moderate coronavirus disease-19 (COVID-19). Materials and Method: A randomized, prospective, interventional pilot study conducted between 8 July and 18 September 2020 used a hospital-based sample of 54 laboratoryconfirmed participants with mild to moderate symptoms of COVID-19. Randomization into a control and two treatment groups ensured all groups received the approved Egyptian COVID-19 management protocol; only treatment group participants received lactoferrin at different doses for seven days. Clinical symptoms and laboratory indices were assessed on Days 0, 2 and 7 after starting treatments. Mean values with standard deviation and one-way analysis of variance with least significant difference of demographic and laboratory data between control and treatment groups were calculated. Results: Our study showed no statistically significant difference among studied groups regarding recovery of symptoms or laboratory improvement. Conclusions: Further research into therapeutic properties particularly related to dosage, duration and follow-up after treatment with lactoferrin in individuals with COVID-19 is required.
Conflicts of Interest: The authors declare that they have no conflict of interest.
Abbreviations SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; COVID-19: Coronavirus disease; Lf: Lactoferrin; Fe: Iron; WHO: World Health Organization; RT-PCR: reverse transcriptase polymerase chain reaction; LDH: Lactate dehydrogenase; ACE2: Angiotensin-converting enzyme 2; HSPGs: Heparan sulfate proteoglycans; IL: Interleukin; TNF alpha: Tumor Necrosis Factor alpha; CRP: C-reactive protein.
References
Angeletti, Benvenuto, Bianchi, Giovanetti, Pascarella et al., COVID-2019: The role of the nsp2 and nsp3 in its pathogenesis, J. Med. Virol, doi:10.1002/jmv.25719
Anurag, Jha, Kumar, Differential white blood cell count in the COVID-19: A cross-sectional study of 148 patients, Diabetes Metab. Syndr. Clin. Res. Rev, doi:10.1016/j.dsx.2020.10.029
Azhar, Mohammadabadi, Babar, Hussain, Milk lactoferrin: A probable immunological agent against sars-cov-2: A review, Basrah J. Agric. Sci, doi:10.37077/25200860.2020.33.2.12
Bajwa, Riaz, Ammar, Farooq, Yousaf, The Dilemma of Renal Involvement in COVID-19: A Systematic Review, Cureus, doi:10.7759/cureus.8632
Bertolini, Van De, Peppel, Bodewes, Moshage et al., Abnormal Liver Function Tests in Patients with COVID-19: Relevance and Potential Pathogenesis, Hepatology, doi:10.1002/hep.31480
Buey, Bellés, Latorre, Abad, Pérez et al., Comparative effect of bovine buttermilk, whey, and lactoferrin on the innate immunity receptors and oxidative status of intestinal epithelial cells, Biochem. Cell Biol
Cai, Huang, Yu, Zhu, Xia et al., COVID-19: Abnormal liver function tests, J. Hepatol, doi:10.1016/j.jhep.2020.04.006
Campione, Lanna, Cosio, Rosa, Conte et al., Lactoferrin as Potential Supplementary Nutraceutical Agent in COVID-19 Patients: In vitro and in vivo Preliminary Evidences, BioRxiv, doi:10.1101/2020.08.11.244996
Cavezzi, Troiani, Corrao, COVID-19: Hemoglobin, Iron, and Hypoxia beyond Inflammation. A Narrative Review, Clin. Pract, doi:10.4081/cp.2020.1271
Chang, Ng, Sun, Lactoferrin as potential preventative and adjunct treatment for COVID-19, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106118
Cheng, Li, Li, Liu, Yan et al., Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis, J. Clin. Lab. Anal, doi:10.1002/jcla.23618
Costagliola, Spada, Comberiati, Peroni, Could nutritional supplements act as therapeutic adjuvants in COVID-19?, Ital. J. Pediatr, doi:10.1186/s13052-021-00990-0
Cutone, Ianiro, Lepanto, Rosa, Valenti et al., Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development, Cancers, doi:10.3390/cancers12123806
Dhama, Sharun, Tiwari, Sircar, Bhat et al., Coronavirus Disease 2019-COVID-19, Clin. Microbiol. Rev, doi:10.1128/CMR.00028-20
El-Khawaga, Abdelmaksoud, Effect of Lactoferrin Supplementation on Iron Deficiency Anemia in Primary School Children, Int. J. Med. Arts, doi:10.21608/ijma.2019.12596.1003
Elmenam, Farouk, Bovine lactoferrin in preterm labor with sterile inflammation, Sci. J. Al-Azhar Med. Fac. Girls
Farid, El Shemy, Nafie, Hegazy, Abdelhiee, Anti-inflammatory, anti-oxidant and hepatoprotective effects of lactoferrin in rats, Drug Chem. Toxicol, doi:10.1080/01480545.2019.1585868
Feng, Fei, Xia, Labropoulou, Swevers et al., Antimicrobial Peptides as Potential Antiviral Factors in Insect Antiviral Immune Response, Front. Immunol, doi:10.3389/fimmu.2020.02030
Hao, Shan, Wei, Ma, Sun, Lactoferrin: Major Physiological Functions and Applications, Curr. Protein Pept. Sci, doi:10.2174/1389203719666180514150921
Henry, Aggarwal, Wong, Benoit, Vikse et al., Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis, Am. J. Emerg. Med, doi:10.1016/j.ajem.2020.05.073
Hsu, Chiu, Lin, Chen, Lee et al., Lactoferrin Contributes a Renoprotective Effect in Acute Kidney Injury and Early Renal Fibrosis, Pharmaceutics, doi:10.3390/pharmaceutics12050434
Hu, Meng, Zhang, Xiang, Wang, The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor, Emerg. Microbes Infect, doi:10.1080/22221751.2021.1888660
Kell, Heyden, Pretorius, The biology of lactoferrin, an iron-binding protein that can help de-fend against viruses and bacteria, Front. Immunol, doi:10.3389/fimmu.2020.01221
Khajeh, Jamshidian-Mojaver, Naeemipour, Farzin, The Identification of a Novel Peptide Derived from Lactoferrin Isolated from Camel Milk with Potential Antimicrobial Activity, Iran. J. Med. Microbiol
Kruzel, Olszewska, Pazdrak, Krupinska, Actor, New insights into the systemic effects of oral lactoferrin: Transcriptome profiling, Biochem. Cell Biol, doi:10.1139/bcb-2020-0069
Liu, Xie, Chen, Zhang, Cheng et al., Kidney Function Indicators Predict Adverse Outcomes of COVID-19, Med, doi:10.1016/j.medj.2020.09.001
Lodhi, Aslam, Sajid, Zulfiqar, Lactoferrin as Nutraceutical Protein from Milk, J. Nutraceuticals Food Sci
Niaz, Saeed, Ahmed, Imran, Maan et al., Lactoferrin (LF): A natural antimicrobial protein, Int. J. Food Prop, doi:10.1080/10942912.2019.1666137
Padrão, Ribeiro, Lanceros-Méndez, Rodrigues, Dourado, Effect of bacterial nanocellulose binding on the bactericidal activity of bovine lactoferrin, Heliyon, doi:10.1016/j.heliyon.2020.e04372
Peroni, Viral infections: Lactoferrin, a further arrow in the quiver of prevention, J. Pediat. Neonatal Individ. Med. (JPNIM
Quintieri, Caputo, Monaci, Cavalluzzi, Denora, Lactoferrin-derived peptides as a con-trol strategy against skinborne staphylococcal biofilms, Biomedicines, doi:10.3390/biomedicines8090323
Salaris, Scarpa, Elli, Bertolini, Guglielmetti et al., Protective effects of lactoferrin against SARS-CoV-2 infection in vitro, Nutrients, doi:10.3390/nu13020328
Serrano, Kochergina, Albors, Diaz, Oroval et al., Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19, Int. J. Res. Health Sci, doi:10.5530/ijrhs.8.1.3
Shahidi, Roshanak, Javadmanesh, Yazdi, Pirkhezranian et al., Evaluation of antimicrobial properties of bovine lactoferrin against foodborne pathogenic microorganisms in planktonic and bio-film forms (in vitro), J. Consum. Prot. Food Saf, doi:10.1007/s00003-020-01280-3
Sienkiewicz, Jaśkiewicz, Tarasiuk, Fichna, Lactoferrin: An overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2021.1895063
Sorour, El-Menshawy, A Proposed Protocol for the Management of COVID-19 in Egypt, AfricArXiv, doi:10.31730/osf.io/3rp97
Sullivan, Weinberg, Keaney, Jr, Common statistical pitfalls in basic science research, J. Am. Heart Assoc, doi:10.1161/JAHA.116.004142
Wang, Timilsena, Blanch, Adhikari, Lactoferrin: Structure, function, denaturation and digestion, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2017.1381583
Wang, Wang, Wang, Luo, Wan et al., Lactoferrin for the treatment of COVID-19, Exp. Ther. Med
Wang, Wu, Zhang, Wu, Yu et al., C-Reactive Protein Level May Predict the Risk of COVID-19 Aggravation, Open Forum Infect. Dis, doi:10.1093/ofid/ofaa153
Wool, Miller, The Impact of COVID-19 Disease on Platelets and Coagulation, Pathobiology, doi:10.1159/000512007
Xu, Dong, An, Lv, Yin et al., Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2, J. Infect
Yang, Cao, Gao, Wu, Wang et al., Discovery, optimization, and target identi-fication of novel potent broad-spectrum antiviral inhibitors, J. Med. Chem, doi:10.1021/acs.jmedchem.9b00091
Zhavoronkov, Aladinskiy, Zhebrak, Zagribelnyy, Terentiev et al., Potential COVID-2019 3C-Like Protease Inhibitors Designed Using Generative Deep Learning Approaches
DOI record:
{
"DOI": "10.3390/medicina57080842",
"ISSN": [
"1648-9144"
],
"URL": "http://dx.doi.org/10.3390/medicina57080842",
"abstract": "<jats:p>Background and Objectives: Preventive, adjunctive and curative properties of lactoferrin have been evaluated since the first wave of severe acute respiratory syndrome coronavirus (SARS-CoV), a viral respiratory disease, emerged 18 years ago. Despite the discovery of new vaccine candidates, there is currently no widely approved treatment for SARS-CoV-2 (COVID-19). Strict adherence to infection prevention and control procedures, as well as vaccines, can, however, prevent the spread of SARS-CoV-2. This study aimed to evaluate the efficacy of lactoferrin treatment in improving clinical symptoms and laboratory indices among individuals with mild to moderate coronavirus disease-19 (COVID-19). Materials and Method: A randomized, prospective, interventional pilot study conducted between 8 July and 18 September 2020 used a hospital-based sample of 54 laboratory-confirmed participants with mild to moderate symptoms of COVID-19. Randomization into a control and two treatment groups ensured all groups received the approved Egyptian COVID-19 management protocol; only treatment group participants received lactoferrin at different doses for seven days. Clinical symptoms and laboratory indices were assessed on Days 0, 2 and 7 after starting treatments. Mean values with standard deviation and one-way analysis of variance with least significant difference of demographic and laboratory data between control and treatment groups were calculated. Results: Our study showed no statistically significant difference among studied groups regarding recovery of symptoms or laboratory improvement. Conclusions: Further research into therapeutic properties particularly related to dosage, duration and follow-up after treatment with lactoferrin in individuals with COVID-19 is required.</jats:p>",
"alternative-id": [
"medicina57080842"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1247-9154",
"affiliation": [],
"authenticated-orcid": false,
"family": "Algahtani",
"given": "Fahad Dhafer",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0138-825X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Elabbasy",
"given": "Mohamed Tharwat",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5837-3921",
"affiliation": [],
"authenticated-orcid": false,
"family": "Samak",
"given": "Mai A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4207-8191",
"affiliation": [],
"authenticated-orcid": false,
"family": "Adeboye",
"given": "Adeniyi A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3669-0210",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yusuf",
"given": "Rafeek A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghoniem",
"given": "Mohamed E.",
"sequence": "additional"
}
],
"container-title": [
"Medicina"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
8,
19
]
],
"date-time": "2021-08-19T04:11:49Z",
"timestamp": 1629346309000
},
"deposited": {
"date-parts": [
[
2021,
8,
25
]
],
"date-time": "2021-08-25T03:09:16Z",
"timestamp": 1629860956000
},
"funder": [
{
"award": [
"COVID-1937."
],
"name": "the Scientific Research Deanship at University of Ha'il, Saudi Arabia"
}
],
"indexed": {
"date-parts": [
[
2021,
8,
27
]
],
"date-time": "2021-08-27T12:43:34Z",
"timestamp": 1630068214742
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1648-9144"
}
],
"issue": "8",
"issued": {
"date-parts": [
[
2021,
8,
19
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2021,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
19
]
],
"date-time": "2021-08-19T00:00:00Z",
"timestamp": 1629331200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1648-9144/57/8/842/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "842",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
8,
19
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
19
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1128/CMR.00028-20",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"key": "ref2",
"unstructured": "Coronavirus (COVID-19) Events as They Happenhttps://www.who.int/emergencies/diseases/novelcoronavirus-2019/events-as-they-happen"
},
{
"author": "Zhavoronkov",
"key": "ref3",
"series-title": "Potential COVID-2019 3C-Like Protease Inhibitors Designed Using Generative Deep Learning Approaches",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25719",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1186/s13052-021-00990-0",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1021/acs.jmedchem.9b00091",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.3389/fimmu.2020.02030",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1080/10408398.2021.1895063",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1139/bcb-2020-0121",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1080/10408398.2017.1381583",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"article-title": "Lactoferrin for the treatment of COVID-19",
"author": "Wang",
"first-page": "1",
"journal-title": "Exp. Ther. Med.",
"key": "ref11",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.5530/ijrhs.8.1.3",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.37077/25200860.2020.33.2.12",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.2174/1389203719666180514150921",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.30699/ijmm.15.3.302",
"article-title": "The Identification of a Novel Peptide Derived from Lactoferrin Isolated from Camel Milk with Potential Antimicrobial Activity",
"author": "Khajeh",
"doi-asserted-by": "crossref",
"first-page": "302",
"journal-title": "Iran. J. Med. Microbiol.",
"key": "ref15",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.01221",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.3390/biomedicines8090323",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1080/01480545.2019.1585868",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1007/s00003-020-01280-3",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.heliyon.2020.e04372",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"article-title": "Lactoferrin as Nutraceutical Protein from Milk",
"author": "Lodhi",
"first-page": "5",
"journal-title": "J. Nutraceuticals Food Sci.",
"key": "ref21",
"volume": "4",
"year": "2019"
},
{
"article-title": "Viral infections: Lactoferrin, a further arrow in the quiver of prevention",
"author": "Peroni",
"first-page": "e090142",
"journal-title": "J. Pediat. Neonatal Individ. Med. (JPNIM)",
"key": "ref22",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2021.1888660",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1101/2020.08.11.244996",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"key": "ref25",
"series-title": "Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases: Interim Guidance, 14 January 2020",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.02.017",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.31730/osf.io/3rp97",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"article-title": "Bovine lactoferrin in preterm labor with sterile inflammation",
"author": "Elmenam",
"first-page": "299",
"journal-title": "Sci. J. Al-Azhar Med. Fac. Girls",
"key": "ref28",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1161/JAHA.116.004142",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106118",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.3390/nu13020328",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1080/10942912.2019.1666137",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1139/bcb-2020-0069",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.4081/cp.2020.1271",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1016/j.dsx.2020.10.029",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1159/000512007",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3390/cancers12123806",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.21608/ijma.2019.12596.1003",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1016/j.jhep.2020.04.006",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1002/hep.31480",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.7759/cureus.8632",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.medj.2020.09.001",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.3390/pharmaceutics12050434",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1093/ofid/ofaa153",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1002/jcla.23618",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.ajem.2020.05.073",
"doi-asserted-by": "publisher",
"key": "ref46"
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"score": 1,
"short-container-title": [
"Medicina"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study"
],
"type": "journal-article",
"volume": "57"
}