Hetero Announces Interim Clinical Results from Phase III Clinical Trials of Molnupiravir conducted in India
, Press Release, Jul 2021
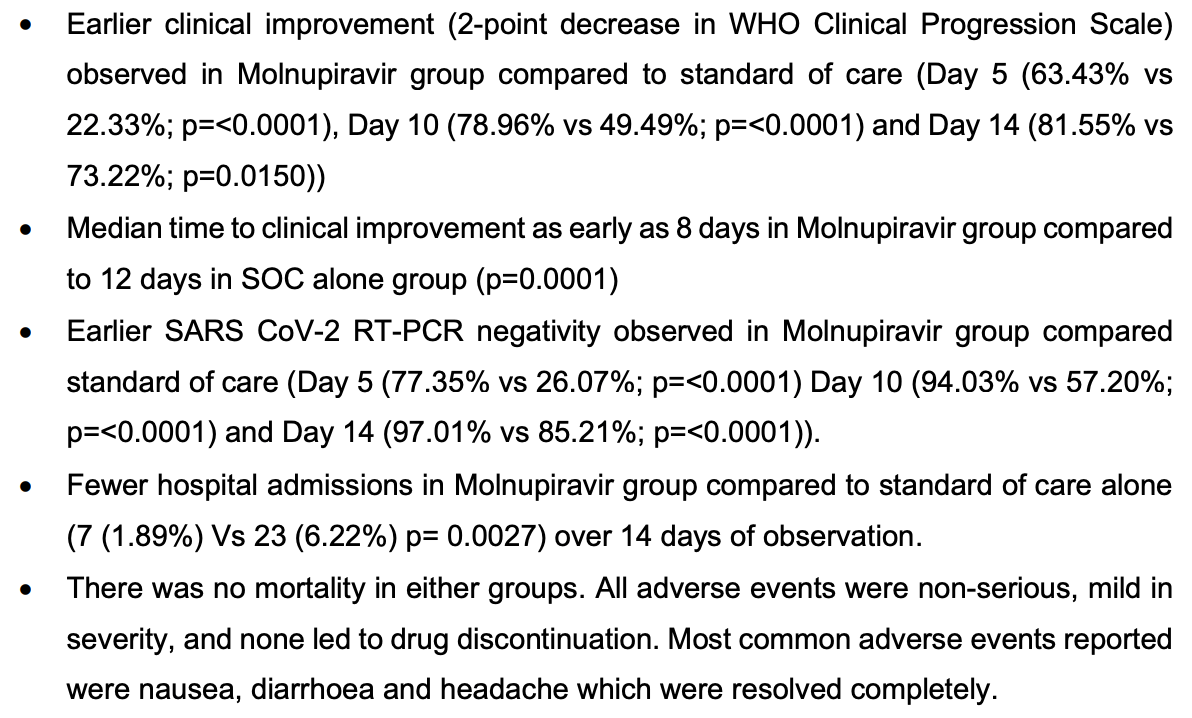
Interim results for CTRI/2021/05/033739, showing lower mortality and faster recovery.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
|
risk of hospitalization, 69.6% lower, RR 0.30, p = 0.003, treatment 7 of 371 (1.9%), control 23 of 370 (6.2%), NNT 23.
|
|
recovery time, 33.3% lower, relative time 0.67, p < 0.001, treatment 371, control 370.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Hetero et al., 9 Jul 2021, Randomized Controlled Trial, India, peer-reviewed, 1 author, licensee.
Hetero Announces Interim Clinical Results from Phase III Clinical Trials of Molnupiravir conducted in India
~ Phase 3 Trial Demonstrates Statistically Significant fewer hospital admissions, Faster Time to Clinical Improvement and early negative SARS CoV-2 RT PCR with Molnupiravir Treatment in Mild COVID 19 Patients Compared to Standard of Care alone~ ~ Hetero has approached the Drug Controller General of India (DCGI) to seek Emergency Use Authorization for Molnupiravir in India ~ India, Hyderabad, 9 th July 2021: Hetero, a globally renowned vertically integrated pharmaceutical organization, today announced the interim clinical results from Phase III Clinical trials of Molnupiravir in mild Covid-19 patients conducted across multiple COVID-19 dedicated hospital sites across India. In April this year, Hetero had entered into a non-exclusive licensing agreement with MSD (tradename of Merck & Co., Inc., Kenilworth, N.J., USA (NYSE: MRK), to manufacture and supply Molnupiravir in India and over 100 low and middle-income countries (LMICs). Molnupiravir is an investigational, orally administered form of a potent ribonucleoside analog, being developed globally by MSD, that inhibits the replication of multiple RNA viruses including SARS-CoV-2, the causative agent of COVID-19 with demonstrated activity against SARS-CoV-2 in human airway epithelial cell cultures and potential to completely eliminate SARS CoV-2 from the body within 5 days. Hetero had commenced a phase-III, comparative, randomized, multicenter clinical trial on mild Covid-19 patients (N=1218). These clinical trials were aimed at evaluating the efficacy and safety of Molnupiravir plus standard of care (test arm) versus standard of care alone (control