Safety and efficacy of favipiravir in COVID-19 patients with pneumonia. A randomized, double-blind, placebo-controlled study (FAVID)
et al., Pneumonia, doi:10.1186/s41479-023-00124-6, FAVID, EudraCT2020-002753-22, Aug 2023 (preprint)
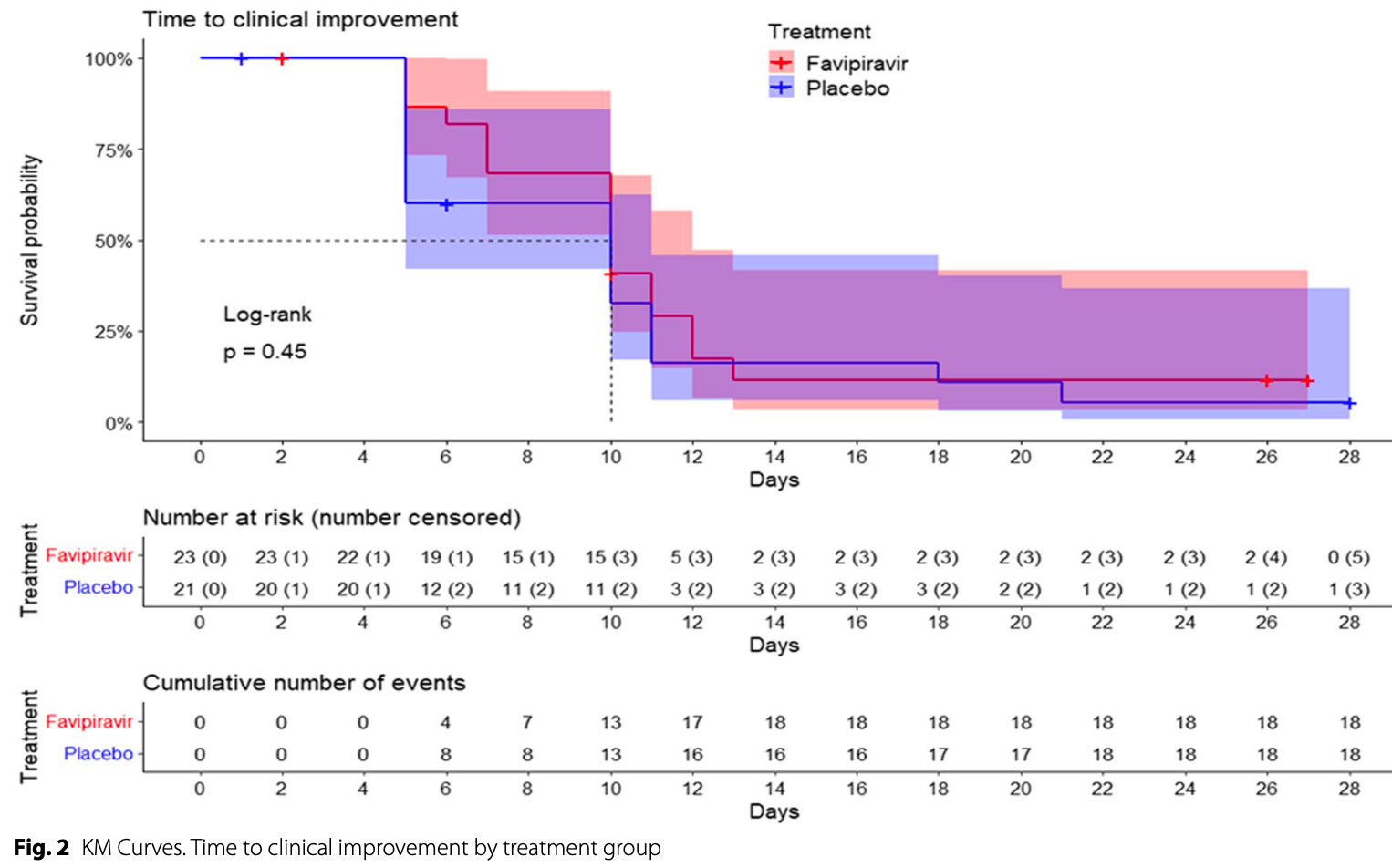
Underpowered RCT with 44 hospitalized patients in Spain, showing no significant difference with favipiravir treatment in the primary outcome of time to clinical improvement, or in the secondary efficacy outcomes. Adverse events were more frequent in the favipiravir group (68%) compared to placebo (32%), but most were mild.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 382.6% higher, RR 4.83, p = 0.49, treatment 2 of 23 (8.7%), control 0 of 21 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 28.
|
|
risk of mechanical ventilation, 37.0% higher, RR 1.37, p = 1.00, treatment 3 of 23 (13.0%), control 2 of 21 (9.5%), day 28.
|
|
time to improvement, no change, relative time 1.00, p = 0.45, treatment 23, control 21.
|
|
dischage or NEWS <3, 16.7% lower, relative time 0.83, p = 0.64, treatment 23, control 21.
|
|
time to viral-, 125.0% higher, relative time 2.25, p = 0.51, treatment 23, control 21.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Horcajada et al., 24 Aug 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, 30 authors, study period November 2020 - October 2021, trial EudraCT2020-002753-22 (FAVID).
Safety and efficacy of favipiravir in COVID-19 patients with pneumonia. A randomized, double-blind, placebo-controlled study (FAVID)
Pneumonia, doi:10.1186/s41479-023-00124-6
Purpose To design a randomized clinical trial to assess the efficacy and safety of favipiravir in patients with COVID-19 disease with pneumonia. Methods A randomized, double blind, placebo-controlled clinical trial of favipiravir in patients with COVID-19 pneumonia was conducted in three Spanish sites. Randomization 1:1 to favipiravir or placebo (in both groups added to the Standard of Care) was performed to treat the patients with COVID-19 pneumonia. The primary endpoint was "time to clinical improvement, " measured as an improvement for ≥ two categories on a 7-point WHO ordinal scale in an up to 28 days' time frame.
Results Forty-four patients were randomized (23 in the favipiravir group and 21 in the placebo group). The median time to clinical improvement was not different between the favipiravir and the placebo arms (10 days for both groups) and none of the secondary endpoints showed significant differences between arms. The proportion of adverse events (both serious and non-serious) was statistically different between the favipiravir group (68.29%) and the placebo group (31.7%) (p = 0.019), but there was insufficient statistical evidence to correlate the degree of severity of the events with the treatment group. Conclusions Favipiravir administered for ten days to patients with COVID-19 and pneumonia did not improve outcomes compared with placebo. Although this is an underpowered negative study, efficacy results align with other randomized trials. However, in the present study, the non-serious adverse events were more frequent in the favipiravir group.
Authors' contributions Conceptualisation: JPH, TAQ, AF, RA and CT. Data collection: MR, SCE, ES, JGJ, ILM, SGZ, SB and MDT. Data quality: RA. Formal analyses: RA. Project administration: AF, RA and CT. Evidence synthesis: JPH, TAQ, RA and CT. Writing-original draft: JPH, TAQ, RA and CT. Funding acquisition: AF, RA and CT. Writing-review and editing: JPH, TAQ, RA and CT. All authors have read and agreed to the published version of the manuscript.
Declarations Ethics approval and consent to participate The competing Ethics Committee of Hospital del Mar, Barcelona, Spain, approved the study protocol on 31 st of July 2020.
Competing interests JPH has received consulting fees from Gilead, Menarini and TFF Pharmaceuticals, and participated in educational activities from MSD, Pfizer and Angelini. All other authors have no conflicts of interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Acanfora, Acanfora, Ciccone, Scicchitano, Bortone et al., The cross-talk between thrombosis and inflammatory storm in acute and long-COVID-19: therapeutic targets and clinical cases, Viruses, doi:10.3390/v13101904
Alqahtani, Kumar, Aljawder, Abdulrahman, Mohamed et al., Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease, Sci Rep, doi:10.1038/s41598-022-08794-w
Bai, Mu, Kargb, Song, Niu et al., Clinical and Virological Characteristics of Ebola Virus Disease Patients Treated With Favipiravir (T-705)-Sierra Leone, Clin Infect Dis
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bosaeed, Alharbi, Alrehily, Bahlaq, Gaifer, Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.12.026
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering, doi:10.1016/j.eng.2020.03.007
Chuah, Chow, Hor, Cheng, Ker et al., Efficacy of Early Treatment With Favipiravir on Disease Progression Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19): A Randomized. Open-Label Clinical Trial, Clin Infect Dis, doi:10.1093/cid/ciab962
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res, doi:10.1016/j.antiviral.2013.09.015
Gowen, Wong, Jung, Sanders, Mendenhall et al., In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections, Antimicrob Agents Chemother
Gök, Bahçecioğlu, Durmuş, Gün, Ersoy et al., The safety profile of favipiravir in COVID-19 patients with severe renal impairment, Int J Clin Pract, doi:10.1111/ijcp.14938
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep, doi:10.1038/s41598-021-90551-6
Horby, Lim, Emberson, Mafham, Bell et al., Dexametasona en pacientes hospitalizados con Covid-19, doi:10.1056/NEJMoa2021436
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, Int J Infect Dis, doi:10.1016/j.ijid.2020.10.069
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis, doi:10.1186/s12879-021-06164-x
Marconi, Ramanan, De Bono, Kartman, Krishnan et al., Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00331-3
Mcelvaney, Nalbandian, Sehgal, Gupta, Madhavan et al., Post-acute COVID-19 syndrome, Nat Med, doi:10.1038/s41591-021-01283-z
Mcmahon, Lau, Coldham, Roney, Hagenauer et al., Favipiravir in early symptomatic COVID-19, a randomised placebocontrolled trial, EClinicalMedicine, doi:10.1016/j.eclinm.2022.101703
Meyerowitz, Richterman, Gandhi, Transmission of SARS-CoV-2: a review of viral host, and environmental factors, Ann Intern Med
Nalbandian, Sehgal, Gupta, Madhavan, Mcgroder et al., Post-acute COVID-19 syndrome, Nat Med, doi:10.1038/s41591-021-01283-z
Oestereich, Lüdtke, Wur, Rieger, Rieger et al., Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model, Antiviral Res
Ozsurekci, Oygar, Gürlevik, Kesici, Ozen et al., Favipiravir use in children with COVID-19 and acute kidney injury: is it safe?, Pediatr Nephrol, doi:10.1007/s00467-021-05111-x
Peiffer-Smadja, Rebeaud, Guihur, Mahamat-Saleh, Fiolet, Hydroxychloroquine and COVID-19: a tale of populism and obscurantism, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30866-5
Schumaker, Bhimraj, Pharmacologic treatment and management of Coronavirus Disease 2019, Infect Dis Clin North Am, doi:10.1016/j.idc.2022.02.001
Shah, Orton, Grinsztejn, Donaldson, Ramírez et al., Favipiravir in patients hospitalised with COVID-19 (PIO-NEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care, Lancet Respir Med, doi:10.1016/S2213-2600(22)00412-X
Shankar-Hari, Vale, Godolphin, Fisher, Higgins et al., WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis, JAMA, doi:10.1001/jama.2021.11330
Shinkai, Tsushima, Tanaka, Hagiwara, Tarumoto et al., Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial, Infect Dis Ther, doi:10.1007/s40121-021-00517-4
Sirijatuphat, Manosuthi, Niyomnaitham, Owen, Copeland et al., Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study, Emerg Microbes Infect, doi:10.1080/22221751.2022.2117092
Sussman, Golberstein, Polosa, Aerial Transmission of the SARS-CoV-2 Virus through Environmental E-Cigarette Aerosols: Implications for Public Policies, Int J Environ Res Public Health, doi:10.3390/ijerph18041437
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Ueda, Tanimoto, Murayama, Ozaki, Kami, Japan's drug regulation during the COVID-19 Pandemic: lessons from a case study of Favipiravir, Clin Pharmacol Ther, doi:10.1002/cpt.2251
Wang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
DOI record:
{
"DOI": "10.1186/s41479-023-00124-6",
"ISSN": [
"2200-6133"
],
"URL": "http://dx.doi.org/10.1186/s41479-023-00124-6",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Purpose</jats:title>\n <jats:p>To design a randomized clinical trial to assess the efficacy and safety of favipiravir in patients with COVID-19 disease with pneumonia.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A randomized, double blind, placebo-controlled clinical trial of favipiravir in patients with COVID-19 pneumonia was conducted in three Spanish sites. Randomization 1:1 to favipiravir or placebo (in both groups added to the Standard of Care) was performed to treat the patients with COVID-19 pneumonia. The primary endpoint was “time to clinical improvement,” measured as an improvement for ≥ two categories on a 7-point WHO ordinal scale in an up to 28 days' time frame.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Forty-four patients were randomized (23 in the favipiravir group and 21 in the placebo group). The median time to clinical improvement was not different between the favipiravir and the placebo arms (10 days for both groups) and none of the secondary endpoints showed significant differences between arms.</jats:p>\n <jats:p>The proportion of adverse events (both serious and non-serious) was statistically different between the favipiravir group (68.29%) and the placebo group (31.7%) (<jats:italic>p</jats:italic> = 0.019), but there was insufficient statistical evidence to correlate the degree of severity of the events with the treatment group.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Favipiravir administered for ten days to patients with COVID-19 and pneumonia did not improve outcomes compared with placebo. Although this is an underpowered negative study, efficacy results align with other randomized trials. However, in the present study, the non-serious adverse events were more frequent in the favipiravir group.</jats:p>\n </jats:sec>",
"alternative-id": [
"124"
],
"article-number": "3",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "20 August 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "11 December 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "25 February 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The competing Ethics Committee of Hospital del Mar, Barcelona, Spain, approved the study protocol on 31<sup>st</sup> of July 2020."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "JPH has received consulting fees from Gilead, Menarini and TFF Pharmaceuticals, and participated in educational activities from MSD, Pfizer and Angelini. All other authors have no conflicts of interests."
}
],
"author": [
{
"affiliation": [],
"family": "Horcajada",
"given": "Juan P.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Aldonza",
"given": "Rebeca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Real",
"given": "Mónica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castañeda-Espinosa",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sendra",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomez-Junyent",
"given": "Joan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López-Montesinos",
"given": "Inmaculada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Zorrilla",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Briansó",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duran-Taberna",
"given": "Montserrat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández",
"given": "Andrés",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tarragó",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auguet-Quintillá",
"given": "Teresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arenas-Miras",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arrieta‐Aldea",
"given": "Itziar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cañas-Ruano",
"given": "Esperanza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Güerri‐Fernandez",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knobel",
"given": "Hernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montero",
"given": "Maria Milagro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pelegrín",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez‐Martínez",
"given": "Francisca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sorlí",
"given": "Luisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villar‐García",
"given": "Judith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alibalic",
"given": "Ajla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camaron",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Febrer",
"given": "Anna Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bertran",
"given": "Laia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barrientos",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the COVID-MAR Research group",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the COVID-HJ23 group",
"sequence": "additional"
}
],
"container-title": "Pneumonia",
"container-title-short": "Pneumonia",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
2,
25
]
],
"date-time": "2024-02-25T01:02:11Z",
"timestamp": 1708822931000
},
"deposited": {
"date-parts": [
[
2024,
2,
25
]
],
"date-time": "2024-02-25T01:02:36Z",
"timestamp": 1708822956000
},
"funder": [
{
"name": "Ferrer Internacional, S.A."
}
],
"indexed": {
"date-parts": [
[
2024,
2,
25
]
],
"date-time": "2024-02-25T01:41:18Z",
"timestamp": 1708825278942
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
2,
25
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
25
]
],
"date-time": "2024-02-25T00:00:00Z",
"timestamp": 1708819200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
25
]
],
"date-time": "2024-02-25T00:00:00Z",
"timestamp": 1708819200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s41479-023-00124-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s41479-023-00124-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s41479-023-00124-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
2,
25
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
25
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.7326/M20-5008",
"author": "EA Meyerowitz",
"doi-asserted-by": "publisher",
"first-page": "69",
"journal-title": "Ann Intern Med",
"key": "124_CR1",
"unstructured": "Meyerowitz EA, Richterman A, Gandhi RT, et al. Transmission of SARS-CoV-2: a review of viral host, and environmental factors. Ann Intern Med. 2021;174:69–79.",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.3390/ijerph18041437",
"author": "RA Sussman",
"doi-asserted-by": "publisher",
"first-page": "1437",
"issue": "4",
"journal-title": "Int J Environ Res Public Health",
"key": "124_CR2",
"unstructured": "Sussman RA, Golberstein E, Polosa R. Aerial Transmission of the SARS-CoV-2 Virus through Environmental E-Cigarette Aerosols: Implications for Public Policies. Int J Environ Res Public Health. 2021;18(4):1437. https://doi.org/10.3390/ijerph18041437.",
"volume": "18",
"year": "2021"
},
{
"key": "124_CR3",
"unstructured": "https://www.worldometers.info/coronavirus/. Accessed 10 Nov 2022."
},
{
"DOI": "10.1016/S1473-3099(20)30866-5",
"author": "N Peiffer-Smadja",
"doi-asserted-by": "publisher",
"first-page": "e121",
"issue": "5",
"journal-title": "Lancet Infect Dis",
"key": "124_CR4",
"unstructured": "Peiffer-Smadja N, Rebeaud ME, Guihur A, Mahamat-Saleh Y, Fiolet T. Hydroxychloroquine and COVID-19: a tale of populism and obscurantism. Lancet Infect Dis. 2021;21(5):e121. https://doi.org/10.1016/S1473-3099(20)30866-5.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "124_CR5",
"unstructured": "Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group. Dexametasona en pacientes hospitalizados con Covid-19. 2021;384(8):693-704. https://doi.org/10.1056/NEJMoa2021436."
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"author": "VC Marconi",
"doi-asserted-by": "publisher",
"first-page": "1407",
"issue": "12",
"journal-title": "Lancet Respir Med.",
"key": "124_CR6",
"unstructured": "Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407–18. https://doi.org/10.1016/S2213-2600(21)00331-3.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.11330",
"author": "M Shankar-Hari",
"doi-asserted-by": "publisher",
"first-page": "499",
"issue": "6",
"journal-title": "JAMA",
"key": "124_CR7",
"unstructured": "Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA. 2021;326(6):499–518. https://doi.org/10.1001/jama.2021.11330.",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"author": "JH Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med.",
"key": "124_CR8",
"unstructured": "Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, ACTT-1 Study Group Members, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813–26. https://doi.org/10.1056/NEJMoa2007764.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.idc.2022.02.001",
"author": "AH Schumaker",
"doi-asserted-by": "publisher",
"first-page": "349",
"issue": "2",
"journal-title": "Infect Dis Clin North Am",
"key": "124_CR9",
"unstructured": "Schumaker AH, Bhimraj A. Pharmacologic treatment and management of Coronavirus Disease 2019. Infect Dis Clin North Am. 2022;36(2):349–64. https://doi.org/10.1016/j.idc.2022.02.001.",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"author": "Y Furuta",
"doi-asserted-by": "publisher",
"first-page": "446",
"issue": "2",
"journal-title": "Antiviral Res.",
"key": "124_CR10",
"unstructured": "Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–54. https://doi.org/10.1016/j.antiviral.2013.09.015.",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1016/j.antiviral.2014.02.014",
"author": "L Oestereich",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "Antiviral Res.",
"key": "124_CR11",
"unstructured": "Oestereich L, Lüdtke A, Wur RS, Rieger T, Rieger T, Muñoz-Fontela C, et al. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–21.",
"volume": "105",
"year": "2014"
},
{
"DOI": "10.1093/cid/ciw571",
"author": "CQ Bai",
"doi-asserted-by": "publisher",
"first-page": "1288",
"issue": "10",
"journal-title": "Clin Infect Dis",
"key": "124_CR12",
"unstructured": "Bai CQ, Mu JS, Kargb OD, Song YB, Niu WK, Nie WM, et al. Clinical and Virological Characteristics of Ebola Virus Disease Patients Treated With Favipiravir (T-705)-Sierra Leone, 2014. Clin Infect Dis. 2016;63(10):1288–94.",
"volume": "63",
"year": "2016"
},
{
"DOI": "10.1128/AAC.00356-07",
"author": "BB Gowen",
"doi-asserted-by": "publisher",
"first-page": "3168",
"journal-title": "Antimicrob Agents Chemother",
"key": "124_CR13",
"unstructured": "Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, et al. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007;51:3168–76 PLoSNegl Trop Dis. 2011;5:e1342.",
"volume": "51",
"year": "2007"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"author": "M Wang",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Cell Res",
"key": "124_CR14",
"unstructured": "Wang M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"author": "Q Cai",
"doi-asserted-by": "publisher",
"first-page": "1192",
"issue": "10",
"journal-title": "Engineering (Beijing)",
"key": "124_CR15",
"unstructured": "Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing). 2020;6(10):1192–8. https://doi.org/10.1016/j.eng.2020.03.007.",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"author": "ZF Udwadia",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "124_CR16",
"unstructured": "Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, Pendse A, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71.",
"volume": "103",
"year": "2021"
},
{
"key": "124_CR17",
"unstructured": "https://www.england.nhs.uk/ourwork/clinical-policy/sepsis/nationalearlywarningscore/. Accessed 10 Nov 2022."
},
{
"DOI": "10.1016/j.eclinm.2022.101703",
"author": "JH McMahon",
"doi-asserted-by": "publisher",
"first-page": "101703",
"issue": "54",
"journal-title": "EClinicalMedicine",
"key": "124_CR18",
"unstructured": "McMahon JH, Lau JSY, Coldham A, Roney J, Hagenauer M, Price S, et al. Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial. EClinicalMedicine. 2022;20(54):101703. https://doi.org/10.1016/j.eclinm.2022.101703.",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2021.12.026",
"author": "M Bosaeed",
"doi-asserted-by": "publisher",
"first-page": "602",
"issue": "4",
"journal-title": "Clin Microbiol Infect",
"key": "124_CR19",
"unstructured": "Bosaeed M, Alharbi A, Mahmoud E, Alrehily S, Bahlaq M, Gaifer Z, et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect. 2022;28(4):602–8. https://doi.org/10.1016/j.cmi.2021.12.026.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciab962",
"author": "CH Chuah",
"doi-asserted-by": "publisher",
"first-page": "e432",
"issue": "1",
"journal-title": "Open-Label Clinical Trial. Clin Infect Dis.",
"key": "124_CR20",
"unstructured": "Chuah CH, Chow TS, Hor CP, Cheng JT, Ker HB, Lee HG, Malaysian Favipiravir Study Group, et al. Efficacy of Early Treatment With Favipiravir on Disease Progression Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19): A Randomized. Open-Label Clinical Trial Clin Infect Dis. 2022;75(1):e432–9. https://doi.org/10.1093/cid/ciab962. PMID: 34849615.",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-08794-w",
"author": "M AlQahtani",
"doi-asserted-by": "publisher",
"first-page": "4925",
"issue": "1",
"journal-title": "Sci Rep",
"key": "124_CR21",
"unstructured": "AlQahtani M, Kumar N, Aljawder D, Abdulrahman A, Mohamed MW, Alnashaba F, et al. Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease. Sci Rep. 2022;12(1):4925. https://doi.org/10.1038/s41598-022-08794-w. (Erratum in: Sci Rep. 2022 Sep 26;12(1):16052).",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"author": "M Shinkai",
"doi-asserted-by": "publisher",
"first-page": "2489",
"issue": "4",
"journal-title": "Infect Dis Ther",
"key": "124_CR22",
"unstructured": "Shinkai M, Tsushima K, Tanaka S, Hagiwara E, Tarumoto N, Kawada I, et al. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial. Infect Dis Ther. 2021;10(4):2489–509. https://doi.org/10.1007/s40121-021-00517-4.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2022.2117092",
"author": "R Sirijatuphat",
"doi-asserted-by": "publisher",
"first-page": "2197",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "124_CR23",
"unstructured": "Sirijatuphat R, Manosuthi W, Niyomnaitham S, Owen A, Copeland KK, Charoenpong L, et al. Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study. Emerg Microbes Infect. 2022;11(1):2197–206. https://doi.org/10.1080/22221751.2022.2117092.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00412-X",
"doi-asserted-by": "publisher",
"key": "124_CR24",
"unstructured": "Shah PL, Orton CM, Grinsztejn B, Donaldson GC, Crabtree Ramírez B, Tonkin J, Santos BR, Cardoso SW, Ritchie AI, Conway F, Riberio MPD, Wiseman DJ, Tana A, Vijayakumar B, Caneja C, Leaper C, Mann B, Samson A, Bhavsar PK, Boffito M, Johnson MR, Pozniak A, Pelly M; PIONEER trial group. Favipiravir in patients hospitalised with COVID-19 (PIONEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care. Lancet Respir Med. 2022;S2213-2600(22)00412-X. https://doi.org/10.1016/S2213-2600(22)00412-X."
},
{
"DOI": "10.1038/s41598-021-90551-6",
"author": "S Hassanipour",
"doi-asserted-by": "publisher",
"first-page": "11022",
"issue": "1",
"journal-title": "Sci Rep",
"key": "124_CR25",
"unstructured": "Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11(1):11022. https://doi.org/10.1038/s41598-021-90551-6.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06164-x",
"author": "T Manabe",
"doi-asserted-by": "publisher",
"first-page": "489",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "124_CR26",
"unstructured": "Manabe T, Kambayashi D, Akatsu H, Kudo K. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):489. https://doi.org/10.1186/s12879-021-06164-x.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1007/s00467-021-05111-x",
"author": "Y Ozsurekci",
"doi-asserted-by": "publisher",
"first-page": "3771",
"issue": "11",
"journal-title": "Pediatr Nephrol",
"key": "124_CR27",
"unstructured": "Ozsurekci Y, Oygar PD, Gürlevik SL, Kesici S, Ozen S, Kurt Sukur ED, Gülhan B, Topaloglu R, Bayrakci B, Cengiz AB. Favipiravir use in children with COVID-19 and acute kidney injury: is it safe? Pediatr Nephrol. 2021;36(11):3771–6. https://doi.org/10.1007/s00467-021-05111-x.",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1111/ijcp.14938",
"author": "S Gök",
"doi-asserted-by": "publisher",
"first-page": "e14938",
"issue": "12",
"journal-title": "Int J Clin Pract",
"key": "124_CR28",
"unstructured": "Gök S, Bahçecioğlu ÖF, Durmuş M, Gün ZÜ, Ersoy Y, Aytemur ZA, Ulutaş Ö. The safety profile of favipiravir in COVID-19 patients with severe renal impairment. Int J Clin Pract. 2021;75(12):e14938. https://doi.org/10.1111/ijcp.14938.",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"author": "OJ McElvaney",
"doi-asserted-by": "publisher",
"first-page": "601",
"issue": "4",
"journal-title": "Nat Med",
"key": "124_CR29",
"unstructured": "McElvaney OJ, Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15. https://doi.org/10.1038/s41591-021-01283-z.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.3390/v13101904",
"author": "D Acanfora",
"doi-asserted-by": "publisher",
"first-page": "1904",
"issue": "10",
"journal-title": "Viruses.",
"key": "124_CR30",
"unstructured": "Acanfora D, Acanfora C, Ciccone MM, Scicchitano P, Bortone AS, Uguccioni M, et al. The cross-talk between thrombosis and inflammatory storm in acute and long-COVID-19: therapeutic targets and clinical cases. Viruses. 2021;13(10):1904. https://doi.org/10.3390/v13101904.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"author": "A Nalbandian",
"doi-asserted-by": "publisher",
"first-page": "601",
"issue": "4",
"journal-title": "Nat Med",
"key": "124_CR31",
"unstructured": "Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15. https://doi.org/10.1038/s41591-021-01283-z.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"author": "S Joshi",
"doi-asserted-by": "publisher",
"first-page": "501",
"journal-title": "Int J Infect Dis",
"key": "124_CR32",
"unstructured": "Joshi S, Parkar J, Ansari A, Vora A, Talwar D, Tiwaskar M, et al. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501–8. https://doi.org/10.1016/j.ijid.2020.10.069.",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2251",
"doi-asserted-by": "publisher",
"key": "124_CR33",
"unstructured": "Ueda M, Tanimoto T, Murayama A, Ozaki A, Kami M. Japan’s drug regulation during the COVID-19 Pandemic: lessons from a case study of Favipiravir. Clin Pharmacol Ther. 2022;111(3):545–7. https://doi.org/10.1002/cpt.2251."
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://pneumonia.biomedcentral.com/articles/10.1186/s41479-023-00124-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Earth and Planetary Sciences",
"General Environmental Science"
],
"subtitle": [],
"title": "Safety and efficacy of favipiravir in COVID-19 patients with pneumonia. A randomized, double-blind, placebo-controlled study (FAVID)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "16"
}