The potential of lactoferrin as antiviral and immune-modulating agent in viral infectious diseases
et al., Frontiers in Immunology, doi:10.3389/fimmu.2024.1402135, Nov 2024
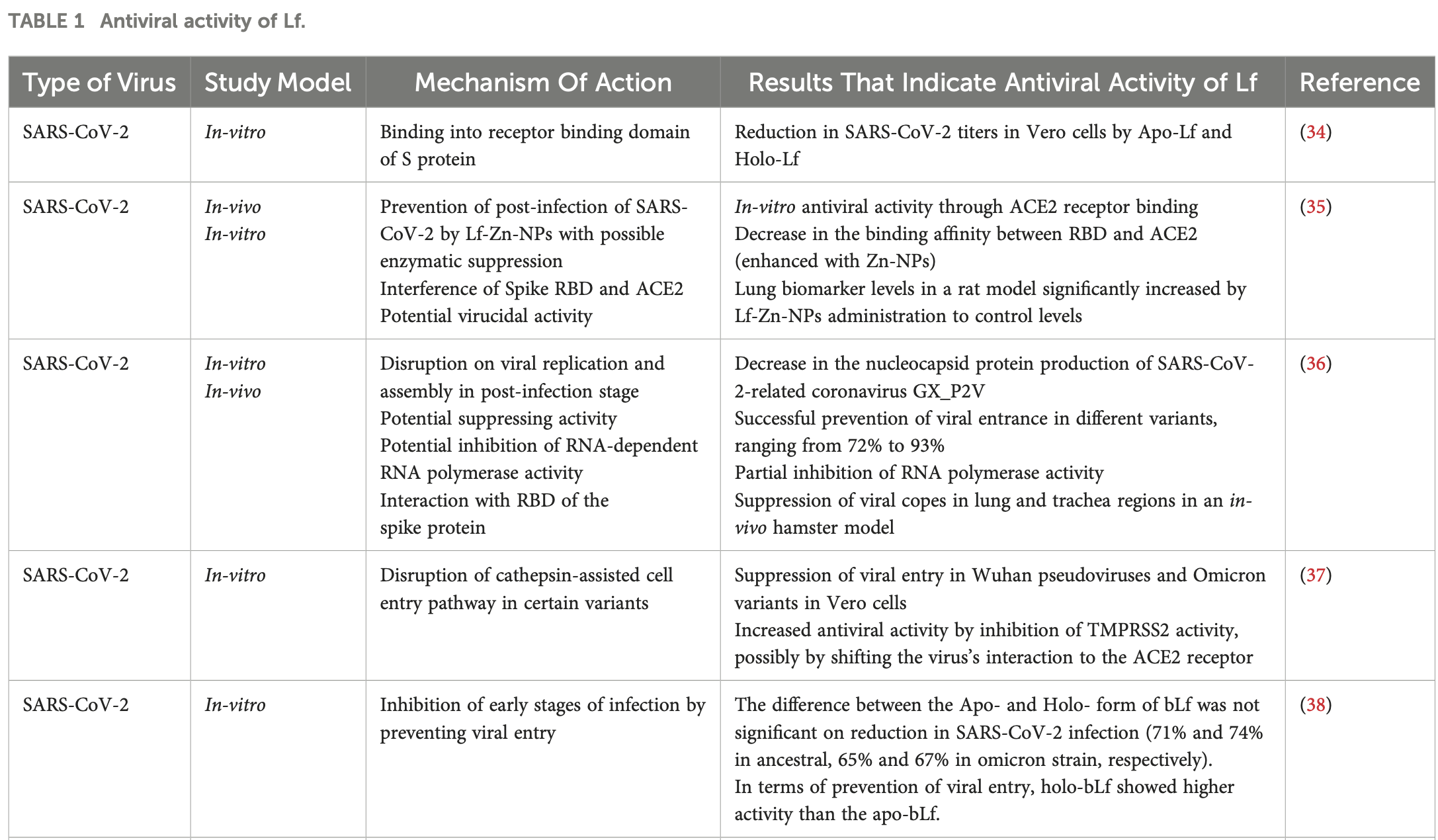
Review of the potential of lactoferrin as an antiviral and immune-modulating agent against various viruses, with a focus on SARS-CoV-2. Authors highlight lactoferrin's ability to interfere with virus-host cell interactions by binding to cell surface receptors like heparan sulfate proteoglycans, competing with viruses. Against SARS-CoV-2, lactoferrin has demonstrated inhibition of viral entry, replication, and assembly in multiple in vitro and in vivo studies. Lactoferrin's antiviral mechanisms include receptor binding competition, direct binding to virus particles, and immunomodulatory effects like regulating cytokine levels. N-terminal lactoferrin peptides have also shown anti-SARS-CoV-2 activity. A clinical trial found oral liposomal bovine lactoferrin reduced symptoms and inflammatory markers in COVID-19 patients.
1.
Palacios-Rosas et al., COVID-19 and Lactoferrin: A Systematic Review and Meta-Analysis, COVID, doi:10.3390/covid5100176.
2.
Eker et al., The potential of lactoferrin as antiviral and immune-modulating agent in viral infectious diseases, Frontiers in Immunology, doi:10.3389/fimmu.2024.1402135.
3.
Manzoni et al., Lactoferrin Supplementation in Preventing and Protecting from SARS-CoV-2 Infection: Is There Any Role in General and Special Populations? An Updated Review of Literature, International Journal of Molecular Sciences, doi:10.3390/ijms251910248.
4.
Rosa et al., An overview on in vitro and in vivo antiviral activity of lactoferrin: its efficacy against SARS-CoV-2 infection, BioMetals, doi:10.1007/s10534-022-00427-z.
5.
Mattar et al., Natural resources to control COVID-19: could lactoferrin amend SARS-CoV-2 infectivity?, PeerJ, doi:10.7717/peerj.11303.
Eker et al., 15 Nov 2024, multiple countries, peer-reviewed, 4 authors.
Contact: sercankarav@comu.edu.tr.
The potential of lactoferrin as antiviral and immune-modulating agent in viral infectious diseases
Frontiers in Immunology, doi:10.3389/fimmu.2024.1402135
Emerging infectious diseases are caused by unpredictable viruses with the dangerous potential to trigger global pandemics. These viruses typically initiate infection by utilizing the anionic structures of host cell surface receptors to gain entry. Lactoferrin (Lf) is a multifunctional glycoprotein with multiple properties such as antiviral, anti-inflammatory and antioxidant activities. Due to its cationic structure, Lf naturally interacts with certain host cell receptors, such as heparan sulfate proteoglycans, as well as viral particles and other receptors that are targeted by viruses. Therefore, Lf may interfere with virus-host cell interactions by acting as a receptor competitor for viruses. Herein we summarize studies in which this competition was investigated with SARS-CoV-2, Zika, Dengue, Hepatitis and Influenza viruses in vitro. These studies have demonstrated not only Lf's competitive properties, but also its potential intracellular impact on host cells, such as enhancing cell survival and reducing infection efficiency by inhibiting certain viral enzymes. In addition, the immunomodulatory effect of Lf is highlighted, as it can influence the activity of specific immune cells and regulate cytokine release, thereby enhancing the host's response to viral infections. Collectively, these properties promote the potential of Lf as a promising candidate for research in viral infectious diseases.
Author contributions
Conflict of interest Author ME was employed by company Uluova Dairy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abad, Vignard, Bouchenot, Graikini, Grasa et al., Dairy byproducts and lactoferrin exert antioxidant and antigenotoxic activity on intestinal and hepatic cells, Foods, doi:10.3390/foods12102073
Albecka, Belouzard, De Beeck, Descamps, Goueslain et al., Role of low-density lipoprotein receptor in the hepatitis C virus life cycle, Hepatology, doi:10.1002/hep.25501
Alves, Azevedo, Dias, Horbach, Setatino et al., Inhibition of SARS-coV-2 infection in vero cells by bovine lactoferrin under different iron-saturation states, Pharmaceuticals, doi:10.3390/PH16101352
Ammendolia, Agamennone, Pietrantoni, Lannutti, Siciliano et al., Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus, Pathog Glob Health, doi:10.1179/2047773212Y.0000000004
Ammendolia, Pietrantoni, Tinari, Valenti, Superti, Bovine lactoferrin inhibits echovirus endocytic pathway by interacting with viral structural polypeptides, Antiviral Res, doi:10.1016/J.ANTIVIRAL.2006.09.002
Andersen, Osbakk, Vorland, Traavik, Gutteberg, Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts, Antiviral Res, doi:10.1016/S0166-3542(01)00146-2
Arslan, Kaplan, Duman, Bayraktar, Ertürk et al., Bovine colostrum and its potential for human health and nutrition, Front Nutr, doi:10.3389/FNUT.2021.651721
Babulic, Cehlar, Ondrovicǒvág, Moskalets, Skrabana et al., Lactoferrin binds through its N-terminus to the receptor-binding domain of the SARS-coV-2 spike protein, Pharmaceuticals, doi:10.3390/PH17081021
Baker, Baker, A structural framework for understanding the multifunctional character of lactoferrin, Biochimie, doi:10.1016/j.biochi.2008.05.006
Baker, Baker, A structural perspective on lactoferrin function, Biochem Cell Biol, doi:10.1139/o11-071
Baker, Baker, Molecular structure, binding properties and dynamics of lactoferrin, Cell Mol Life Sci, doi:10.1007/s00018-005-5368-9
Baker, Mahmud, Miller, Rajeev, Rasambainarivo et al., Infectious disease in an era of global change, Nat Rev Microbiol, doi:10.1038/s41579-021-00639-z
Barth, Schäfer, Adah, Zhang, Linhardt et al., Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate, J Biol Chem, doi:10.1074/jbc.M302267200
Beljaars, Van Der Strate, Bakker, Reker-Smit, Van Loenen-Weemaes et al., Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo, Antiviral Res, doi:10.1016/J.ANTIVIRAL.2004.05.002
Björkström, Strunz, Ljunggren, Natural killer cells in antiviral immunity, Nat Rev Immunol, doi:10.1038/s41577-021-00558-3
Bolat, Eker, Kaplan, Duman, Lactoferrin for COVID-prevention, treatment, and recovery, Fronti Nutr, doi:10.3389/fnut.2022.992733
Bukowska-Osḱo, Popiel, Kowalczyk, The immunological role of the placenta in sars-cov-2 infection-viral transmission, immune regulation, and lactoferrin activity, Int J Mol Sci, doi:10.3390/ijms22115799
Cagno, Tseligka, Jones, Tapparel, Heparan sulfate proteoglycans and viral attachment: True receptors or adaptation bias?, Viruses, doi:10.3390/v11070596
Campione, Lanna, Cosio, Rosa, Conte et al., Lactoferrin as antiviral treatment in COVID-19 management: Preliminary evidence, Int J Environ Res Public Health, doi:10.3390/ijerph182010985
Cao, Ren, Lu, Wang, Wu et al., Lactoferrin: A glycoprotein that plays an active role in human health, Front Nutr, doi:10.3389/fnut.2022.1018336
Carabelli, Peacock, Thorne, Harvey, Hughes et al., SARS-CoV-2 variant biology: immune escape, transmission and fitness, Nat Rev Microbiol, doi:10.1038/s41579-022-00841-7
Carvalho, Casseb, Goncalves, Silva, Gomes et al., Bovine lactoferrin activity against chikungunya and zika viruses, J Gen Virol, doi:10.1099/jgv.0.000849
Castaneda, Gonzalez, Alomari, Tandon, Zervos et al., Hepatitis C virus-apolipoprotein interactions: molecular mechanisms and clinical impact, World J Gastroenterol, doi:10.1080/14789450.2017.1344102
Chen, Fan, Lin, Chen, Hsu et al., Bovine lactoferrin inhibits dengue virus infectivity by interacting with heparan sulfate, low-density lipoprotein receptor, and DC-SIGN, Int J Mol Sci, doi:10.3390/ijms18091957
Cheńeau, Eichholz, Tran, Tran, Paris et al., Lactoferrin retargets human adenoviruses to TLR4 to induce an abortive NLRP3associated pyroptotic response in human phagocytes, Front Immunol, doi:10.3389/FIMMU.2021.685218/BIBTEX
Chien, Chen, Hsu, Chiou, Bovine lactoferrin inhibits Japanese encephalitis virus by binding to heparan sulfate and receptor for low density lipoprotein, Virology, doi:10.1016/J.VIROL.2008.06.017
Cutone, Rosa, Di Patti, Iacovelli, Conte et al., Lactoferrin binding to SARS-coV-2 spike glycoprotein blocks pseudoviral entry and relieves iron protein dysregulation in several in vitro models, Pharmaceutics, doi:10.3390/pharmaceutics14102111
D'souza, Lau, Coffin, Patel, Zimmermann et al., Breast milk microbiota: A review of the factors that influence composition, World J Gastroenterol, doi:10.1016/j.jinf.2020.01.023
Dixon, Stockwell, The role of iron and reactive oxygen species in cell death, Nat Chem Biol, doi:10.1038/nchembio.1416
Duman, Karav, Bovine colostrum and its potential contributions for treatment and prevention of COVID-19, Front Immunol, doi:10.3389/FIMMU.2023.1214514/BIBTEX
Dutta, Langenburg, A perspective on current flavivirus vaccine development: A brief review, Viruses, doi:10.3390/v15040860
Eker, Akda¸sci, Duman, Mert, Yalcinta¸s et al., Antimicrobial properties of colostrum and milk, Antibiotics, doi:10.3390/ANTIBIOTICS13030251
Eker, Bolat, Pekdemir, Duman, Karav, Lactoferrin: neuroprotection against Parkinson's disease and secondary molecule for potential treatment, Front Aging Neurosci, doi:10.3389/fnagi.2023.1204149
El-Fakharany, El-Gendi, El-Maradny, Abu-Serie, Kg et al., Inhibitory effect of lactoferrin-coated zinc nanoparticles on SARS-CoV-2 replication and entry along with improvement of lung fibrosis induced in adult male albino rats, Int J Biol Macromol, doi:10.1016/j.ijbiomac.2023.125552
El-Fakharany, Sańchez, Al-Mehdar, Redwan, Effectiveness of human, camel, bovine and sheep lactoferrin on the hepatitis C virus cellular infectivity: comparison study, Virol J, doi:10.1186/1743-422X-10-199
Florian, Macovei, Lazar, Milac, Sokolowska et al., Characterization of the anti-HBV activity of HLP1-23, a human lactoferrin-derived peptide, J Med Virol, doi:10.1002/JMV.23549
Gonzaĺez-Chavez, Arevalo-Gallegos, Cruz, Lactoferrin: structure, function and applications, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2008.07.020
Hu, Meng, Zhang, Xiang, Wang, The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor, Emerg Microbes Infect, doi:10.1080/22221751.2021.1888660
Hyatt, Prevost, Devos, Mycroft-West, Skidmore et al., Molecular changes in dengue envelope protein domain III upon interaction with glycosaminoglycans, Pathogens, doi:10.3390/pathogens9110935
Jeon, Blacklow, Structure and physiologic function of the low-density lipoprotein receptor, Annu Rev Biochem, doi:10.1146/annurev.biochem.74.082803.133354
Juffrie, Van Der Meer, Hack, Haasnoot, Veerman et al., Inflammatory mediators in dengue virus infection in children: interleukin-8 and its relationship to neutrophil degranulation
Kaito, Iwasa, Fujita, Kobayashi, Kojima et al., Effect of lactoferrin in patients with chronic hepatitis C: Combination therapy with interferon and ribavirin, J Gastroenterol Hepatol, doi:10.1111/J.1440-1746.2007.04858.X
Kaplan, Sahutoglu, Sarıtaşs, Duman, Arslan et al., Role of milk glycome in prevention, treatment, and recovery of COVID-19, Front Nutr, doi:10.3389/FNUT.2022.1033779/BIBTEX
Karav, Casaburi, Arslan, Kaplan, Sucu et al., Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria, Appl Environ Microbiol, doi:10.1128/AEM.00547-16/ASSET/8DFAD725-4DE7-4159-AA5C-9DAEB7283E69/ASSETS/GRAPHIC/ZAM9991171820005.JPEG
Karav, German, Rouquiéc, Parc, Barile, Studying lactoferrin Nglycosylation, Int J Mol Sci, doi:10.3390/IJMS18040870
Kell, Heyden, Pretorius, The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria, Front Immunol, doi:10.3389/fimmu.2020.01221
Kim, Li, Linhardt, Pathogenesis and inhibition of flaviviruses from a carbohydrate perspective, Pharmaceuticals, doi:10.3390/ph10020044
Kobayashi-Sakamoto, Maeda, Yusa, Shimada, Tani et al., Bovine lactoferrin suppresses the cathepsin-dependent pathway of SARS-CoV-2 entry in vitro, Int Dairy J, doi:10.1016/j.idairyj.2023.105805
Kuhara, Tanaka, Yamauchi, Iwatsuki, Bovine lactoferrin ingestion protects against inflammation via IL-11 induction in the small intestine of mice with hepatitis, Br J Nutr, doi:10.1017/S0007114513004315
Lai, Yu, Xian, Ye, Ju et al., Identified human breast milk compositions effectively inhibit SARS-coV-2 and variants infection and replication, iScience, doi:10.1016/j.isci.2022.104136
Legrand, Overview of lactoferrin as a natural immune modulator, J Pediatr, doi:10.1016/j.jpeds.2016.02.071
Liu, Liu, Lin, Tsai, Hsu, Serum neutrophil gelatinase-associated lipocalin and resistin are associated with dengue infection in adults, BMC Infect Dis, doi:10.1186/s12879-016-1759-9
Luo, Xiang, Liu, Song, Feng et al., Inhibition of in vitro infection of hepatitis B virus by human breastmilk, Nutrients, doi:10.3390/nu14081561
Mancinelli, Rosa, Cutone, Lepanto, Franchitto et al., Viral hepatitis and iron dysregulation: Molecular pathways and the role of lactoferrin, Molecules, doi:10.3390/molecules25081997
Mathew, Defining the role of NK cells during dengue virus infection, Immunology, doi:10.1111/imm.12928
Mcarthur, Emerging infectious diseases, Nurs Clinics North America, doi:10.1016/j.cnur.2019.02.006
Meganck, Baric, Developing therapeutic approaches for twenty-firstcentury emerging infectious viral diseases, Nat Med, doi:10.1038/s41591-021-01282-0
Moreno-Expośito, Illescas-Montes, Melguizo-Rodrıǵuez, Ruiz, Ramos-Torrecillas et al., Multifunctional capacity and therapeutic potential of lactoferrin, Life Sci, doi:10.1016/j.lfs.2018.01.002
Msemburi, Karlinsky, Knutson, Aleshin-Guendel, Chatterji et al., The WHO estimates of excess mortality associated with the COVID-19 pandemic, Nature, doi:10.1038/s41586-022-05522-2
Nanaware, Banerjee, Bagchi, Bagchi, Mukherjee, Dengue virus infection: A tale of viral exploitations and host responses, Viruses, doi:10.3390/v13101967
Naqvi, Mohammad, Fatima, Singh, Singh, Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach, Biochim Biophys Acta Mol Basis Dis, doi:10.1016/j.bbadis.2020.165878
Niaz, Saeed, Ahmed, Imran, Maan et al., Lactoferrin (LF): a natural antimicrobial protein, Int J Food Prop, doi:10.1080/10942912.2019.1666137
Ogasawara, Imase, Oda, Wakabayashi, Ishii, Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: A possible role in protection against oxidative DNA damage, Int J Mol Sci, doi:10.3390/ijms15011003
Ohradanova-Repic, Prazěnicovár, Gebetsberger, Moskalets, Skrabana et al., Time to kill and time to heal: the multifaceted role of lactoferrin and lactoferricin in host defense, Pharmaceutics, doi:10.3390/PHARMACEUTICS15041056
Ohradanova-Repic, Skrabana, Gebetsberger, Tajti, Barath et al., Blockade of TMPRSS2-mediated priming of SARS-coV-2 by lactoferricin, Front Immunol, doi:10.3389/FIMMU.2022.958581/BIBTEX
Pagani, Ottoboni, Panina-Bordignon, Martino, Poli et al., Heparin precursors with reduced anticoagulant properties retain antiviral and protective effects that potentiate the efficacy of sofosbuvir against zika virus infection in human neural progenitor cells, Pharmaceuticals, doi:10.3390/ph16101385
Picard-Jean, Bouchard, Lariveé, Bisaillon, The intracellular inhibition of HCV replication represents a novel mechanism of action by the innate immune Lactoferrin protein, Antiviral Res, doi:10.1016/j.antiviral.2014.08.012
Pietrantoni, Ammendolia, Superti, Bovine lactoferrin: Involvement of metal saturation and carbohydrates in the inhibition of influenza virus infection, Biochem Cell Biol, doi:10.1139/o11-072
Pietrantoni, Dofrelli, Tinari, Ammendolia, Puzelli et al., Bovine lactoferrin inhibits Influenza A virus induced programmed cell death in vitro, BioMetals, doi:10.1007/s10534-010-9323-3
Prieto-Fernańdez, Egia-Mendikute, Vila-Vecilla, Bosch, Barreira-Manrique et al., Hypoxia reduces cell attachment of SARS-coV-2 spike protein by modulating the expression of ACE2, neuropilin-1, syndecan-1 and cellular heparan, Emerg Microbes Infections, doi:10.1080/22221751.2021.1932607
Qian, Qi, Mosquito-borne flaviviruses and current therapeutic advances, Viruses, doi:10.3390/v14061226
Redwan, El-Fakharany, Uversky, Linjawi, Screening the anti infectivity potentials of native N-and C-lobes derived from the camel lactoferrin against hepatitis C virus, BMC Complement Altern Med, doi:10.1186/1472-6882-14-219
Rosa, Cutone, Conte, Campione, Bianchi et al., An overview on in vitro and in vivo antiviral activity of lactoferrin: its efficacy against SARS-CoV-2 infection, BioMetals, doi:10.1007/s10534-022-00427-z
Ryu, Nogami, Kitakaze, Harada, Suzuki et al., Lactoferrin induces tropoelastin expression by activating the lipoprotein receptor-related protein 1-mediated phosphatidylinositol 3-kinase/Akt pathway in human dermal fibroblasts, Cell Biol Int, doi:10.1002/cbin.10845
Salaris, Scarpa, Elli, Bertolini, Guglielmetti et al., Protective effects of lactoferrin against sars-cov-2 infection in vitro, Nutrients, doi:10.3390/nu13020328
Sarrazin, Lamanna, Esko, Heparan sulfate proteoglycans, Cold Spring Harb Perspect Biol, doi:10.1101/cshperspect.a004952
Schuurs, Hammond, Elli, Rudd, Mycroft-West et al., Evidence of a putative glycosaminoglycan binding site on the glycosylated SARS-CoV-2 spike protein N-terminal domain, Comput Struct Biotechnol J, doi:10.1016/j.csbj.2021.05.002
Sherman, Pritzl, Xia, Miller, Zaghouani et al., Lactoferrin acts as an adjuvant during influenza vaccination of neonatal mice, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2015.10.067
Sienkiewicz, Jasḱiewicz, Tarasiuk, Fichna, Lactoferrin: an overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance, Crit Rev Food Sci Nutr, doi:10.1080/10408398.2021.1895063
Sokolov, Isakova-Sivak, Mezhenskaya, Kostevich, Gorbunov et al., Molecular mimicry of the receptor-binding domain of the SARS-CoV-2 spike protein: from the interaction of spike-specific antibodies with transferrin and lactoferrin to the antiviral effects of human recombinant lactoferrin, BioMetals, doi:10.1007/s10534-022-00458-6
Sorin, Kuhn, Stasiak, Stehle, Structural insight into non-enveloped virus binding to glycosaminoglycan receptors: A review, Viruses, doi:10.3390/v13050800
St, Qin, Guan, Liu, Hong et al., Bovine lactoferrin inhibits SARS-CoV-2 and SARS-CoV-1 by targeting the RdRp complex and alleviates viral infection in the hamster model, J Med Virol, doi:10.1002/jmv.28281
Superti, Agamennone, Pietrantoni, Ammendolia, Bovine lactoferrin prevents influenza a virus infection by interfering with the fusogenic function of viral hemagglutinin, Viruses, doi:10.3390/v11010051
Superti, Lactoferrin from bovine milk: A protective companion for life, Nutrients, doi:10.3390/nu12092562
Syed, Tang, Khan, Hassanein, Liu et al., Hepatitis C virus stimulates low-density lipoprotein receptor expression to facilitate viral propagation, J Virol, doi:10.1128/jvi.02727-13
Tahmoorespur, Azghandi, Javadmanesh, Meshkat, Sekhavati, A Novel chimeric anti-HCV peptide derived from camel lactoferrin and molecular level insight on its interaction with E2, Int J Pept Res Ther, doi:10.22103/JAB.2022.18992.1382
Tapper, Emerging viral diseases and infectious disease risks, Haemophilia, doi:10.1111/j.1365-2516.2006.01194.x
Vitetta, Coulson, Beck, Gramotnev, Du et al., The clinical efficacy of a bovine lactoferrin/whey protein Ig-rich fraction (Lf/IgF) for the common cold: A double blind randomized study, Complement Ther Med, doi:10.1016/j.ctim.2012.12.006
Wakabayashi, Oda, Yamauchi, Abe, Lactoferrin for prevention of common viral infections, J Infection Chemotherapy, doi:10.1016/j.jiac.2014.08.003
Wang, Bi, Liu, Song, Xie, The protective effect of lactoferrin on ventral mesencephalon neurons against MPP+ is not connected with its iron binding ability, Sci Rep, doi:10.1038/srep10729
Wang, Timilsena, Blanch, Adhikari, Lactoferrin: Structure, function, denaturation and digestion, Crit Rev Food Sci Nutr, doi:10.1080/10408398.2017.1381583
Wang, Yue, Dang, Yang, Chen et al., Role of sialylated glycans on bovine lactoferrin against influenza virus, Glycoconj J, doi:10.1007/s10719-021-10029-5
Williams, Mackenzie, Bingham, None, Flaviviruses. Dis Swine, doi:10.1002/9781119350927.CH33
Wu, Tian, Wei, Developmental and functional control of natural killer cells by cytokines, Front Immunol, doi:10.3389/fimmu.2017.00930
Wu, Zang, Wang, Qiao, Zhao et al., Lactoferricin, an antimicrobial motif derived from lactoferrin with food preservation potential, Crit Rev Food Sci Nutr, doi:10.1080/10408398.2023.2207650
Xie, Wang, Cai, Bai, Shao et al., Optimum fermentation conditions for bovine lactoferricin-lactoferrampin-encoding limosiLactobacillus reuteri and regulation of intestinal inflammation, Foods, doi:10.3390/FOODS12224068/S1
Yi, Kaneko, Yu, Murakami, Hepatitis C virus envelope proteins bind lactoferrin, J Virol, doi:10.1128/JVI.71.8.5997-6002.1997
Zarzosa-Moreno, Avalos-Goḿez, Ramıŕez-Texcalco, Torres-Loṕez, Ramıŕez-Mondragoń et al., Lactoferrin and its derived peptides: an alternative for combating virulence mechanisms developed by pathogens, Molecules, doi:10.3390/MOLECULES25245763
Zhao, Li, Yu, Wu, Ding et al., Identification of lactoferrin-derived peptides as potential inhibitors against the main protease of SARS-coV-2, LWT, doi:10.1016/J.LWT.2021.112684
Zhou, Zhu, Liu, Zhang, Han, Effect of iron saturation of bovine lactoferrin on the inhibition of hepatitis B virus in vitro, PeerJ, doi:10.7717/PEERJ.17302/SUPP-2
Zong, Cao, Wang, Zhao, Lu et al., Porcine lactoferrin-derived peptide LFP-20 modulates immune homoeostasis to defend lipopolysaccharidetriggered intestinal inflammation in mice, Br J Nutr, doi:10.1017/S0007114519000485
Zong, Hu, Song, Li, Du et al., Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response, Biochem Pharmacol, doi:10.1016/J.BCP.2016.01.009
Zumla, Hui, Emerging and reemerging infectious diseases: global overview, Infect Dis Clin North Am, doi:10.1016/j.idc.2019.09.001
DOI record:
{
"DOI": "10.3389/fimmu.2024.1402135",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2024.1402135",
"abstract": "<jats:p>Emerging infectious diseases are caused by unpredictable viruses with the dangerous potential to trigger global pandemics. These viruses typically initiate infection by utilizing the anionic structures of host cell surface receptors to gain entry. Lactoferrin (Lf) is a multifunctional glycoprotein with multiple properties such as antiviral, anti-inflammatory and antioxidant activities. Due to its cationic structure, Lf naturally interacts with certain host cell receptors, such as heparan sulfate proteoglycans, as well as viral particles and other receptors that are targeted by viruses. Therefore, Lf may interfere with virus-host cell interactions by acting as a receptor competitor for viruses. Herein we summarize studies in which this competition was investigated with SARS-CoV-2, Zika, Dengue, Hepatitis and Influenza viruses <jats:italic>in vitro</jats:italic>. These studies have demonstrated not only Lf’s competitive properties, but also its potential intracellular impact on host cells, such as enhancing cell survival and reducing infection efficiency by inhibiting certain viral enzymes. In addition, the immunomodulatory effect of Lf is highlighted, as it can influence the activity of specific immune cells and regulate cytokine release, thereby enhancing the host’s response to viral infections. Collectively, these properties promote the potential of Lf as a promising candidate for research in viral infectious diseases.</jats:p>",
"alternative-id": [
"10.3389/fimmu.2024.1402135"
],
"author": [
{
"affiliation": [],
"family": "Eker",
"given": "Furkan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Duman",
"given": "Hatice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ertürk",
"given": "Melih",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karav",
"given": "Sercan",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2024,
11,
15
]
],
"date-time": "2024-11-15T06:18:09Z",
"timestamp": 1731651489000
},
"deposited": {
"date-parts": [
[
2024,
11,
15
]
],
"date-time": "2024-11-15T06:18:14Z",
"timestamp": 1731651494000
},
"indexed": {
"date-parts": [
[
2024,
11,
16
]
],
"date-time": "2024-11-16T05:13:45Z",
"timestamp": 1731734025487,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
11,
15
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
15
]
],
"date-time": "2024-11-15T00:00:00Z",
"timestamp": 1731628800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2024.1402135/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2024,
11,
15
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
15
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1111/j.1365-2516.2006.01194.x",
"article-title": "Emerging viral diseases and infectious disease risks",
"author": "Tapper",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Haemophilia",
"key": "B1",
"volume": "12",
"year": "2006"
},
{
"DOI": "10.1016/j.cnur.2019.02.006",
"article-title": "Emerging infectious diseases",
"author": "McArthur",
"doi-asserted-by": "publisher",
"first-page": "297",
"journal-title": "Nurs Clinics North America",
"key": "B2",
"volume": "54",
"year": "2019"
},
{
"DOI": "10.1007/s00018-005-5368-9",
"article-title": "Molecular structure, binding properties and dynamics of lactoferrin",
"author": "Baker",
"doi-asserted-by": "publisher",
"journal-title": "Cell Mol Life Sci",
"key": "B3",
"volume": "62",
"year": "2005"
},
{
"DOI": "10.1080/10408398.2017.1381583",
"article-title": "Lactoferrin: Structure, function, denaturation and digestion",
"author": "Wang",
"doi-asserted-by": "publisher",
"journal-title": "Crit Rev Food Sci Nutr",
"key": "B4",
"volume": "59",
"year": "2019"
},
{
"DOI": "10.1080/22221751.2021.1888660",
"article-title": "The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor",
"author": "Hu",
"doi-asserted-by": "publisher",
"journal-title": "Emerg Microbes Infect",
"key": "B5",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3390/nu12092562",
"article-title": "Lactoferrin from bovine milk: A protective companion for life",
"author": "Superti",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nutrients",
"key": "B6",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/IJMS18040870",
"article-title": "Studying lactoferrin N-glycosylation",
"author": "Karav",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "B7",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.1080/10942912.2019.1666137",
"article-title": "Lactoferrin (LF): a natural antimicrobial protein",
"author": "Niaz",
"doi-asserted-by": "publisher",
"journal-title": "Int J Food Prop",
"key": "B8",
"volume": "22",
"year": "2019"
},
{
"DOI": "10.1016/j.lfs.2018.01.002",
"article-title": "Multifunctional capacity and therapeutic potential of lactoferrin",
"author": "Moreno-Expósito",
"doi-asserted-by": "publisher",
"journal-title": "Life Sci",
"key": "B9",
"volume": "195",
"year": "2018"
},
{
"DOI": "10.3389/FIMMU.2023.1214514/BIBTEX",
"article-title": "Bovine colostrum and its potential contributions for treatment and prevention of COVID-19",
"author": "Duman",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B10",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/S0166-3542(01)00146-2",
"article-title": "Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts",
"author": "Andersen",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "B11",
"volume": "51",
"year": "2001"
},
{
"DOI": "10.1016/J.ANTIVIRAL.2004.05.002",
"article-title": "Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo",
"author": "Beljaars",
"doi-asserted-by": "publisher",
"first-page": "197",
"journal-title": "Antiviral Res",
"key": "B12",
"volume": "63",
"year": "2004"
},
{
"DOI": "10.3389/FIMMU.2021.685218/BIBTEX",
"article-title": "Lactoferrin retargets human adenoviruses to TLR4 to induce an abortive NLRP3-associated pyroptotic response in human phagocytes",
"author": "Chéneau",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B13",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/J.VIROL.2008.06.017",
"article-title": "Bovine lactoferrin inhibits Japanese encephalitis virus by binding to heparan sulfate and receptor for low density lipoprotein",
"author": "Chien",
"doi-asserted-by": "publisher",
"journal-title": "Virology",
"key": "B14",
"volume": "379",
"year": "2008"
},
{
"DOI": "10.1016/J.ANTIVIRAL.2006.09.002",
"article-title": "Bovine lactoferrin inhibits echovirus endocytic pathway by interacting with viral structural polypeptides",
"author": "Ammendolia",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "B15",
"volume": "73",
"year": "2007"
},
{
"DOI": "10.1080/10408398.2021.1895063",
"article-title": "Lactoferrin: an overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance",
"author": "Sienkiewicz",
"doi-asserted-by": "publisher",
"journal-title": "Crit Rev Food Sci Nutr",
"key": "B16",
"volume": "62",
"year": "2022"
},
{
"DOI": "10.1016/j.jpeds.2016.02.071",
"article-title": "Overview of lactoferrin as a natural immune modulator",
"author": "Legrand",
"doi-asserted-by": "publisher",
"journal-title": "J Pediatr",
"key": "B17",
"volume": "173",
"year": "2016"
},
{
"DOI": "10.3390/ANTIBIOTICS13030251",
"article-title": "Antimicrobial properties of colostrum and milk",
"author": "Eker",
"doi-asserted-by": "publisher",
"journal-title": "Antibiotics",
"key": "B18",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.3389/FNUT.2021.651721",
"article-title": "Bovine colostrum and its potential for human health and nutrition",
"author": "Arslan",
"doi-asserted-by": "publisher",
"journal-title": "Front Nutr",
"key": "B19",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1111/J.1440-1746.2007.04858.X",
"article-title": "Effect of lactoferrin in patients with chronic hepatitis C: Combination therapy with interferon and ribavirin",
"author": "Kaito",
"doi-asserted-by": "publisher",
"journal-title": "J Gastroenterol Hepatol",
"key": "B20",
"volume": "22",
"year": "2007"
},
{
"DOI": "10.3389/fnut.2022.1018336",
"article-title": "Lactoferrin: A glycoprotein that plays an active role in human health",
"author": "Cao",
"doi-asserted-by": "publisher",
"journal-title": "Front Nutr",
"key": "B21",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1016/j.biochi.2008.05.006",
"article-title": "A structural framework for understanding the multifunctional character of lactoferrin",
"author": "Baker",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Biochimie",
"key": "B22",
"volume": "91",
"year": "2009"
},
{
"DOI": "10.1038/srep10729",
"article-title": "The protective effect of lactoferrin on ventral mesencephalon neurons against MPP+ is not connected with its iron binding ability",
"author": "Wang",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "B23",
"volume": "5",
"year": "2015"
},
{
"DOI": "10.1016/j.ijantimicag.2008.07.020",
"article-title": "Lactoferrin: structure, function and applications",
"author": "González-Chávez",
"doi-asserted-by": "publisher",
"journal-title": "Int J Antimicrob Agents",
"key": "B24",
"volume": "33",
"year": "2009"
},
{
"DOI": "10.1080/10408398.2023.2207650",
"article-title": "Lactoferricin, an antimicrobial motif derived from lactoferrin with food preservation potential",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "Crit Rev Food Sci Nutr",
"key": "B25",
"volume": "64",
"year": "2023"
},
{
"DOI": "10.3390/MOLECULES25245763",
"article-title": "Lactoferrin and its derived peptides: an alternative for combating virulence mechanisms developed by pathogens",
"author": "Zarzosa-Moreno",
"doi-asserted-by": "publisher",
"journal-title": "Molecules",
"key": "B26",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.3390/PHARMACEUTICS15041056",
"article-title": "Time to kill and time to heal: the multifaceted role of lactoferrin and lactoferricin in host defense",
"author": "Ohradanova-Repic",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceutics",
"key": "B27",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1139/o11-071",
"article-title": "A structural perspective on lactoferrin function",
"author": "Baker",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Cell Biol",
"key": "B28",
"volume": "90",
"year": "2012"
},
{
"DOI": "10.1038/nchembio.1416",
"article-title": "The role of iron and reactive oxygen species in cell death",
"author": "Dixon",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "Nat Chem Biol",
"key": "B29",
"volume": "10",
"year": "2014"
},
{
"DOI": "10.3390/ijms15011003",
"article-title": "Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: A possible role in protection against oxidative DNA damage",
"author": "Ogasawara",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "B30",
"volume": "15",
"year": "2014"
},
{
"DOI": "10.3390/foods12102073",
"article-title": "Dairy by-products and lactoferrin exert antioxidant and antigenotoxic activity on intestinal and hepatic cells",
"author": "Abad",
"doi-asserted-by": "publisher",
"journal-title": "Foods",
"key": "B31",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1016/j.antiviral.2014.08.012",
"article-title": "The intracellular inhibition of HCV replication represents a novel mechanism of action by the innate immune Lactoferrin protein",
"author": "Picard-Jean",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "Antiviral Res",
"key": "B32",
"volume": "111",
"year": "2014"
},
{
"DOI": "10.1016/j.jiac.2014.08.003",
"article-title": "Lactoferrin for prevention of common viral infections",
"author": "Wakabayashi",
"doi-asserted-by": "publisher",
"journal-title": "J Infection Chemotherapy",
"key": "B33",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1007/s10534-022-00458-6",
"article-title": "Molecular mimicry of the receptor-binding domain of the SARS-CoV-2 spike protein: from the interaction of spike-specific antibodies with transferrin and lactoferrin to the antiviral effects of human recombinant lactoferrin",
"author": "Sokolov",
"doi-asserted-by": "publisher",
"journal-title": "BioMetals",
"key": "B34",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.1016/j.ijbiomac.2023.125552",
"article-title": "Inhibitory effect of lactoferrin-coated zinc nanoparticles on SARS-CoV-2 replication and entry along with improvement of lung fibrosis induced in adult male albino rats",
"author": "El-Fakharany",
"doi-asserted-by": "publisher",
"journal-title": "Int J Biol Macromol",
"key": "B35",
"volume": "245",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28281",
"article-title": "Bovine lactoferrin inhibits SARS-CoV-2 and SARS-CoV-1 by targeting the RdRp complex and alleviates viral infection in the hamster model",
"author": "He",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "B36",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.idairyj.2023.105805",
"article-title": "Bovine lactoferrin suppresses the cathepsin-dependent pathway of SARS-CoV-2 entry in vitro",
"author": "Kobayashi-Sakamoto",
"doi-asserted-by": "publisher",
"first-page": "105805",
"journal-title": "Int Dairy J",
"key": "B37",
"volume": "148",
"year": "2024"
},
{
"DOI": "10.3390/PH16101352",
"article-title": "Inhibition of SARS-coV-2 infection in vero cells by bovine lactoferrin under different iron-saturation states",
"author": "Alves",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceuticals",
"key": "B38",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1002/hep.25501",
"article-title": "Role of low-density lipoprotein receptor in the hepatitis C virus life cycle",
"author": "Albecka",
"doi-asserted-by": "publisher",
"first-page": "998",
"journal-title": "Hepatology",
"key": "B39",
"volume": "55",
"year": "2012"
},
{
"DOI": "10.1099/jgv.0.000849",
"article-title": "Bovine lactoferrin activity against chikungunya and zika viruses",
"author": "Carvalho",
"doi-asserted-by": "publisher",
"journal-title": "J Gen Virol",
"key": "B40",
"volume": "98",
"year": "2017"
},
{
"DOI": "10.1007/s10719-021-10029-5",
"article-title": "Role of sialylated glycans on bovine lactoferrin against influenza virus",
"author": "Wang",
"doi-asserted-by": "publisher",
"journal-title": "Glycoconj J",
"key": "B41",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.3390/v11010051",
"article-title": "Bovine lactoferrin prevents influenza a virus infection by interfering with the fusogenic function of viral hemagglutinin",
"author": "Superti",
"doi-asserted-by": "publisher",
"first-page": "51",
"journal-title": "Viruses",
"key": "B42",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1007/s10534-010-9323-3",
"article-title": "Bovine lactoferrin inhibits Influenza A virus induced programmed cell death in vitro",
"author": "Pietrantoni",
"doi-asserted-by": "publisher",
"first-page": "23",
"journal-title": "BioMetals",
"key": "B43",
"year": "2010"
},
{
"DOI": "10.1179/2047773212Y.0000000004",
"article-title": "Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus",
"author": "Ammendolia",
"doi-asserted-by": "publisher",
"journal-title": "Pathog Glob Health",
"key": "B44",
"volume": "106",
"year": "2012"
},
{
"DOI": "10.1139/o11-072",
"article-title": "Bovine lactoferrin: Involvement of metal saturation and carbohydrates in the inhibition of influenza virus infection",
"author": "Pietrantoni",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Cell Biol",
"key": "B45",
"volume": "90",
"year": "2012"
},
{
"DOI": "10.1186/1472-6882-14-219",
"article-title": "Screening the anti infectivity potentials of native N- and C-lobes derived from the camel lactoferrin against hepatitis C virus",
"author": "Redwan",
"doi-asserted-by": "publisher",
"first-page": "209",
"journal-title": "BMC Complement Altern Med",
"key": "B46",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1186/1743-422X-10-199",
"article-title": "Effectiveness of human, camel, bovine and sheep lactoferrin on the hepatitis C virus cellular infectivity: comparison study",
"author": "El-Fakharany",
"doi-asserted-by": "publisher",
"journal-title": "Virol J",
"key": "B47",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.3390/nu14081561",
"article-title": "Inhibition of in vitro infection of hepatitis B virus by human breastmilk",
"author": "Luo",
"doi-asserted-by": "publisher",
"journal-title": "Nutrients",
"key": "B48",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1002/JMV.23549",
"article-title": "Characterization of the anti-HBV activity of HLP1–23, a human lactoferrin-derived peptide",
"author": "Florian",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "B49",
"volume": "85",
"year": "2013"
},
{
"DOI": "10.7717/PEERJ.17302/SUPP-2",
"article-title": "Effect of iron saturation of bovine lactoferrin on the inhibition of hepatitis B virus in vitro",
"author": "Zhou",
"doi-asserted-by": "publisher",
"journal-title": "PeerJ",
"key": "B50",
"volume": "12",
"year": "2024"
},
{
"DOI": "10.3390/v13050800",
"article-title": "Structural insight into non-enveloped virus binding to glycosaminoglycan receptors: A review",
"author": "Sorin",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B51",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1101/cshperspect.a004952",
"article-title": "Heparan sulfate proteoglycans",
"author": "Sarrazin",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Cold Spring Harb Perspect Biol",
"key": "B52",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.3389/fimmu.2020.01221",
"article-title": "The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria",
"author": "Kell",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B53",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3390/v11070596",
"article-title": "Heparan sulfate proteoglycans and viral attachment: True receptors or adaptation bias",
"author": "Cagno",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B54",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.3389/fnagi.2023.1204149",
"article-title": "Lactoferrin: neuroprotection against Parkinson’s disease and secondary molecule for potential treatment",
"author": "Eker",
"doi-asserted-by": "publisher",
"journal-title": "Front Aging Neurosci",
"key": "B55",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1146/annurev.biochem.74.082803.133354",
"article-title": "Structure and physiologic function of the low-density lipoprotein receptor",
"author": "Jeon",
"doi-asserted-by": "publisher",
"journal-title": "Annu Rev Biochem",
"key": "B56",
"volume": "74",
"year": "2005"
},
{
"DOI": "10.1002/cbin.10845",
"article-title": "Lactoferrin induces tropoelastin expression by activating the lipoprotein receptor-related protein 1-mediated phosphatidylinositol 3-kinase/Akt pathway in human dermal fibroblasts",
"author": "Ryu",
"doi-asserted-by": "publisher",
"journal-title": "Cell Biol Int",
"key": "B57",
"volume": "41",
"year": "2017"
},
{
"DOI": "10.3390/ijms18091957",
"article-title": "Bovine lactoferrin inhibits dengue virus infectivity by interacting with heparan sulfate, low-density lipoprotein receptor, and DC-SIGN",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "B58",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.1128/jvi.02727-13",
"article-title": "Hepatitis C virus stimulates low-density lipoprotein receptor expression to facilitate viral propagation",
"author": "Syed",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B59",
"volume": "88",
"year": "2014"
},
{
"DOI": "10.1016/j.csbj.2021.05.002",
"article-title": "Evidence of a putative glycosaminoglycan binding site on the glycosylated SARS-CoV-2 spike protein N-terminal domain",
"author": "Schuurs",
"doi-asserted-by": "publisher",
"journal-title": "Comput Struct Biotechnol J",
"key": "B60",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1007/s10534-022-00427-z",
"article-title": "An overview on in vitro and in vivo antiviral activity of lactoferrin: its efficacy against SARS-CoV-2 infection",
"author": "Rosa",
"doi-asserted-by": "publisher",
"journal-title": "BioMetals",
"key": "B61",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.1128/JVI.71.8.5997-6002.1997",
"article-title": "Hepatitis C virus envelope proteins bind lactoferrin",
"author": "Yi",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B62",
"volume": "71",
"year": "1997"
},
{
"DOI": "10.1074/jbc.M302267200",
"article-title": "Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate",
"author": "Barth",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Chem",
"key": "B63",
"volume": "278",
"year": "2003"
},
{
"DOI": "10.1038/s41577-021-00558-3",
"article-title": "Natural killer cells in antiviral immunity",
"author": "Björkström",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Immunol",
"key": "B64",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2017.00930",
"article-title": "Developmental and functional control of natural killer cells by cytokines",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B65",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1111/imm.12928",
"article-title": "Defining the role of NK cells during dengue virus infection",
"author": "Mathew",
"doi-asserted-by": "publisher",
"journal-title": "Immunology",
"key": "B66",
"volume": "154",
"year": "2018"
},
{
"DOI": "10.3390/ijms22115799",
"article-title": "The immunological role of the placenta in sars-cov-2 infection—viral transmission, immune regulation, and lactoferrin activity",
"author": "Bukowska-Ośko",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "B67",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1017/S0007114513004315",
"article-title": "Bovine lactoferrin ingestion protects against inflammation via IL-11 induction in the small intestine of mice with hepatitis",
"author": "Kuhara",
"doi-asserted-by": "publisher",
"journal-title": "Br J Nutr",
"key": "B68",
"volume": "111",
"year": "2014"
},
{
"DOI": "10.1016/j.bbrc.2015.10.067",
"article-title": "Lactoferrin acts as an adjuvant during influenza vaccination of neonatal mice",
"author": "Sherman",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Biophys Res Commun",
"key": "B69",
"volume": "467",
"year": "2015"
},
{
"DOI": "10.1016/j.ctim.2012.12.006",
"article-title": "The clinical efficacy of a bovine lactoferrin/whey protein Ig-rich fraction (Lf/IgF) for the common cold: A double blind randomized study",
"author": "Vitetta",
"doi-asserted-by": "publisher",
"journal-title": "Complement Ther Med",
"key": "B70",
"volume": "21",
"year": "2013"
},
{
"DOI": "10.3390/nu13020328",
"article-title": "Protective effects of lactoferrin against sars-cov-2 infection in vitro",
"author": "Salaris",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nutrients",
"key": "B71",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/FOODS12224068/S1",
"article-title": "Optimum fermentation conditions for bovine lactoferricin-lactoferrampin-encoding limosiLactobacillus reuteri and regulation of intestinal inflammation",
"author": "Xie",
"doi-asserted-by": "publisher",
"journal-title": "Foods",
"key": "B72",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1017/S0007114519000485",
"article-title": "Porcine lactoferrin-derived peptide LFP-20 modulates immune homoeostasis to defend lipopolysaccharide-triggered intestinal inflammation in mice",
"author": "Zong",
"doi-asserted-by": "publisher",
"journal-title": "Br J Nutr",
"key": "B73",
"volume": "121",
"year": "2019"
},
{
"DOI": "10.1016/J.BCP.2016.01.009",
"article-title": "Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response",
"author": "Zong",
"doi-asserted-by": "publisher",
"first-page": "74",
"journal-title": "Biochem Pharmacol",
"key": "B74",
"volume": "104",
"year": "2016"
},
{
"DOI": "10.1038/s41591-021-01282-0",
"article-title": "Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases",
"author": "Meganck",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B75",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/j.idc.2019.09.001",
"article-title": "Emerging and reemerging infectious diseases: global overview",
"author": "Zumla",
"doi-asserted-by": "publisher",
"journal-title": "Infect Dis Clin North Am",
"key": "B76",
"volume": "33",
"year": "2019"
},
{
"DOI": "10.1038/s41579-021-00639-z",
"article-title": "Infectious disease in an era of global change",
"author": "Baker",
"doi-asserted-by": "publisher",
"first-page": "193",
"journal-title": "Nat Rev Microbiol",
"key": "B77",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1016/j.bbadis.2020.165878",
"article-title": "Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach",
"author": "Naqvi",
"doi-asserted-by": "publisher",
"journal-title": "Biochim Biophys Acta Mol Basis Dis",
"key": "B78",
"volume": "1866",
"year": "2020"
},
{
"DOI": "10.1038/s41579-022-00841-7",
"article-title": "SARS-CoV-2 variant biology: immune escape, transmission and fitness",
"author": "Carabelli",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Microbiol",
"key": "B79",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1038/s41586-022-05522-2",
"article-title": "The WHO estimates of excess mortality associated with the COVID-19 pandemic",
"author": "Msemburi",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B80",
"volume": "613",
"year": "2023"
},
{
"DOI": "10.3390/pharmaceutics14102111",
"article-title": "Lactoferrin binding to SARS-coV-2 spike glycoprotein blocks pseudoviral entry and relieves iron protein dysregulation in several in vitro models",
"author": "Cutone",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceutics",
"key": "B81",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.isci.2022.104136",
"article-title": "Identified human breast milk compositions effectively inhibit SARS-coV-2 and variants infection and replication",
"author": "Lai",
"doi-asserted-by": "publisher",
"journal-title": "iScience",
"key": "B82",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2021.1932607",
"article-title": "Hypoxia reduces cell attachment of SARS-coV-2 spike protein by modulating the expression of ACE2, neuropilin-1, syndecan-1 and cellular heparan",
"author": "Prieto-Fernández",
"doi-asserted-by": "publisher",
"journal-title": "Emerg Microbes Infections",
"key": "B83",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/J.LWT.2021.112684",
"article-title": "Identification of lactoferrin-derived peptides as potential inhibitors against the main protease of SARS-coV-2",
"author": "Zhao",
"doi-asserted-by": "publisher",
"journal-title": "LWT",
"key": "B84",
"volume": "154",
"year": "2022"
},
{
"DOI": "10.3390/PH17081021",
"article-title": "Lactoferrin binds through its N-terminus to the receptor-binding domain of the SARS-coV-2 spike protein",
"author": "Babulic",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceuticals",
"key": "B85",
"volume": "17",
"year": "2024"
},
{
"DOI": "10.3389/FIMMU.2022.958581/BIBTEX",
"article-title": "Blockade of TMPRSS2-mediated priming of SARS-coV-2 by lactoferricin",
"author": "Ohradanova-Repic",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B86",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/fnut.2022.992733",
"article-title": "Lactoferrin for COVID- prevention, treatment, and recovery",
"author": "Bolat",
"doi-asserted-by": "publisher",
"journal-title": "Fronti Nutr",
"key": "B87",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.3390/ijerph182010985",
"article-title": "Lactoferrin as antiviral treatment in COVID-19 management: Preliminary evidence",
"author": "Campione",
"doi-asserted-by": "publisher",
"journal-title": "Int J Environ Res Public Health",
"key": "B88",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.3390/v14061226",
"article-title": "Mosquito-borne flaviviruses and current therapeutic advances",
"author": "Qian",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B89",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1002/9781119350927.CH33",
"article-title": "Flaviviruses",
"author": "Williams",
"doi-asserted-by": "publisher",
"journal-title": "Dis Swine",
"key": "B90",
"year": "2019"
},
{
"DOI": "10.3390/ph10020044",
"article-title": "Pathogenesis and inhibition of flaviviruses from a carbohydrate perspective",
"author": "Kim",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceuticals",
"key": "B91",
"volume": "10",
"year": "2017"
},
{
"DOI": "10.3390/ph16101385",
"article-title": "Heparin precursors with reduced anticoagulant properties retain antiviral and protective effects that potentiate the efficacy of sofosbuvir against zika virus infection in human neural progenitor cells",
"author": "Pagani",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceuticals",
"key": "B92",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.3390/v15040860",
"article-title": "A perspective on current flavivirus vaccine development: A brief review",
"author": "Dutta",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B93",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.3390/v13101967",
"article-title": "Dengue virus infection: A tale of viral exploitations and host responses",
"author": "Nanaware",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B94",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/pathogens9110935",
"article-title": "Molecular changes in dengue envelope protein domain III upon interaction with glycosaminoglycans",
"author": "Hyatt",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Pathogens",
"key": "B95",
"volume": "9",
"year": "2020"
},
{
"key": "B96",
"unstructured": "Inflammatory mediators in dengue virus infection in children: interleukin-8 and its relationship to neutrophil degranulation\n \n \n \n Juffrie\n M\n \n \n van der Meer\n GM\n \n \n Hack\n CE\n \n \n Haasnoot\n K\n \n \n P Veerman\n AJ\n \n \n Thijs\n LG\n \n \n \n 2000"
},
{
"DOI": "10.1186/s12879-016-1759-9",
"article-title": "Serum neutrophil gelatinase-associated lipocalin and resistin are associated with dengue infection in adults",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "441",
"journal-title": "BMC Infect Dis",
"key": "B97",
"volume": "16",
"year": "2016"
},
{
"DOI": "10.3390/molecules25081997",
"article-title": "Viral hepatitis and iron dysregulation: Molecular pathways and the role of lactoferrin",
"author": "Mancinelli",
"doi-asserted-by": "publisher",
"journal-title": "Molecules",
"key": "B98",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.3748/wjg.v26.i38.5759",
"article-title": "Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma",
"author": "D’souza",
"doi-asserted-by": "publisher",
"journal-title": "World J Gastroenterol",
"key": "B99",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.01.023",
"article-title": "Breast milk microbiota: A review of the factors that influence composition",
"author": "Zimmermann",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "J Infection",
"key": "B100",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.3389/FNUT.2022.1033779/BIBTEX",
"article-title": "Role of milk glycome in prevention, treatment, and recovery of COVID-19",
"author": "Kaplan",
"doi-asserted-by": "publisher",
"journal-title": "Front Nutr",
"key": "B101",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/J.JFF.2019.103485",
"article-title": "N-glycans from human milk glycoproteins are selectively released by an infant gut symbiont in vivo",
"author": "Karav",
"doi-asserted-by": "publisher",
"journal-title": "J Funct Foods",
"key": "B102",
"volume": "61",
"year": "2019"
},
{
"DOI": "10.1128/AEM.00547-16/ASSET/8DFAD725-4DE7-4159-AA5C-9DAEB7283E69/ASSETS/GRAPHIC/ZAM9991171820005.JPEG",
"article-title": "Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria",
"author": "Karav",
"doi-asserted-by": "publisher",
"journal-title": "Appl Environ Microbiol",
"key": "B103",
"volume": "82",
"year": "2016"
},
{
"DOI": "10.3748/wjg.v27.i16.1691",
"article-title": "From hepatitis A to E: A critical review of viral hepatitis",
"author": "Castaneda",
"doi-asserted-by": "publisher",
"journal-title": "World J Gastroenterol",
"key": "B104",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1080/14789450.2017.1344102",
"article-title": "Hepatitis C virus–apolipoprotein interactions: molecular mechanisms and clinical impact",
"author": "Crouchet",
"doi-asserted-by": "publisher",
"first-page": "593",
"journal-title": "Expert Rev Proteomics",
"key": "B105",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1007/S10989-019-09972-7/FIGURES/6",
"article-title": "A Novel chimeric anti-HCV peptide derived from camel lactoferrin and molecular level insight on its interaction with E2",
"author": "Tahmoorespur",
"doi-asserted-by": "publisher",
"journal-title": "Int J Pept Res Ther",
"key": "B106",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.22103/JAB.2022.18992.1382",
"article-title": "Comparison of antiviral effect of camel lactoferrin peptide (CLF36) and new generation drugs against hepatitis C virus",
"doi-asserted-by": "publisher",
"journal-title": "Agric Biotechnol J",
"key": "B107",
"volume": "14",
"year": "2022"
}
],
"reference-count": 107,
"references-count": 107,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2024.1402135/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The potential of lactoferrin as antiviral and immune-modulating agent in viral infectious diseases",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "15"
}