Relationship between selenium status, selenoproteins and COVID-19 and other inflammatory diseases: A critical review
et al., Journal of Trace Elements in Medicine and Biology, doi:10.1016/j.jtemb.2022.127099, Jan 2023
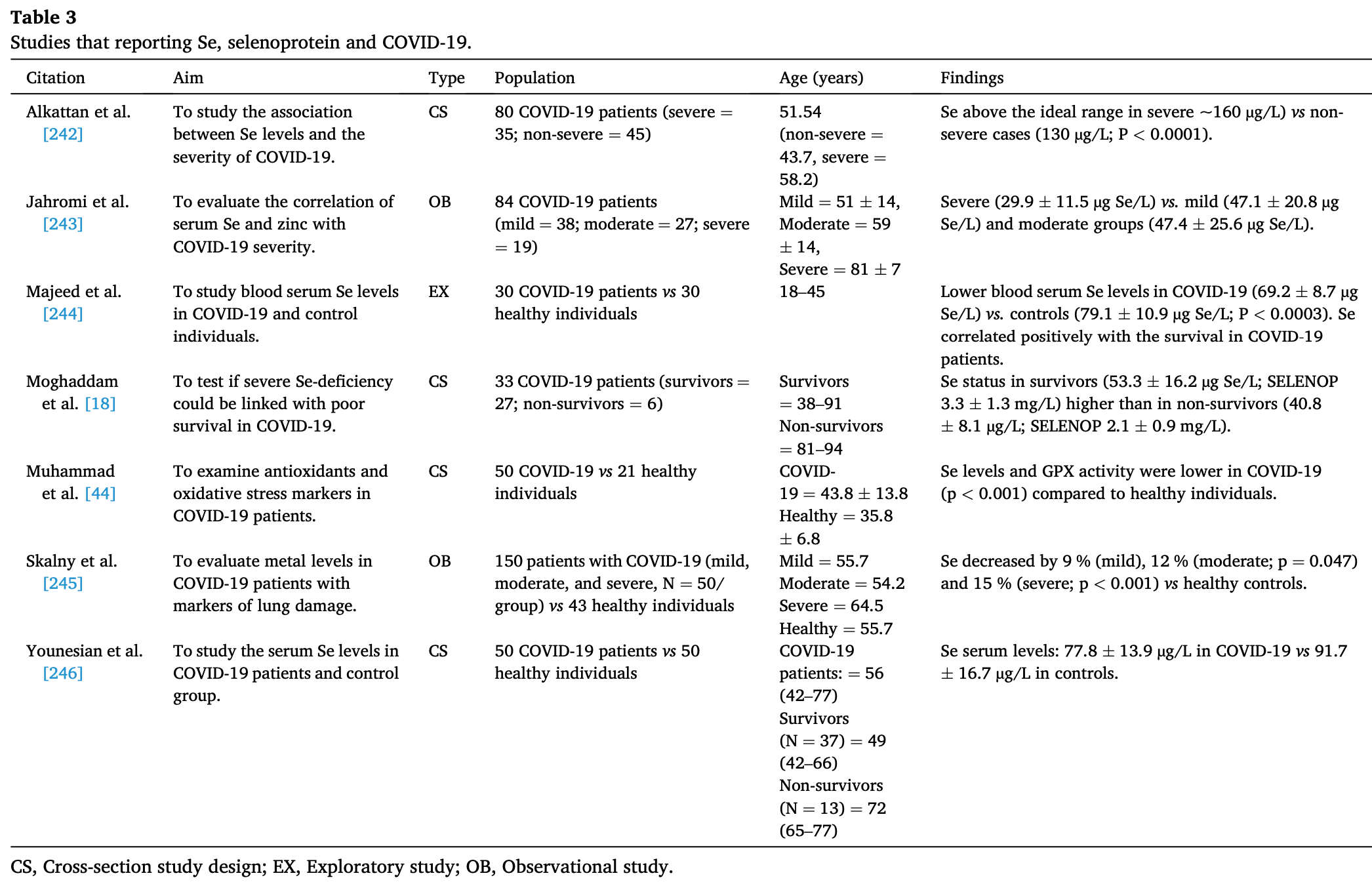
Review of the relationship between selenium, selenoproteins, COVID-19, and other inflammatory and viral diseases. Authors found that COVID-19 severity and mortality have been associated with selenium deficiency in some studies, though data is limited and a causal role is unclear. Selenium has important roles in regulating immune and inflammatory responses, mainly through antioxidant selenoproteins. However, specific molecular mechanisms are poorly understood. Selenium deficiency has been linked to increased severity of some viral infections, but evidence for a direct effect on viral replication is lacking.
1.
Smail et al., Antioxidant and oxidative enzymes, genetic variants, and cofactors as prognostic biomarkers of COVID-19 severity and mortality: a systematic review, Frontiers in Molecular Biosciences, doi:10.3389/fmolb.2025.1700263.
2.
Sarker et al., Selenium as a Nutritional Shield in Viral Defense: A Narrative Review, MDPI AG, doi:10.20944/preprints202502.2251.v1.
3.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
4.
Fazli et al., Possible Link between Gut Microbiota, Diet, and COVID-19 Infection, Journal of Medical Bacteriology, 12:4, jmb.tums.ac.ir/index.php/jmb/article/view/525.
5.
Xie et al., The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death, Cellular & Molecular Biology Letters, doi:10.1186/s11658-024-00659-6.
6.
Kaushal, A., Nutraceuticals and pharmacological to balance the transitional microbiome to extend immunity during COVID-19 and other viral infections, Journal of Translational Medicine, doi:10.1186/s12967-024-05587-9.
7.
Mu et al., Anti-inflammatory and Nutritional Interventions Against SARS-CoV-2: A Comprehensive Review, Journal of Agriculture and Food Research, doi:10.1016/j.jafr.2024.101422.
8.
Maia et al., Selenium—More than Just a Fortuitous Sulfur Substitute in Redox Biology, Molecules, doi:10.3390/molecules29010120.
9.
Yuan et al., The role of cell death in SARS-CoV-2 infection, Signal Transduction and Targeted Therapy, doi:10.1038/s41392-023-01580-8.
10.
Golin et al., Relationship between selenium status, selenoproteins and COVID-19 and other inflammatory diseases: A critical review, Journal of Trace Elements in Medicine and Biology, doi:10.1016/j.jtemb.2022.127099.
11.
Foshati et al., Antioxidants and clinical outcomes of patients with coronavirus disease 2019: A systematic review of observational and interventional studies, Food Science & Nutrition, doi:10.1002/fsn3.3034.
12.
Khatiwada et al., A Mechanistic Link Between Selenium and Coronavirus Disease 2019 (COVID-19), Current Nutrition Reports, doi:10.1007/s13668-021-00354-4.
Golin et al., 31 Jan 2023, peer-reviewed, 5 authors.
Contact: jbtrocha@yahoo.com.br.
Relationship between selenium status, selenoproteins and COVID-19 and other inflammatory diseases: A critical review
Journal of Trace Elements in Medicine and Biology, doi:10.1016/j.jtemb.2022.127099
The antioxidant effects of selenium as a component of selenoproteins has been thought to modulate host immunity and viral pathogenesis. Accordingly, the association of low dietary selenium status with inflammatory and immunodeficiency has been reported in the literature; however, the causal role of selenium deficiency in chronic inflammatory diseases and viral infection is still undefined. The COVID-19, characterized by acute respiratory syndrome and caused by the novel coronavirus 2, SARS-CoV-2, has infected millions of individuals worldwide since late 2019. The severity and mortality from COVID-19 have been associated with several factor, including age, sex and selenium deficiency. However, available data on selenium status and COVID-19 are limited, and a possible causative role for selenium deficiency in COVID-19 severity has yet to be fully addressed. In this context, we review the relationship between selenium, selenoproteins, COVID-19, immune and inflammatory responses, viral infection, and aging. Regardless of the role of selenium in immune and inflammatory responses, we emphasize that selenium supplementation should be indicated after a selenium deficiency be detected, particularly, in view of the critical role played by selenoproteins in human health. In addition, the levels of selenium should be monitored after the start of supplementation and discontinued as soon as normal levels are reached. Periodic assessment of selenium levels after supplementation is a critical issue to avoid over production of toxic metabolites of selenide because under normal conditions, selenoproteins attain saturated expression levels that limits their potential deleterious metabolic effects.
CRediT authorship contribution statement Anieli
Declaration of interest The authors declare that there are no conflicts of interest.
References
Addinsall, Wright, Andrikopoulos, Van Der Poel, Stupka, Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease, Biochem. J, doi:10.1042/BCJ20170920
Aggarwal, Gathwala, Yadav, Kumar, Selenium supplementation for prevention of late-onset sepsis in very low birth weight preterm neonates, J. Trop. Pediatr, doi:10.1093/tropej/fmv096
Akbaraly, Arnaud, Hininger-Favier, Gourlet, Roussel et al., Selenium and mortality in the elderly: results from the EVA study, Clin. Chem, doi:10.1373/clinchem.2005.055301
Akbaraly, Hininger-Favier, Carrière, Anaud, Gourrlet et al., Plasma selenium over time and cognitive decline in the elderly, Epidemiology, doi:10.1097/01.ede.0000248202.83695.4e
Alehagen, Aaseth, Lindahl, Larsson, Alexander, Dietary supplementation with selenium and coenzyme Q10 prevents increase in plasma ddimer while lowering cardiovascular mortality in an Elderly SwedisH Population, Nutrients, doi:10.3390/nu13041344
Alehagen, Johansson, Björnstedt, Rosén, Post et al., Relatively high mortality risk in elderly Swedish subjects with low selenium status, Eur. J. Clin. Nutr, doi:10.1038/ejcn.2015.92
Alehagen, Opstad, Alexander, Larsson, Aaseth, Impact of selenium on biomarkers and clinical aspects related to ageing. a review, Biomolecules, doi:10.3390/biom1110478
Alexander, Tinkov, Strand, Alehagen, Skalny et al., Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19, Nutrients, doi:10.3390/nu12082358
Alhazzani, Jacobi, Sindi, Hartog, Reinhart et al., The effect of selenium therapy on mortality in patients with sepsis syndrome: a systematic review and meta-analysis of randomized controlled trials, Crit. Care Med, doi:10.1097/ccm.0b013e31828a24c6
Alhussien, Dang, Potential roles of neutrophils in maintaining the health and productivity of dairy cows during various physiological and physiopathological conditions: a review, Immunol. Res, doi:10.1007/s12026-019-9064-5
Alkattan, Alabdulkareem, Kamel, Abdelseed, Almutairi et al., Correlation between micronutrients plasma concentration and disease severity in COVID-19 patients, Alex. J. Med, doi:10.1080/20905068.2020.1870788
Almeida, Gajewska, Duro, Costa, Pinto, Trace element imbalances in patients undergoing chronic hemodialysis therapy -report of an observational study in a cohort of Portuguese patients, J. Trace Elem. Med. Biol, doi:10.1016/j.jtemb.2020.126580
Anaya, Herrán, Beltrán, Rojas, Is post-COVID syndrome and autoimmune disease?, Expert Rev. Clin. Immunol, doi:10.1080/1744666X.2022.2085561
Arnaud, De Lorgeril, Akbaraly, Salen, Arnout et al., Gender differences in copper, zinc and selenium status in diabetic-free metabolic syndrome European population-The IMMIDIET study, Nutr. Metab. Cardiovasc. Dis, doi:10.1016/j.numecd.2010.09.005
Arthur, Mckenzie, Beckett, Selenium in the immune system, J. Nutr, doi:10.1093/jn/133.5.1457S
Avery, Hoffmann, Selenium, selenoproteins, and Immunity, Nutrients, doi:10.3390/nu10091203
Aziz, Klesius, Effect of selenium deficiency on caprine polymorphonuclear leukocyte production of leukotriene B4 and its neutrophil chemotactic activity, Am. J. Vet. Res
Aziz, Klesius, Frandsen, Effects of selenium on polymorphonuclear leukocyte function in goats, Am. J. Vet. Res
Aziz, Klesius, The effect of selenium deficiency in goats on lymphocyte production of leukocyte migration inhibitory factor, Vet. Immunol. Immunopathol, doi:10.1016/0165-2427(85)90026-1
Bae, Kim, Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19, Molecules, doi:10.3390/molecules25225346
Balboni, Zagnoli, Filippini, Fairweather-Tait, Vinceti, Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies, J. Trace Elem. Med. Biol, doi:10.1016/j.jtemb.2022.126956
Barchielli, Capperucci, Tanini, The role of selenium in pathologies: an updated review, Antioxidants, doi:10.3390/antiox1102025
Barrett, Reddy, Short, Motley, Lintel et al., Selenoprotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage, J. Clin. Investig, doi:10.1172/JCI76099
Baum, Miguez-Burbano, Campa, Shor-Posner, Selenium and interleukins in persons infected with human immunodeficiency virus type 1, J. Infect. Dis, doi:10.1086/315911
Baum, Shor-Posner, Lai, Zhang, Lai et al., High risk of HIV-related mortality is associated with selenium deficiency, JAIDS J. Acquir. Immune Defic. Syndr, doi:10.1097/00042560-199708150-00007
Beck, Esworthy, Ho, Chu, Glutathione peroxidase protects mice from viral-induced myocarditis, FASEB J, doi:10.1096/fasebj.12.12.1143
Beck, Handy, Levander, Host nutritional status: the neglected virulence factor, Trends Microbiol, doi:10.1016/j.tim.2004.07.007
Beck, Kolbeck, Rohr, Shi, Morris et al., Benign human enterovirus becomes virulent in selenium-deficient mice, J. Med. Virol, doi:10.1002/jmv.1890430213
Beck, Levander, Handy, Selenium deficiency and viral infection, The Journal of Nutrition, doi:10.1093/jn/133.5.1463S
Beck, Nelson, Shi, Van Dael, Schiffrin et al., Selenium deficiency increases the pathology of an influenza virus infection, Fed. Am. Soc. Exp. Biol, doi:10.1096/fj.00-0721fje
Beck, Selenium and host defense towards viruses, Proc. Nutr. Soc, doi:10.1017/s0029665199000920
Beck, Shi, Morris, Levander, Rapid genomic evolution of a nonvirulent Coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates, Nat. Med, doi:10.1038/nm0595-433
Beligaswatta, Sudusinghe, Silva, Davenport, Prevalence and correlates of low plasma selenium concentrations in peritoneal dialysis patients, J. Trace Elem. Med. Biol, doi:10.1016/j.jtemb.2021.126899
Belsky, Wira, Jacob, Sather, Lee, A review of micronutrients in sepsis: the role of thiamine, L-carnitine, vitamin C, selenium and vitamin D, Nutr. Res. Rev, doi:10.1017/S0954422418000124
Bermano, Méplan, Mercer, Hesketh, Selenium and viral infection: are there lessons for COVID-19, Br. J. Nutr, doi:10.1017/S0007114520003128
Bleys, Navas-Acien, Guallar, Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults, JAMA Intern. Med, doi:10.1001/archinternmed.2007.74
Bloos, Trips, Nierhaus, Briegel, Heyland et al., Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial, JAMA Intern. Med, doi:10.1001/jamainternmed.2016.2514
Bomer, Beverborg, Hoes, Streng, Vermeer et al., Selenium and outcome in heart failure, Eur. J. Heart Fail, doi:10.1002/ejhf.1644
Bomer, Pavez-Giani, Deiman, Linders, Hoes et al., Selenoprotein DIO2 is a regulator of mitochondrial function, morphology and UPRmt in human cardiomyocytes, Int. J. Mol. Sci, doi:10.3390/ijms222111906
Bonilla, Oettgen, Adaptive immunity, J. Allergy Clin. Immunol, doi:10.1016/j.jaci.2009.09.017
Bosschaerts, Guilliams, Noel, Herin, Burk et al., Alternatively activated myeloid cells limit pathogenicity associated with African trypanosomiasis through the IL-10 inducible gene selenoprotein p, J. Immunol, doi:10.4049/jimmunol.180.9.6168
Boyne, Arthur, Alterations of neutrophil function in selenium-deficient cattle, J. Comp. Pathol, doi:10.1016/0021-9975(79)90018-5
Brigelius-Flohé, Glutathione peroxidases and redox-regulated transcription factors, Biol. Chem, doi:10.1515/BC.2006.166
Brummer, Hayes, Adam, Horohov, Dawson et al., The effect of selenium supplementation on vaccination response and immune function in adult horses, J. Anim. Sci, doi:10.2527/jas.2012-5819
Cai, Zhang, Li, Selenium, aging and aging-related diseases, Aging Clin. Exp. Res, doi:10.1007/s40520-018-1086-7
Campa, Shor-Posner, Indacochea, Zhang, Lai et al., Mortality risk in selenium-deficient HIV-positive children, J. Acquir. Immune Defic. Syndr. Hum. retrovirol, doi:10.1097/00042560-199904150-00015
Cao, Fan, Chen, Li, Xing et al., Inflammatory response occurs in veins of broiler chickens treated with a selenium deficiency diet, Biol. Trace Elem. Res, doi:10.1007/s12011-017-1145-5
Cao, Reddy, Sordillo, Altered eicosanoid biosynthesis in selenium deficient endothelial cells, Free Radic, Biol. Med, doi:10.1016/s0891-5849(99)00251-8
Cardoso, Hare, Macpherson, Sex-dependent association between selenium status and cognitive performance in older adults, Eur. J. Nutr, doi:10.1007/s00394-020-02384-0
Carlson, Yoo, Sano, Sengupta, Kim et al., Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression, BMC Immunol, doi:10.1186/1471-2172-10-57
Carlson, Yoo, Shrimali, Irons, Gladyshev et al., Role of selenium-containing proteins in the function of T cells and macrophages, Proc. Nutr. Soc, doi:10.1017/S002966511000176X
Chen, An original discovery: selenium deficiency and Keshan disease (an endemic heart disease), Asia Pac, J. Clin. Nutr
Cheng, Bolognesi, Kraus, DIO2 modifies inflammatory responses in chondrocytes, Osteoarthr. Cartil, doi:10.1016/j.joca.2012.02.006
Chi, Zhang, Lu, Zhang, Xu et al., Roles of selenoprotein S in reactive oxygen species-dependent neutrophil extracellular trap formation induced by selenium-deficient arteritis, Redox Biol, doi:10.1016/j.redox.2021.102003
Christoffolete, Doleschall, Egri, Liposits, Zavacki et al., Regulation of thyroid hormone activation via the liver X-receptor/ retinoid X-receptor pathway, J. Endocrinol, doi:10.1677/JOE-09-0448
Cirelli, Ciardi, De Simone, Sorice, Giordano et al., Serum selenium concentration and disease progress in patients with HIV infection, Clin. Biochem, doi:10.1016/0009-9120(91)90601-a
Cumpstey, Clark, Santolini, Jackson, Feelisch, COVID-19: A redox disease-What a stress pandemic can teach us about resilience and what we may learn from the reactive species interactome about its treatment, Antioxid. Redox Signal, doi:10.1089/ars.2021.0017
Da Silva, Rocha, Morsch, Zanin, Kaizer et al., Oxidative stress and delta-ALA-D activity in chronic renal failure patients, Biomed. Pharmacother, doi:10.1016/j.biopha.2006.12.007
Darras, Van Herck, Iodothyronine deiodinase structure and function: from ascidians to humans, J. Endocrinol, doi:10.1530/JOE-12-0204
Delgado-Rizo, Martinez-Guzman, Iñiguez-Gutierrez, García-Orozco, Alvarado-Navarro et al., Neutrophil extracellular traps and its implications in inflammation: an overview, Front. Immunol, doi:10.3389/fimmu.2017.00081
Dhanya, Swathy, Indira, Selenium downregulates oxidative stressinduced activation of leukotriene pathway in experimental rats with diabetic cardiac hypertrophy, Biol. Trace Elem. Res, doi:10.1007/s12011-014-0076-7
Dharmalingam, Birdi, Tomo, Sreenivasulu, Charan et al., Trace elements as immunoregulators in SARS-CoV-2 and other viral infections, Indian J. Clin. Biochem, doi:10.1007/s12291-021-00961-6
Di-Bella, Grilli, Cataldo, Petrosillo, Selenium deficiency and HIV infection, Infect. Dis. Rep, doi:10.4081/idr.2010.e18
Domingo, Marquès, The effects of some essential and toxic metals/ metalloids in COVID-19: a review, Food Chem. Toxicol, doi:10.1016/j.fct.2021.112161
Duntas, Hubalewska-Dydejczyk, Selenium and inflammation-potential use and perspectives, US Endocrinol, doi:10.17925/USE.2015.11.02.97
Duntas, Selenium and inflammation: underlying anti-inflammatory mechanisms, Horm. Metab. Res, doi:10.1055/s-0029-1220724
Dworkin, Selenium deficiency in HIV infection and the acquired immunodeficiency syndrome (AIDS), Chem. Biol. Interact, doi:10.1016/0009-2797(94)90038-8
Eming, Wynn, Martin, Inflammation and metabolism in tissue repair and regeneration, Science, doi:10.1126/science.aam7928
Engin, Engin, Engin, Can iron, zinc, copper and selenium status be a prognostic determinant in COVID-19 patients, Environ. Toxicol. Pharmacol, doi:10.1016/j.etap.2022.103937
Epp, Ladenstein, Wendel, The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution, Eur. J. Biochem, doi:10.1111/j.1432-1033.1983.tb07429.x
Fairweather-Tait, Bao, Broadley, Collings, Ford et al., Selenium in human health and disease, Antioxid. Redox Signal, doi:10.1089/ars.2010.3275
Fang, Goeijenbier, Zuo, Wang, Liang et al., The association between Hantavirus infection and selenium deficiency in Mainland China, Viruses, doi:10.3390/v7010333
Ferrucci, Fabbri, Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty, Nat. Rev. Cardiol, doi:10.1038/s41569-018-0064-2
Fodor, Te, Velthuis, Structure and function of the influenza virus transcription and replication machinery, Cold Spring Harb. Perspect. Med, doi:10.1101/cshperspect.a038398
Forceville, Laviolle, Annane, Vitoux, Bleichner et al., Effects of high doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study, Crit. Care, doi:10.1186/cc5960
Fredericks, Hoffmann, Rose, Osterheld, Hess et al., Stable expression and function of the inositol 1,4,5-triphosphate receptor requires palmitoylation by a DHHC6/selenoprotein K complex, Proc. Natl. Acad. Sci. USA, doi:10.1073/pnas.1417176111
Fujishima, Ohsawa, Itai, Kato, Tanno et al., Serum selenium levels are inversely associated with death risk among hemodialysis patients, Nephrol., Dial., Transplant, doi:10.1093/ndt/gfq859
Gasmi, Tippairote, Mujawdiya, Peana, Menzel et al., Micronutrients as immunomodulatory tools for COVID-19 management, Clin. Immunol, doi:10.1016/j.clim.2020.108545
Giacconi, Chiodi, Boccoli, Costarelli, Piacenza et al., Reduced levels of plasma selenium are associated with increased inflammation and cardiovascular disease in an Italian elderly population, Exp. Gerontol, doi:10.1016/j.exger.2020.111219
Gilmore, The Rel/NR-κB signal transduction pathway introduction, Oncogene, doi:10.1038/sj.onc.1203237
Grasso, Scholz, Erskine, Eberhart, Phagocytosis, bactericidal activity, and oxidative metabolism of milk neutrophils from dairy cows fed selenium-supplemented and selenium-deficient diets, Am. J. Vet. Res
Gu, Wang, He, Zhao, Zhang et al., MiR-1656 targets GPX4 to trigger pyroptosis in broilers kidney tissues by activating NLRP3 inflammasome under Se deficiency, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2022.109001
Guillin, Vindry, Ohlmann, Chavatte, Selenium, selenoproteins and viral infection, Nutrients, doi:10.3390/nu11092101
Hackler, Heller, Sun, Schwarzer, Diegmann et al., Relation of serum copper status to survival in COVID-19, Nutrients, doi:10.3390/nu13061898
Handy, Lubos, Yang, Galbraith, Kelly et al., Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses, J. Biol. Chem, doi:10.1074/jbc.M900392200
Hannon, Fairfield, Adams, Kyle, Crow et al., Use and abuse of dietary supplements in persons with diabetes, Nutr. Diabetes, doi:10.1038/s41387-020-0117-6
Hardy, Hardy, Manzanares, Selenium supplementation in the critical ill, Nutr. Clin. Pract, doi:10.1177/0884533611434116
Hefnawy, Tórtora-Pérez, The importance of selenium and the effects of its deficiency in animal health, Small Rumin. Res, doi:10.1016/j.smallrumres.2009.12.042
Heller, Sun, Hackler, Seelig, Seibert et al., Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker, Redox Biol, doi:10.1016/j.redox.2020.101764
Hernandez, Germain, Thyroid hormone deiodinases: physiology and clinical disorders, Endocrinol. Metab, doi:10.1097/00008480-200308000-00011
Hiffler, Rakotoambinina, Selenium and RNA virus interactions: Potential implications for SARS-CoV-2 infection (COVID-19, Front. Nutr, doi:10.3389/fnut.2020.00164
Hoffmann, Berry, The influence of selenium on immune responses, Mol. Nutr. Food Res, doi:10.1002/mnfr.200700330
Hoffmann, Hashimoto, Shafer, Dow, Berry et al., Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols, J. Nutr. Nutr. Immunol, doi:10.3945/jn.109.120725
Holmgren, Lu, Thioredoxin and thioredoxin reductase: current research with special reference to human disease, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2010.03.083
Holzer, Bockenkamp, Booker, Newland, Ciotti et al., The impact of cardiopulmonary bypass on selenium status, thyroid function, and oxidative defense in children, Pediatr. Cardiol, doi:10.1007/s00246-004-0659-8
Hong, Li, Burgess, Chang, Salem et al., The role of selenium-dependent and selenium-independent glutathione peroxidases in the formation of prostaglandin F 2α, J. Biol. Chem, doi:10.1016/S0021-9258(18)80071-0
Hoque, Shi, Association between selenium intake, diabetes and mortality in adults: finding from National Health and Nutrition Examination Survey (NHANES) 2003-2014, Br. J. Nutr, doi:10.1017/S000711452100177X
Huang, Rose, Hoffmann, The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities, Antioxid. Redox Signal, doi:10.1089/ars.2011.4145
Hurwitz, Klaus, Llabre, Gonzalez, Lawrence et al., Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial, Arch. Intern. Med, doi:10.1001/archinte.167.2.148
Im, Le, Baek, Chung, Kwon, Nutritional status of patients with COVID-19, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.08.018
Imai, Narashima, Arai, Sakamoto, Chibas et al., Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase, J. Biol. Chem, doi:10.1074/jbc.273.4.1990
Ioannou, Locke, Green, Berry, O'hare et al., Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.22310
Ivory, Prieto, Spinks, Armah, Goldson et al., Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults, Clin. Nutr, doi:10.1016/j.clnu.2015.12.003
Jablonska, Vinceti, Selenium and human health: witnessing a Copernican revolution?, J. Environ. Sci. Health, doi:10.1080/10590501.2015.1055163
Jahromi, Tabriz, Togha, Ariyanfar, Gorbani et al., The correlation between serum selenium, zinc, and COVID-19 severity: an observational study, BMC Infect. Dis, doi:10.1186/s12879-021-06617-3
Jayawardena, Sooriyaarachchi, Chourdakis, Jeewandara, Ranasinghe, Enhancing immunity in viral infections, with special emphasis on COVID-19: a review, Diabetes Metab. Syndr, doi:10.1016/j.dsx.2020.04.015
Jofré, Rodriguez-Benitez, López-Gómez, Pérez-Garcia, Inflammatory syndrome in patients on hemodialysis, J. Am. Soc. Nephrol, doi:10.1681/ASN.2006080926
Jubelt, Lipton, Enterovirus/picornavirus infections, Handb. Clin. Neurol, doi:10.1016/B978-0-444-53488-0.00018-3
Junior, Leite, Konstantyner, Selenium and selenoproteins: from endothelial cytoprotection to clinical outcomes, Transl. Res, doi:10.1016/j.trsl.2019.01.004
Kalantar-Zadeh, Block, Mcallister, Humphreys, Kopple, Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients, Am. J. Clin. Nutr, doi:10.1093/ajcn/80.2.299
Kamwesiga, Mutabazi, Kayumba, Tayari, Uwimbabazi et al., Effect of selenium supplementation on CD4+ T=cell recovery, viral suppression and morbidity of HIV-infected patients in Rwanda: a randomized controlled trial, AIDS, doi:10.1097/QAD.0000000000000673
Kang, Lee, Jung, Carlson, Chang et al., Selenophosphate synthetase 1 deficiency exacerbates osteoarthritis by dysregulating redox homeostasis, Nat. Commun, doi:10.1038/s41467-022-28385-7
Kaushal, Gandhi, Nelson, Narayan, Prabhu, Selenium and inflammation, doi:10.1007/978-1-4614-1025-6_35
Khalili, Soudbakhsh, Hajiabdolbaghi, Dashti-Khavidaki, Poorzare et al., Nutritional status and serum zinc and selenium levels in Iranian HIV infected individuals, BMC Infect. Dis, doi:10.1186/1471-2334-8-165
Khatiwada, Subedi, A mechanistic link between selenium and coronavirus disease 2019 (COVID-19, Curr. Nutr. Rep, doi:10.1007/s13668-021-00354-4
Khoso, Yang, Liu, Li, Selenium deficiency downregulates selenoproteins and suppresses immune function in chicken thymus, Biol. Trace Elem. Res, doi:10.1007/s12011-015-0282-y
Khoso, Zhang, Yin, Teng, Li, Selenium deficiency affects immune function by influencing selenoprotein and cytokine expression in chicken spleen, Biol. Trace Elem. Res, doi:10.1007/s12011-018-1396-9
Kieliszek, Lipinski, Selenium supplementation in the prevention of coronavirus infections (COVID-19, Med. Hypotheses, doi:10.1016/j.mehy.2020.109878
Kipp, Banning, Van Schothorst, Méplan, Coort et al., Marginal selenium deficiency downregulates inflammation-related genes in splenic leukocytes of the mouse, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2011.06.011
Kiremidjian-Schumacher, Roy, Wishe, Cohen, Stotzky, Selenium and immune cell function. I. Effect on lymphocyte proliferation and production of interleukin 1 and interleukin 2, Proc. Soc. Exp. Biol. Med, doi:10.3181/00379727-193-43014
Kiremidjian-Schumacher, Stotzky, Selenium and immune responses, Environ. Res, doi:10.1016/S0013-9351(87)80194-9
Koeberle, Gollowitzer, Laokili, Kranenburg, Werz et al., Distinct and overlapping functions of glutathione peroxidases 1 and 2 in limiting NF-κB-driven inflammation through redox-active mechanisms, Redox Biol, doi:10.1016/j.redox.2019.101388
Koeberle, Kipp, Selenium and inflammatory mediators, doi:10.1007/978-3-319-95390-8_7
Kohler, Foote, Kelley, Florea, Shelly et al., Selenium and type 2 diabetes: systematic review, Nutrients, doi:10.3390/nu10121924
Korompoki, Gavriatopoulou, Fotiou, Ntanasis-Stathopoulos, Dimopoulos et al., Late-onset hematological complications post COVID-19: an emerging medical problem for the hematologist, Am. J. Hematol, doi:10.1002/ajh.26384
Korwar, Hossain, Lee, Shay, Basrur et al., Selenium-dependent metabolic reprogramming during inflammation and resolution, J. Biol. Chem, doi:10.1016/j.jbc.2021.100410
Kumar, Gupta, Kaushik, Jyoti, Neutrophil extracellular traps: formation and involvement in disease progression, Iran, J. Allergy Asthma Immunol
Köhrle, Brigelius-Flohé, Böck, Gärtner, Meyer et al., Selenium in biology: facts and medical perspectives, Biol. Chem, doi:10.1515/BC.2000.107
Köhrle, Selenium and the control of thyroid hormone metabolism, Thyroid, doi:10.1089/thy.2005.15.841
Labunskyy, Hatfield, Gladyshev, Selenoproteins: molecular pathways and physiological roles, Physiol. Rev, doi:10.1152/physrev.00039.2013
Labunskyy, Lee, Handy, Loscalzo, Hatfield et al., Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice, Antioxid. Redox Signal, doi:10.1089/ars.2010.3526
Laing, Petrovic, Lachat, De Boevre, Klingenberg et al., Course and survival of COVID-19 patients with comorbidities in relation to the trace element status at hospital admission, Nutrients, doi:10.3390/nu13103304
Lands, Biological consequences of fatty acid oxygenase reaction mechanisms, Prostaglandins Leukot. Med, doi:10.1016/0262-1746(84)90100-8
Larsen, Tollersund, Effect of dietary vitamin E and selenium on phytohaemagglutinin response of pig lymphocytes, Res. Vet. Sci
Lee, Péterfi, Hoffmann, Moore, Kaya et al., MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation, Mol. Cell, doi:10.1016/j.molcel.2013.06.019
Lee, Takahashi, Matsuzaka, Yamai, Danjo et al., The relationship between serum selenium concentration and neutrophil function in peripheral blood, Biol. Trace Elem. Res, doi:10.1007/s12011-011-9108-8
Levander, Beck, Selenium and viral virulence, Br. Med. Bull, doi:10.1258/0007142991902592
Li, Jiang, Li, Li, Zhou et al., Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity, Nat. Immunol, doi:10.1038/s41590-021-00993-3
Li, Liang, Mao, Deng, Zhang, Effects of B-lymphocyte dysfunction on the serum copper, selenium and zinc levels of rheumatoid arthritis patients, Pak. J. Med. Sci, doi:10.12669/pjms.305.5214
Li, Liu, Wu, Peng, Wang et al., Influenza-associated excess respiratory mortality in China, 2010-15: a population-based study, Lancet Public Health, doi:10.1016/S2468-2667(19)30163-X
Li, Sun, Zhang, Zhu, Jia et al., Selenium deficiency induces spleen pathological changes in pigs by decreasing selenoprotein expression, evoking oxidative stress, and activating inflammation and apoptosis, J. Anim. Sci. Biotechnol, doi:10.1186/s40104-021-00587-x
Li, Tang, Gui, Zou, Ao et al., A meta-analysis of randomized controlled trials: efficacy of selenium treatment for sepsis, Medicine, doi:10.1097/MD.0000000000014733
Liu, Chiba, Inaba, Kondo, Keshan disease-a review from the aspect of history and etiology (Japanese), Nihon Eiseigaku Zasshi, doi:10.1265/jjh.56.641
Liu, Zhao, Ma, Mu, Wang et al., Selenium (Se) plays a key role in the biological effects of some viruses: implications for COVID-19, Environ. Res, doi:10.1016/j.envres.2021.110984
Loscalzo, Keshan disease, selenium deficiency, and the selenoproteome, N. Engl. J. Med, doi:10.1056/NEJMcibr1402199
Luan, Zhao, Yao, Shao, Fan et al., Selenium deficiency influences the mRNA expression of selenoproteins and cytokines in chicken erythrocytes, Biol. Trace Elem. Res, doi:10.1007/s12011-015-0536-8
Lymbury, Tinggi, Griffiths, Rosenfeldt, Perkins, Selenium status of the Australian population: effect of age, gender and cardiovascular disease, Biol. Trace Elem. Res, doi:10.1007/s12011-008-8208-6
López-Pereira, Iturrante, Cámara, Cardeñoso, Alegre et al., Can COVID-19 cause severe neutropenia?, Clin. Case Rep, doi:10.1002/ccr3.3369
Ma, Hoffmann, Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism, Semin. Cell Dev. Biol, doi:10.1016/j.semcdb.2020.11.006
Maddox, Aherne, Reddy, Sordillo, Increased neutrophil adherence and adhesion molecule mRNA expression in endothelial cells during selenium deficiency, J. Leukoc. Biol, doi:10.1002/jlb.65.5.658
Mahomed, Williams, Woelk, Mudzamiri, Madzime et al., Leukocyte selenium, zinc, and copper concentrations in preeclamptic and normotensive pregnant women, Biol. Trace Elem. Res, doi:10.1385/BTER:75:1-3:107
Majeed, Nagabhushanam, Gowda, Mundkur, An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: the case for adequate selenium status, Nutrition, doi:10.1016/j.nut.2020.111053
Mank, Mank, Ogle, Roberts, Delayed, transient and self-resolving neutropenia following CPVOD-19 pneumonia, BMJ Case Rep, doi:10.1136/bcr-2021-242596
Mantzarlis, Tsolaki, Zakynthinos, Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies, Oxid. Med. Cell. Longev, doi:10.1155/2017/5985209
Manzanares, Biestro, Galusso, Torre, Mañay et al., Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill?, Intensive Care Med, doi:10.1007/s00134-008-1356-5
Mao, Zhang, Huang, Selenium supplementation and the risk of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials, Endocrine, doi:10.1007/s12020-014-0298-7
Mattmiller, Carlson, Gandy, Sordillo, Reduced macrophage selenoprotein expression alters oxidized lipid metabolite biosynthesis from arachidonic and linoleic acid, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2014.02.005
Mattmiller, Carlson, Sordillo, Regulation of inflammation by selenium and selenoproteins: impact on eicosanoid biosynthesis, J. Nutr. Sci, doi:10.1017/jns.2013.17
Mccarty, Can dietary selenium reduce leukotriene production, Med. Hypotheses, doi:10.1016/0306-9877(84)90129-4
Mcclung, Roneker, Mu, Lisk, Langlais et al., Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase, Proc. Natl. Acad. Sci. USA, doi:10.1073/pnas.0308096101
Mckenzie, Rafferty, Beckett, Arthur, Effects of selenium on immunity and aging, doi:10.1007/978-1-4615-1609-5_21
Mertens, Lowes, Webster, Talib, Hall et al., Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation, BJA, Br. J. Anaesth, doi:10.1093/bja/aev073
Miller, Walker, Arthur, Nicol, Pickard et al., Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase, Clin. Sci
Misu, Takamura, Takayama, Hayashi, Matsuzawa-Nagata et al., A liver-derived secretory protein, selenoprotein P, causes insulin resistance, Cell Metab, doi:10.1016/j.cmet.2010.09.015
Mita, Nakayama, Inari, Nishito, Yoshioka et al., Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models, Nat. Commun, doi:10.1038/s41467-017-01863-z
Moghaddam, Heller, Sun, Seelig, Cherkezov et al., Selenium deficiency is associated with mortality risk from COVID-19, Nutrients, doi:10.3390/nu12072098
Muhammad, Kani, Muhammad, Benji, Ahmad et al., Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: a cross-sectional comparative study in Jigawa, Northwestern Nigeria, SAGE Open Med, doi:10.1177/2050312121991246
Munir, Jahangeer, Hussain, Mahmood, Ashiq et al., Hantavirus diseases pathophysiology, their diagnostic strategies and therapeutic approaches: A review, Clin. Exp. Pharmacol. Physiol, doi:10.1111/1440-1681.13403
Mutua, Gershwin, A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics, Clin. Rev. Allergy Immunol, doi:10.1007/s12016-020-08804-7
Nair, Schwartz, Immunoregulation of natural and lymphokine-activated killer cells by selenium, Immunopharmacology, doi:10.1016/0162-3109(90)90067-o
Negre-Salvayre, Auge, Basaga, Boada, Brenke et al., Pathological aspects of lipid peroxidation, Free Radic. Res, doi:10.3109/10715762.2010.498478
Nelson, Lei, Prabhu, Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages, J. Nutr, doi:10.3945/jn.111.141176
Netea, Domínguez-Andrés, Barreiro, Chavakis, Divangahi et al., Defining trained immunity and its role in health and disease, Nat. Rev. Immunol, doi:10.1038/s41577-020-0285-6
Nettleford, Prabhu, Selenium and selenoproteins in gut inflammation: a review, Antioxidants, doi:10.3390/antiox7030036
Nordberg, Arnér, Reactive oxygen species, antioxidants, and the mammalian thioredoxin system, Free Radic, Biol. Med, doi:10.1016/s0891-5849(01)00724-9
Norton, Fredericks, Huang, Fay, Hoffmann et al., Selenoprotein K regulation of palmitoylation and calpain cleavage of ASAP2 is required for efficient Fcγ-R mediated phagocytosis, J. Leukoc. Biol, doi:10.1189/jlb.2A0316-156RR
Oliveira, Piccoli, Nogara, Pereira, Carvalho et al., Selenium neuroprotection in neurodegenerative disorders
Oliveira, Viana, Gonçalves, Silva, Vieira, Therapeutic use of intravenous selenium in respiratory and immunological diseases: a narrative review, Adv. Respir. Med, doi:10.5603/ARM.a2022.0018
Olivieri, Stanzial, Girelli, Trevisan, Guatini et al., Selenium status, fatty acids, vitamins A and E, and aging: the novel study, Am. J. Clin. Nutr, doi:10.1093/ajcn/60.4.510
Oo, Misu, Saito, Tanaka, Kato et al., Serum selenoprotein P, but not selenium, predicts future hyperglycemia in a general Japanese population, Sci. Rep, doi:10.1038/s41598-018-35067-2
Pan, Liu, Tan, Zhang, Li, Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency, Biol. Trace Elem. Res, doi:10.1007/s12011-017-1110-3
Papp, Lu, Holmgren, Khanna, From selenium to selenoproteins: synthesis, identity, and their role in human health, Antioxid. Redox Signal, doi:10.1089/ars.2007.1528
Pawlak, Ho, Kuchroo, Cytokines and transcription factors in the differentiation of CD4 + T helper cell subsets and induction of tissue inflammation and autoimmunity, Curr. Opin. Immunol, doi:10.1016/j.coi.2020.09.001
Peretz, Nève, Famaey, Selenium in rheumatic diseases, Semin. Arthritis Rheum, doi:10.1016/0049-0172(91)90031-t
Perona, Schiavon, Guidi, Veneri, Minuz, Selenium dependent glutathione peroxidase: a physiological regulatory system for platelet function, Thromb. Haemost
Pincemail, Cavalier, Charlier, Cheramy-Bien, Brevers et al., Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study, Antioxidants, doi:10.3390/antiox10020257
Pitts, Hoffmann, Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis, Cell Calcium, doi:10.1016/j.ceca.2017.05.001
Pleschaka, Overview of influenza viruses, Curr. Top. Microbiol. Immunol, doi:10.1007/82_2012_272
Pulikkot, Hu, Chen, Sun, Fan, Integrin regulators in neutrophils, Cells, doi:10.3390/cells11132025
Qian, Misra, Prabhu, Selenium and selenoproteins in prostanoid metabolism and immunity, Crit. Rev. Biochem. Mol. Biol, doi:10.1080/10409238.2020.1717430
Qiu, Geng, Wan, Lu, Guo et al., Serum selenium concentrations and risk of all-cause and heart disease mortality among individuals with type 2 diabetes, Am. J. Clin. Nutr, doi:10.1093/ajcn/nqab241
Raphael, Nalawade, Eagar, Forsthuber, T cell subsets and their signature cytokines in autoimmune and inflammatory disease, Cytokine, doi:10.1016/j.cyto.2014.09.011
Rayman, Selenium and human health, Lancet, doi:10.1016/S0140-6736(11)61452-9
Rayman, Stranges, Epidemiology of selenium and type 2 diabetes: can we make sense of it, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2013.04.003
Ríos-López, González, Hernández-Bello, Sánchez-González, Avoiding the trap: mechanisms developed by pathogens to scape neutrophil extracellular traps, Microbiol. Res, doi:10.1016/j.micres.2020.126644
Sahebari, Rezaieyazdi, Khodashahi, Selenium and autoimmune diseases: a review article, Curr. Rheumatol. Rev, doi:10.2174/1573397114666181016112342
Saito, Selenoprotein P as a significant regulator of pancreatic beta cell function, J. Biochem, doi:10.1093/jb/mvz061
Santesmasses, Castro, Zenin, Shindyapina, Gerashchenko et al., COVID-19 is an emergent disease of aging, Aging Cell, doi:10.1111/acel.13230
Schrauzer, Sacher, Selenium in the maintenance and therapy of HIVinfected patients, Chem. Biol. Interact, doi:10.1016/0009-2797(94)90040-x
Seale, Torres, Berry, Pitts, A role for selenium-dependent GPX1 in SARS-CoV-2 virulence, Am. J. Clin. Nutr, doi:10.1093/ajcn/nqaa177
Shakoor, Feehan, Al Dhaheri, Ali, Platat et al., Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19, Maturitas, doi:10.1016/j.maturitas.2020.08.003
Shivakoti, Gupte, Yang, Mwelase, Kanyama et al., Pre-antiretroviral therapy serum selenium concentrations predict WHO stages 3, 4 or death but not virologic failure post-antiretroviral therapy, Nutrients, doi:10.3390/nu6115061
Shrimali, Irons, Carlson, Sano, Gladyshev et al., Selenoproteins mediate T cell immunity through an antioxidant mechanism, J. Biol. Chem, doi:10.1074/jbc.M802559200
Singer, Deutschman, Seymour, Shankar-Hari, Annane et al., The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), JAMA, doi:10.1001/jama.2016.0287
Skaf, Pattison, Morgan, Bachana, Jain et al., Selenium-containing amino acids are targets for myeloperoxidasederived hypothiocyanous acid: determination of absolute rate constants and implications for biological damage, Biochem. J, doi:10.1042/BJ20101762
Skalny, Timashev, Aschner, Aaseth, Chernova et al., Serum zinc, cooper and other biometals are associated with COVID-19 severity markers, Metabolites, doi:10.3390/metabo11040244
Smith, Hogan, Weiss, Dietary vitamin E and selenium affect mastitis and milk quality, J. Anim. Sci, doi:10.2527/1997.7561659x
Solovyev, Drobyshev, Bjørklund, Dubrovskii, Lysiuk et al., Selenium, selenoprotein P, and Alzheimer's disease: is there a link, Free Radic, Biol. Med, doi:10.1016/j.freeradbiomed.2018.02.030
Stefanowicz, Gashut, Talwar, Duncan, Beulshausen et al., Assessment of plasma and red trace element concentrations, disease severity, and outcome in patients with critical illness, J. Crit. Care, doi:10.1016/j.jcrc.2013.10.012
Stefanowicz, Talwar, O'reilly, Dickinson, Atkinson et al., Erythrocyte selenium concentration as a marker of selenium status, Clin. Nutr, doi:10.1016/j.clnu.2013.01.005
Steinbrenner, Al-Quraishy, Dkhil, Wunderlich, Sies, Dietary selenium in adjuvant therapy of viral and bacterial infections, Adv. Nutr, doi:10.3945/an.114.007575
Steinbrenner, Duntas, Rayman, The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities, Redox Biol, doi:10.1016/j.redox.2022.102236
Steinbrenner, Klotz, Selenium and zinc: "antioxidants" for healthy aging?, Z. für Gerontol. und Geriatr, doi:10.1007/s00391-020-01735-0
Stone, Kawai, Kupka, Fawzi, Role of selenium in HIV infection, Nutr. Rev, doi:10.1111/j.1753-4887.2010.00337.x
Sugimoto, Sousa, Pinho, Perretti, Teixeira, Resolution of inflammation: what controls its onset?, Front. Immunol, doi:10.3389/fimmu.2016.00160
Sun, Hackler, Hilger, Gluschke, Muric et al., Selenium and copper as biomarkers for pulmonary arterial hypertension in systemic sclerosis, Nutrients, doi:10.3390/nu12061894
Swain, Johri, Majumdar, Effect of supplementation of vitamin E, selenium and their different combinations on the performance and immune response of broilers, Br. Poult. Sci, doi:10.1080/713654938
Takamura, Hepatokine selenoprotein P-mediated reductive stress causes resistance to intracellular signal transduction, Antioxid. Redox Signal, doi:10.1089/ars.2020.8087
Tang, Wang, Jia, Liu, Chen et al., The hydroxy-analogue of selenomethionine alleviated lipopolysaccharide-induced inflammatory responses is associated with recover expression of several selenoprotein encoding in the spleens of Kunming mice, R. Soc. Chem. Adv, doi:10.1039/C9RA07260H
Tarp, Selenium and the selenium-dependent glutathione peroxidase in rheumatoid arthritis, Dan. Med. Bull
Taylor, Radding, Understanding selenium and glutathione as antiviral factors in COVID-19: does the viral M pro protease target host selenoproteins and glutathione synthesis, Front. Nutr, doi:10.3389/fnut.2020.00143
Taylor, Selenium and cellular immunity. Evidence that selenoproteins may be encoded in the +1 reading frame overlapping the human CD4, CD8, and HLA-DR genes, Biol. Trace Elem. Res, doi:10.1007/BF02788958
Terpiłowska, Siwicki, Review paper. The role of selected microelements: selenium, zinc, chromium and iron in immune system, Cent. Eur. J. Immunol, doi:10.3390/nu12010236
Tinkov, Ajsuvakova, Filippini, Zhou, Lei et al., Selenium and selenoproteins in adipose tissue physiology and obesity, Biomolecules, doi:10.3390/biom10040658
Tolando, Jovanović, Brigelius-Flohé, Ursini, Maiorino, Reactive oxygen species and proinflammatory cytokine signaling in endothelial cells: effect of selenium supplementation, Free Radic, Biol. Med, doi:10.1016/s0891-5849(00)00183-0
Tomo, Saikiran, Banerjee, Paul, Selenium to selenoproteins -role in COVID-19, EXCLI J, doi:10.17179/excli2021-3530
Tsuji, Carlson, Anderson, Seifried, Hatfield et al., Dietary selenium levels affect selenoprotein expression and support the interferon-γ and IL-6 immune response pathways in mice, Nutrients, doi:10.3390/nu7085297
Tsuji, Santesmasses, Lee, Gladyshev, Hatfield, Historical roles of selenium and selenoproteins in health and development: the good, the bad and the ugly, Int. J. Mol. Sci, doi:10.3390/ijms23010005
Turner, Wheatley, Beck, Stimulatory effects of selenium on mitogen responses in lambs, Vet. Immunol. Immunopathol, doi:10.1016/0165-2427(85)90115-1
Turrubiates-Hernández, Márquez-Sandoval, González-Estevez, Reyes-Castillo, Muñoz-Valle, The relevance of selenium status in rheumatoid arthritis, Nutrients, doi:10.3390/nu12103007
Ulfig, Leichert, The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens, Cell. Mol. Life Sci, doi:10.1007/s00018-020-03591-y
Ursini, Maiorino, Gregolin, The selenoenzyme phospholipid hydroperoxide glutathione peroxide, Biochim. et Biophysuca Acta, doi:10.1016/0304-4165(85)90182-5
Vaghari-Tabari, Jafari-Gharabaghlou, Sadeghsoltani, Hassanpour, Qujeq et al., Zinc and Selenium in Inflammatory Bowel Disease: Trace Elements with Key Roles?, Biol. Trace Elem. Res, doi:10.1007/s12011-020-02444-w
Vaheri, Strandin, Hepojoki, Sironen, Henttonen et al., Uncovering the mysteries of hantavirus infections, Nat. Rev. Microbiol, doi:10.1038/nrmicro3066
Vavougios, Ntoskas, Doskas, Impairment in selenocysteine synthesis as a candidate mechanism of inducible coagulopathy in COVID-19 patients, Med. Hypotheses, doi:10.1016/j.mehy.2020.110475
Veras, Pontelli, Silva, Toller-Kawahisa, De Lima et al., SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology, J. Exp. Med, doi:10.1084/jem.20201129
Verma, Hoffmann, Kumar, Huang, Roe et al., Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses, J. Immunol, doi:10.4049/jimmunol.1002878
Verma, Molina, Lo, Cropp, Nakano et al., In vitro effects of selenium deficiency on West Nile virus replication and cytopathogenicity, Virol. J, doi:10.1186/1743-422X-5-66
Vinceti, Bonaccio, Filippini, Costanzo, Wise et al., Moli-sani Study Investigators, Dietary selenium intake and risk of hospitalization for type 2 diabetes in the Moli-sani study cohort, Nutr., Metab. Cardiovasc. Dis, doi:10.1016/j.numecd.2021.02.016
Vinceti, Filippini, Del Giovane, Dennert, Zwahlen et al., Selenium for preventing cancer, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD005195.pub4
Vinceti, Filippini, Jablonska, Saito, Wise, Safety of selenium exposure and limitations of selenoprotein maximization: molecular and epidemiologic perspectives, Environ. Res, doi:10.1016/j.envres.2022.113092
Vinceti, Filippini, Malagoli, Violi, Mandrioli et al., Amyotrophic lateral sclerosis incidence following exposure to inorganic selenium in drinking water: A long-term follow-up, Environ. Res, doi:10.1016/j.envres.2019.108742
Vinceti, Filippini, Wise, Environmental selenium and human health: an update, Curr. Environ. Health Rep, doi:10.1007/s40572-018-0213-0
Vinceti, Filippini, Wise, Rothman, A systematic review and doseresponse meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies, Environ. Res, doi:10.1016/j.envres.2021.111210
Vitoux, Chappuis, Arnaud, Bost, Accominotti et al., Selenium, glutathione peroxidase, peroxides and platelet functions, Ann.de Biol. Clin
Vlahos, Stambas, Selemidis, Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy, Trends Pharmacol. Sci, doi:10.1016/j.tips.2011.09.001
Vunta, Belda, Arner, Reddy, Heuvel et al., Selenium attenuates pro-inflammatory gene expression in macrophages, Mol. Nutr, doi:10.1002/mnfr.200700346
Vunta, Davis, Palempalli, Bhat, Arner et al., The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages, J. Biol. Chem, doi:10.1074/jbc.M703075200
Wang, Huang, Sun, He, Li et al., SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, ER stress and DNA synthesis, Food Chem. Toxicol, doi:10.1016/j.fct.2021.112286
Wang, Seo, Park, Serum selenium and non-alcoholic fatty liver disease (NAFLS) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2011-2016, Environ. Res, doi:10.1016/j.envres.2021.111190
Watson, Moriguchi, Mcrae, Tobin, Mayberry et al., Effects of selenium in vitro on human T-lymphocyte functions and K-564 tumor cell growth, J. Leukoc. Biol, doi:10.1002/jlb.39.4.447
Wiehe, Cremer, Wisniewska, Becker, Rijntjes et al., Selenium status in neonates with connatal infection, Br. J. Nutr, doi:10.1017/S0007114516002208
Wiercinska-Drapalo, Jaroszewicz, Tarasow, Flisiak, Prokopowicz, Transforming growth factor beta(1) and prostaglandin E2 concentrations are associated with bone formation markers in ulcerative colitis patients, Prostaglandins Other Lipid Mediat, doi:10.1016/j.prostaglandins.2005.06.006
Wolfram, Weidenbach, Adolf, Schwarz, Schädel et al., The trace element selenium is important for redox signaling in phorbol ester-differentiated THP-1 macrophages, Int. J. Mol. Sci, doi:10.3390/ijms222011060
Wright, Gibson, Simpson, Mcdonald, Baines, Neutrophil extracellular traps are associated with inflammation in chronic airway disease, Respirology, doi:10.1111/resp.12730
Xie, Wang, Zhang, Chen, Dietary and serum selenium in coronary heart disease and all-cause mortality: an international perspective, Asia Pac, J. Clin. Nutr, doi:10.3316/informit.647067211509963
Xu, Gong, Sun, Cai, Liu et al., Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages, Biol. Trace Elem. Res, doi:10.1007/s12011-019-01775-7
Yang, Zhao, Liu, Zhang, Wang et al., Oxidative stress induced by Se-deficient high-energy diet implicates neutrophil dysfunction via Nrf2 pathway suppression in swine, Oncotarget, doi:10.18632/oncotarget.14550
Yatmaz, Seow, Gualano, Wong, Stambas et al., Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation, Am. J. Respir. Cell Mol. Biol, doi:10.1165/rcmb.2011-0345OC
Younesian, Khodabakhshi, Abdolahi, Norouzi, Behnampour et al., Decreased serum selenium levels of COVID-19 patients in comparison with healthy individuals, Biol. Trace Elem, doi:10.1007/s12011-021-02797-w
Yu, Sun, Nan, Zhu, Protection from H1N1 influenza virus infections in mice by supplementation with selenium: a comparison with selenium-deficient mice, Biol. Trace Elem. Res, doi:10.1007/s12011-010-8726-x
Zamamiri-Davis, Lu, Thompson, Prabhu, Reddy et al., Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency, Free Radic, Biol. Med, doi:10.1016/s0891-5849(02)00775-x
Zeng, Du, Zhou, Huang, Role of SelS in lipopolysaccharide-induced inflammatory response in hepatoma HepG2 cells, Arch. Biochem. Biophys, doi:10.1016/j.abb.2008.07.016
Zhang, Li, Zhao, Li, Han et al., Multi-Omics profiling reveals Se deficiency-induced redox imbalance, metabolic reprogramming, and inflammation in pig muscle, J. Nutr, doi:10.1093/jn/nxac016
Zhang, Saad, Taylor, Rayman, Selenium and selenoproteins in viral infection with potential relevance to COVID-19, Redox Biol, doi:10.1016/j.redox.2020.101715
Zhang, Taylor, Bennett, Saad, Rayman, Association between regional selenium and reported outcome of COVID-19 cases in China, Am. J. Clin. Nutr, doi:10.1093/ajcn/nqaa095
Zhang, Yu, Hargrove, Greenspan, Dean et al., Inhibition of TNF-alpha induced ICAM-1, VCAM-1 and E-selectin expression by selenium, Atherosclerosis, doi:10.1016/s0021-9150(01)00672-4
Zhou, Wang, Lian, Wang, Wu, Effect of copper, zinc and selenium on the formation of bovine neutrophil extracellular traps, Biol. Trace Elem. Res, doi:10.1007/s12011-020-02477-1
Zhou, Zhang, Chen, Li, He et al., Micronutrient level is negatively correlated with the neutrophil-lymphocyte ratio in patients with severe COVI-19, Int. J. Clin. Pract, doi:10.1155/2022/6498794
Zhu, Chen, Liu, NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and beyond, Front. Immunol, doi:10.3389/fimmu.2022.838011
Zuo, Yalavarthi, Shi, Gockman, Zuo et al., Neutrophil extracellular traps in COVID-19, JCI Insight
Čobanová, Faix, Plachá, Mihaliková, Váradyová et al., Effects of different dietary selenium sources on antioxidant status and blood phagocytic activity in sheep, Biol. Trace Elem. Res, doi:10.1007/s12011-016-0794-0
DOI record:
{
"DOI": "10.1016/j.jtemb.2022.127099",
"ISSN": [
"0946-672X"
],
"URL": "http://dx.doi.org/10.1016/j.jtemb.2022.127099",
"alternative-id": [
"S0946672X22001791"
],
"article-number": "127099",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Relationship between selenium status, selenoproteins and COVID-19 and other inflammatory diseases: A critical review"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Trace Elements in Medicine and Biology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jtemb.2022.127099"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Elsevier GmbH. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Golin",
"given": "Anieli",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tinkov",
"given": "Alexey A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aschner",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farina",
"given": "Marcelo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Rocha",
"given": "João Batista Teixeira",
"sequence": "additional"
}
],
"container-title": "Journal of Trace Elements in Medicine and Biology",
"container-title-short": "Journal of Trace Elements in Medicine and Biology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T07:57:02Z",
"timestamp": 1667462222000
},
"deposited": {
"date-parts": [
[
2024,
4,
27
]
],
"date-time": "2024-04-27T14:17:50Z",
"timestamp": 1714227470000
},
"funder": [
{
"DOI": "10.13039/501100003593",
"doi-asserted-by": "publisher",
"name": "CNPq"
},
{
"DOI": "10.13039/501100004263",
"doi-asserted-by": "publisher",
"name": "FAPERGS"
}
],
"indexed": {
"date-parts": [
[
2024,
7,
10
]
],
"date-time": "2024-07-10T20:05:23Z",
"timestamp": 1720641923827
},
"is-referenced-by-count": 13,
"issued": {
"date-parts": [
[
2023,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "http://www.elsevier.com/open-access/userlicense/1.0/",
"content-version": "am",
"delay-in-days": 314,
"start": {
"date-parts": [
[
2023,
11,
11
]
],
"date-time": "2023-11-11T00:00:00Z",
"timestamp": 1699660800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0946672X22001791?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0946672X22001791?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "127099",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/0304-4165(85)90182-5",
"article-title": "The selenoenzyme phospholipid hydroperoxide glutathione peroxide",
"author": "Ursini",
"doi-asserted-by": "crossref",
"first-page": "62",
"issue": "1",
"journal-title": "Biochim. et Biophysuca Acta",
"key": "10.1016/j.jtemb.2022.127099_bib1",
"volume": "839",
"year": "1985"
},
{
"DOI": "10.1002/jlb.65.5.658",
"article-title": "Increased neutrophil adherence and adhesion molecule mRNA expression in endothelial cells during selenium deficiency",
"author": "Maddox",
"doi-asserted-by": "crossref",
"first-page": "658",
"issue": "5",
"journal-title": "J. Leukoc. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib2",
"volume": "65",
"year": "1999"
},
{
"DOI": "10.1016/S0891-5849(01)00724-9",
"article-title": "Reactive oxygen species, antioxidants, and the mammalian thioredoxin system",
"author": "Nordberg",
"doi-asserted-by": "crossref",
"first-page": "1287",
"issue": "11",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib3",
"volume": "31",
"year": "2001"
},
{
"DOI": "10.1177/0884533611434116",
"article-title": "Selenium supplementation in the critical ill",
"author": "Hardy",
"doi-asserted-by": "crossref",
"first-page": "21",
"issue": "1",
"journal-title": "Nutr. Clin. Pract.",
"key": "10.1016/j.jtemb.2022.127099_bib4",
"volume": "27",
"year": "2012"
},
{
"article-title": "The hydroxy-analogue of selenomethionine alleviated lipopolysaccharide-induced inflammatory responses is associated with recover expression of several selenoprotein encoding in the spleens of Kunming mice",
"author": "Tang",
"first-page": "40462",
"journal-title": "R. Soc. Chem. Adv.",
"key": "10.1016/j.jtemb.2022.127099_bib5",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/j.molcel.2013.06.019",
"article-title": "MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "397",
"issue": "3",
"journal-title": "Mol. Cell",
"key": "10.1016/j.jtemb.2022.127099_bib6",
"volume": "51",
"year": "2013"
},
{
"DOI": "10.1016/j.abb.2008.07.016",
"article-title": "Role of SelS in lipopolysaccharide-induced inflammatory response in hepatoma HepG2 cells",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Arch. Biochem. Biophys.",
"key": "10.1016/j.jtemb.2022.127099_bib7",
"volume": "478",
"year": "2008"
},
{
"DOI": "10.1111/j.1432-1033.1983.tb07429.x",
"article-title": "The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution",
"author": "Epp",
"doi-asserted-by": "crossref",
"first-page": "51",
"issue": "1",
"journal-title": "Eur. J. Biochem.",
"key": "10.1016/j.jtemb.2022.127099_bib8",
"volume": "133",
"year": "1983"
},
{
"article-title": "Selenium neuroprotection in neurodegenerative disorders",
"author": "Oliveira",
"first-page": "35",
"key": "10.1016/j.jtemb.2022.127099_bib9",
"volume": "1",
"year": "2021"
},
{
"first-page": "506",
"key": "10.1016/j.jtemb.2022.127099_bib10",
"series-title": "Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids",
"year": "2000"
},
{
"DOI": "10.1373/clinchem.2005.055301",
"article-title": "Selenium and mortality in the elderly: results from the EVA study",
"author": "Akbaraly",
"doi-asserted-by": "crossref",
"first-page": "2117",
"issue": "11",
"journal-title": "Clin. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib11",
"volume": "51",
"year": "2005"
},
{
"DOI": "10.1001/archinternmed.2007.74",
"article-title": "Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults",
"author": "Bleys",
"doi-asserted-by": "crossref",
"first-page": "404",
"issue": "4",
"journal-title": "JAMA Intern. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib12",
"volume": "168",
"year": "2008"
},
{
"DOI": "10.1093/ndt/gfq859",
"article-title": "Serum selenium levels are inversely associated with death risk among hemodialysis patients",
"author": "Fujishima",
"doi-asserted-by": "crossref",
"first-page": "3331",
"issue": "10",
"journal-title": "Nephrol., Dial., Transplant.",
"key": "10.1016/j.jtemb.2022.127099_bib13",
"volume": "26",
"year": "2011"
},
{
"DOI": "10.1089/ars.2010.3526",
"article-title": "Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice",
"author": "Labunskyy",
"doi-asserted-by": "crossref",
"first-page": "2327",
"issue": "12",
"journal-title": "Antioxid. Redox Signal.",
"key": "10.1016/j.jtemb.2022.127099_bib14",
"volume": "14",
"year": "2011"
},
{
"article-title": "Selenium and human health: witnessing a Copernican revolution?",
"author": "Jablonska",
"first-page": "328",
"issue": "3",
"journal-title": "J. Environ. Sci. Health",
"key": "10.1016/j.jtemb.2022.127099_bib15",
"volume": "33",
"year": "2015"
},
{
"DOI": "10.1016/S0140-6736(11)61452-9",
"article-title": "Selenium and human health",
"author": "Rayman",
"doi-asserted-by": "crossref",
"first-page": "1256",
"issue": "9822",
"journal-title": "Lancet",
"key": "10.1016/j.jtemb.2022.127099_bib16",
"volume": "379",
"year": "2012"
},
{
"DOI": "10.1016/j.freeradbiomed.2013.04.003",
"article-title": "Epidemiology of selenium and type 2 diabetes: can we make sense of it",
"author": "Rayman",
"doi-asserted-by": "crossref",
"first-page": "1557",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib17",
"volume": "65",
"year": "2013"
},
{
"DOI": "10.3390/nu12072098",
"article-title": "Selenium deficiency is associated with mortality risk from COVID-19",
"author": "Moghaddam",
"doi-asserted-by": "crossref",
"first-page": "2098",
"issue": "7",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib18",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.redox.2020.101715",
"article-title": "Selenium and selenoproteins in viral infection with potential relevance to COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"journal-title": "Redox Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib19",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.1016/j.envres.2021.111190",
"article-title": "Serum selenium and non-alcoholic fatty liver disease (NAFLS) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2011-2016",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "Environ. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib20",
"volume": "197",
"year": "2021"
},
{
"DOI": "10.1007/s40572-018-0213-0",
"article-title": "Environmental selenium and human health: an update",
"author": "Vinceti",
"doi-asserted-by": "crossref",
"first-page": "464",
"issue": "4",
"journal-title": "Curr. Environ. Health Rep.",
"key": "10.1016/j.jtemb.2022.127099_bib21",
"volume": "5",
"year": "2018"
},
{
"article-title": "Selenium for preventing cancer",
"author": "Vinceti",
"issue": "1",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "10.1016/j.jtemb.2022.127099_bib22",
"volume": "1",
"year": "2018"
},
{
"DOI": "10.1016/j.envres.2019.108742",
"article-title": "Amyotrophic lateral sclerosis incidence following exposure to inorganic selenium in drinking water: A long-term follow-up",
"author": "Vinceti",
"doi-asserted-by": "crossref",
"journal-title": "Environ. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib23",
"volume": "179",
"year": "2019"
},
{
"DOI": "10.1016/j.numecd.2021.02.016",
"article-title": "Dietary selenium intake and risk of hospitalization for type 2 diabetes in the Moli-sani study cohort",
"author": "Vinceti",
"doi-asserted-by": "crossref",
"first-page": "1738",
"issue": "6",
"journal-title": "Nutr., Metab. Cardiovasc. Dis.",
"key": "10.1016/j.jtemb.2022.127099_bib24",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1016/j.envres.2021.111210",
"article-title": "A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies",
"author": "Vinceti",
"doi-asserted-by": "crossref",
"journal-title": "Environ. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib25",
"volume": "197",
"year": "2021"
},
{
"DOI": "10.1007/s12020-014-0298-7",
"article-title": "Selenium supplementation and the risk of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials",
"author": "Mao",
"doi-asserted-by": "crossref",
"first-page": "758",
"issue": "3",
"journal-title": "Endocrine",
"key": "10.1016/j.jtemb.2022.127099_bib26",
"volume": "47",
"year": "2014"
},
{
"DOI": "10.1093/ajcn/nqab241",
"article-title": "Serum selenium concentrations and risk of all-cause and heart disease mortality among individuals with type 2 diabetes",
"author": "Qiu",
"doi-asserted-by": "crossref",
"first-page": "53",
"issue": "1",
"journal-title": "Am. J. Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib27",
"volume": "115",
"year": "2022"
},
{
"DOI": "10.1038/s41387-020-0117-6",
"article-title": "Use and abuse of dietary supplements in persons with diabetes",
"author": "Hannon",
"doi-asserted-by": "crossref",
"first-page": "14",
"issue": "1",
"journal-title": "Nutr. Diabetes",
"key": "10.1016/j.jtemb.2022.127099_bib28",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.3390/nu10121924",
"article-title": "Selenium and type 2 diabetes: systematic review",
"author": "Kohler",
"doi-asserted-by": "crossref",
"first-page": "1024",
"issue": "12",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib29",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1016/j.redox.2022.102236",
"article-title": "The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities",
"author": "Steinbrenner",
"doi-asserted-by": "crossref",
"journal-title": "Redox Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib30",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1017/S000711452100177X",
"article-title": "Association between selenium intake, diabetes and mortality in adults: finding from National Health and Nutrition Examination Survey (NHANES) 2003-2014",
"author": "Hoque",
"doi-asserted-by": "crossref",
"first-page": "1098",
"issue": "7",
"journal-title": "Br. J. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib31",
"volume": "127",
"year": "2021"
},
{
"key": "10.1016/j.jtemb.2022.127099_bib32",
"unstructured": "WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available in https://covid19.who.int/ (Accessed on 02 March 2022)."
},
{
"DOI": "10.1001/jamanetworkopen.2020.22310",
"article-title": "Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection",
"author": "Ioannou",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.jtemb.2022.127099_bib33",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1093/ajcn/nqaa095",
"article-title": "Association between regional selenium and reported outcome of COVID-19 cases in China",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1297",
"issue": "6",
"journal-title": "Am. J. Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib34",
"volume": "111",
"year": "2020"
},
{
"DOI": "10.1002/jmv.1890430213",
"article-title": "Benign human enterovirus becomes virulent in selenium-deficient mice",
"author": "Beck",
"doi-asserted-by": "crossref",
"first-page": "166",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.jtemb.2022.127099_bib35",
"volume": "43",
"year": "1994"
},
{
"article-title": "Selenium deficiency increases the pathology of an influenza virus infection",
"author": "Beck",
"first-page": "1481",
"issue": "8",
"journal-title": "Fed. Am. Soc. Exp. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib36",
"volume": "15",
"year": "2001"
},
{
"DOI": "10.3390/nu10091203",
"article-title": "Selenium, selenoproteins, and Immunity",
"author": "Avery",
"doi-asserted-by": "crossref",
"first-page": "1203",
"issue": "9",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib37",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.3390/nu11092101",
"article-title": "Selenium, selenoproteins and viral infection",
"author": "Guillin",
"doi-asserted-by": "crossref",
"first-page": "2101",
"issue": "9",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib38",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.3390/antiox11020251",
"article-title": "The role of selenium in pathologies: an updated review",
"author": "Barchielli",
"doi-asserted-by": "crossref",
"first-page": "251",
"issue": "2",
"journal-title": "Antioxidants",
"key": "10.1016/j.jtemb.2022.127099_bib39",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.fct.2021.112161",
"article-title": "The effects of some essential and toxic metals/metalloids in COVID-19: a review",
"author": "Domingo",
"doi-asserted-by": "crossref",
"journal-title": "Food Chem. Toxicol.",
"key": "10.1016/j.jtemb.2022.127099_bib40",
"volume": "152",
"year": "2021"
},
{
"article-title": "Selenium to selenoproteins - role in COVID-19",
"author": "Tomo",
"first-page": "781",
"journal-title": "EXCLI J.",
"key": "10.1016/j.jtemb.2022.127099_bib41",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1007/s00391-020-01735-0",
"article-title": "Selenium and zinc: “antioxidants” for healthy aging?",
"author": "Steinbrenner",
"doi-asserted-by": "crossref",
"first-page": "295",
"issue": "4",
"journal-title": "Z. für Gerontol. und Geriatr.",
"key": "10.1016/j.jtemb.2022.127099_bib42",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.3390/antiox10020257",
"article-title": "Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study",
"author": "Pincemail",
"doi-asserted-by": "crossref",
"first-page": "257",
"issue": "2",
"journal-title": "Antioxidants",
"key": "10.1016/j.jtemb.2022.127099_bib43",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1177/2050312121991246",
"article-title": "Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: a cross-sectional comparative study in Jigawa, Northwestern Nigeria",
"author": "Muhammad",
"doi-asserted-by": "crossref",
"journal-title": "SAGE Open Med.",
"key": "10.1016/j.jtemb.2022.127099_bib44",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1089/ars.2021.0017",
"article-title": "COVID-19: A redox disease-What a stress pandemic can teach us about resilience and what we may learn from the reactive species interactome about its treatment",
"author": "Cumpstey",
"doi-asserted-by": "crossref",
"first-page": "1226",
"issue": "14",
"journal-title": "Antioxid. Redox Signal.",
"key": "10.1016/j.jtemb.2022.127099_bib45",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.redox.2019.101388",
"article-title": "Distinct and overlapping functions of glutathione peroxidases 1 and 2 in limiting NF-κB-driven inflammation through redox-active mechanisms",
"author": "Koeberle",
"doi-asserted-by": "crossref",
"journal-title": "Redox Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib46",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1080/10409238.2020.1717430",
"article-title": "Selenium and selenoproteins in prostanoid metabolism and immunity",
"author": "Qian",
"doi-asserted-by": "crossref",
"first-page": "484",
"issue": "6",
"journal-title": "Crit. Rev. Biochem. Mol. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib47",
"volume": "54",
"year": "2019"
},
{
"DOI": "10.1016/j.jaci.2009.09.017",
"article-title": "Adaptive immunity",
"author": "Bonilla",
"doi-asserted-by": "crossref",
"first-page": "S33",
"issue": "2",
"journal-title": "J. Allergy Clin. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib48",
"volume": "125",
"year": "2010"
},
{
"DOI": "10.1016/j.cyto.2014.09.011",
"article-title": "T cell subsets and their signature cytokines in autoimmune and inflammatory disease",
"author": "Raphael",
"doi-asserted-by": "crossref",
"first-page": "5",
"issue": "1",
"journal-title": "Cytokine",
"key": "10.1016/j.jtemb.2022.127099_bib49",
"volume": "74",
"year": "2015"
},
{
"DOI": "10.1016/j.coi.2020.09.001",
"article-title": "Cytokines and transcription factors in the differentiation of CD4+ T helper cell subsets and induction of tissue inflammation and autoimmunity",
"author": "Pawlak",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Curr. Opin. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib50",
"volume": "67",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0285-6",
"article-title": "Defining trained immunity and its role in health and disease",
"author": "Netea",
"doi-asserted-by": "crossref",
"first-page": "375",
"issue": "6",
"journal-title": "Nat. Rev. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib51",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2016.00160",
"article-title": "Resolution of inflammation: what controls its onset?",
"author": "Sugimoto",
"doi-asserted-by": "crossref",
"first-page": "160",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib52",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1007/978-3-319-95390-8_7",
"article-title": "Selenium and inflammatory mediators",
"author": "Koeberle",
"doi-asserted-by": "crossref",
"key": "10.1016/j.jtemb.2022.127099_bib53",
"series-title": "Selenium. Molecular and Integrative Toxicology",
"year": "2018"
},
{
"DOI": "10.1016/S0013-9351(87)80194-9",
"article-title": "Selenium and immune responses",
"author": "Kiremidjian-Schumacher",
"doi-asserted-by": "crossref",
"first-page": "277",
"issue": "2",
"journal-title": "Environ. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib54",
"volume": "42",
"year": "1987"
},
{
"DOI": "10.3181/00379727-193-43014",
"article-title": "Selenium and immune cell function. I. Effect on lymphocyte proliferation and production of interleukin 1 and interleukin 2",
"author": "Kiremidjian-Schumacher",
"doi-asserted-by": "crossref",
"first-page": "136",
"journal-title": "Proc. Soc. Exp. Biol. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib55",
"volume": "193",
"year": "1990"
},
{
"article-title": "Selenium and inflammation",
"author": "Kaushal",
"first-page": "443",
"key": "10.1016/j.jtemb.2022.127099_bib56",
"series-title": "Selenium",
"year": "2011"
},
{
"DOI": "10.1016/j.clnu.2015.12.003",
"article-title": "Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults",
"author": "Ivory",
"doi-asserted-by": "crossref",
"first-page": "407",
"issue": "2",
"journal-title": "Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib57",
"volume": "36",
"year": "2017"
},
{
"DOI": "10.1016/S0034-5288(18)32461-5",
"article-title": "Effect of dietary vitamin E and selenium on phytohaemagglutinin response of pig lymphocytes",
"author": "Larsen",
"doi-asserted-by": "crossref",
"first-page": "301",
"journal-title": "Res. Vet. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib58",
"volume": "31",
"year": "1981"
},
{
"DOI": "10.1016/0165-2427(85)90115-1",
"article-title": "Stimulatory effects of selenium on mitogen responses in lambs",
"author": "Turner",
"doi-asserted-by": "crossref",
"first-page": "119",
"journal-title": "Vet. Immunol. Immunopathol.",
"key": "10.1016/j.jtemb.2022.127099_bib59",
"volume": "8",
"year": "1985"
},
{
"DOI": "10.1089/ars.2011.4145",
"article-title": "The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "705",
"issue": "7",
"journal-title": "Antioxid. Redox Signal.",
"key": "10.1016/j.jtemb.2022.127099_bib60",
"volume": "16",
"year": "2012"
},
{
"DOI": "10.1002/jlb.39.4.447",
"article-title": "Effects of selenium in vitro on human T-lymphocyte functions and K-564 tumor cell growth",
"author": "Watson",
"doi-asserted-by": "crossref",
"first-page": "447",
"issue": "4",
"journal-title": "J. Leukoc. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib61",
"volume": "39",
"year": "1986"
},
{
"DOI": "10.1016/0162-3109(90)90067-O",
"article-title": "Immunoregulation of natural and lymphokine-activated killer cells by selenium",
"author": "Nair",
"doi-asserted-by": "crossref",
"first-page": "177",
"issue": "3",
"journal-title": "Immunopharmacology",
"key": "10.1016/j.jtemb.2022.127099_bib62",
"volume": "19",
"year": "1990"
},
{
"DOI": "10.1016/j.semcdb.2020.11.006",
"article-title": "Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "54",
"journal-title": "Semin. Cell Dev. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib63",
"volume": "115",
"year": "2021"
},
{
"DOI": "10.1189/jlb.2A0316-156RR",
"article-title": "Selenoprotein K regulation of palmitoylation and calpain cleavage of ASAP2 is required for efficient Fcγ-R mediated phagocytosis",
"author": "Norton",
"doi-asserted-by": "crossref",
"first-page": "439",
"issue": "2",
"journal-title": "J. Leukoc. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib64",
"volume": "101",
"year": "2017"
},
{
"DOI": "10.1073/pnas.1417176111",
"article-title": "Stable expression and function of the inositol 1,4,5-triphosphate receptor requires palmitoylation by a DHHC6/selenoprotein K complex",
"author": "Fredericks",
"doi-asserted-by": "crossref",
"first-page": "16478",
"issue": "46",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "10.1016/j.jtemb.2022.127099_bib65",
"volume": "111",
"year": "2014"
},
{
"DOI": "10.1080/713654938",
"article-title": "Effect of supplementation of vitamin E, selenium and their different combinations on the performance and immune response of broilers",
"author": "Swain",
"doi-asserted-by": "crossref",
"first-page": "287",
"issue": "3",
"journal-title": "Br. Poult. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib66",
"volume": "41",
"year": "2000"
},
{
"DOI": "10.1007/s12011-017-1110-3",
"article-title": "Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "364",
"issue": "2",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib67",
"volume": "182",
"year": "2017"
},
{
"DOI": "10.1017/S0029665199000920",
"article-title": "Selenium and host defense towards viruses",
"author": "Beck",
"doi-asserted-by": "crossref",
"first-page": "707",
"issue": "3",
"journal-title": "Proc. Nutr. Soc.",
"key": "10.1016/j.jtemb.2022.127099_bib68",
"volume": "58",
"year": "1999"
},
{
"article-title": "Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols",
"author": "Hoffmann",
"issue": "6",
"journal-title": "J. Nutr. Nutr. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib69",
"volume": "140",
"year": "2010"
},
{
"DOI": "10.1017/S002966511000176X",
"article-title": "Role of selenium-containing proteins in the function of T cells and macrophages",
"author": "Carlson",
"doi-asserted-by": "crossref",
"first-page": "300",
"issue": "3",
"journal-title": "Proc. Nutr. Soc.",
"key": "10.1016/j.jtemb.2022.127099_bib70",
"volume": "69",
"year": "2010"
},
{
"DOI": "10.3945/jn.111.141176",
"article-title": "Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages",
"author": "Nelson",
"doi-asserted-by": "crossref",
"first-page": "1754",
"issue": "9",
"journal-title": "J. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib71",
"volume": "141",
"year": "2011"
},
{
"DOI": "10.1007/s12011-010-8726-x",
"article-title": "Protection from H1N1 influenza virus infections in mice by supplementation with selenium: a comparison with selenium-deficient mice",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "254",
"issue": "1–3",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib72",
"volume": "141",
"year": "2011"
},
{
"DOI": "10.1007/s12011-019-01775-7",
"article-title": "Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "237",
"issue": "1",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib73",
"volume": "194",
"year": "2020"
},
{
"DOI": "10.1016/j.jbc.2021.100410",
"article-title": "Selenium-dependent metabolic reprogramming during inflammation and resolution",
"author": "Korwar",
"doi-asserted-by": "crossref",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib74",
"volume": "296",
"year": "2021"
},
{
"DOI": "10.1007/s12011-016-0794-0",
"article-title": "Effects of different dietary selenium sources on antioxidant status and blood phagocytic activity in sheep",
"author": "Čobanová",
"doi-asserted-by": "crossref",
"first-page": "339",
"issue": "2",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib75",
"volume": "175",
"year": "2017"
},
{
"DOI": "10.1016/0021-9975(79)90018-5",
"article-title": "Alterations of neutrophil function in selenium-deficient cattle",
"author": "Boyne",
"doi-asserted-by": "crossref",
"first-page": "151",
"journal-title": "J. Comp. Pathol.",
"key": "10.1016/j.jtemb.2022.127099_bib76",
"volume": "89",
"year": "1979"
},
{
"DOI": "10.2527/1997.7561659x",
"article-title": "Dietary vitamin E and selenium affect mastitis and milk quality",
"author": "Smith",
"doi-asserted-by": "crossref",
"first-page": "1659",
"issue": "6",
"journal-title": "J. Anim. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib77",
"volume": "75",
"year": "1997"
},
{
"article-title": "Effects of selenium on polymorphonuclear leukocyte function in goats",
"author": "Aziz",
"first-page": "1715",
"issue": "9",
"journal-title": "Am. J. Vet. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib78",
"volume": "45",
"year": "1984"
},
{
"DOI": "10.1016/0165-2427(85)90026-1",
"article-title": "The effect of selenium deficiency in goats on lymphocyte production of leukocyte migration inhibitory factor",
"author": "Aziz",
"doi-asserted-by": "crossref",
"first-page": "381",
"issue": "4",
"journal-title": "Vet. Immunol. Immunopathol.",
"key": "10.1016/j.jtemb.2022.127099_bib79",
"volume": "10",
"year": "1985"
},
{
"article-title": "Effect of selenium deficiency on caprine polymorphonuclear leukocyte production of leukotriene B4 and its neutrophil chemotactic activity",
"author": "Aziz",
"first-page": "426",
"issue": "2",
"journal-title": "Am. J. Vet. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib80",
"volume": "47",
"year": "1986"
},
{
"DOI": "10.2527/jas.2012-5819",
"article-title": "The effect of selenium supplementation on vaccination response and immune function in adult horses",
"author": "Brummer",
"doi-asserted-by": "crossref",
"first-page": "3702",
"issue": "8",
"journal-title": "J. Anim. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib81",
"volume": "91",
"year": "2013"
},
{
"DOI": "10.18632/oncotarget.14550",
"article-title": "Oxidative stress induced by Se-deficient high-energy diet implicates neutrophil dysfunction via Nrf2 pathway suppression in swine",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "13428",
"issue": "8",
"journal-title": "Oncotarget",
"key": "10.1016/j.jtemb.2022.127099_bib82",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1016/0009-2797(94)90038-8",
"article-title": "Selenium deficiency in HIV infection and the acquired immunodeficiency syndrome (AIDS)",
"author": "Dworkin",
"doi-asserted-by": "crossref",
"first-page": "181",
"issue": "2–3",
"journal-title": "Chem. Biol. Interact.",
"key": "10.1016/j.jtemb.2022.127099_bib83",
"volume": "91",
"year": "1994"
},
{
"DOI": "10.1086/315911",
"article-title": "Selenium and interleukins in persons infected with human immunodeficiency virus type 1",
"author": "Baum",
"doi-asserted-by": "crossref",
"first-page": "S69",
"issue": "1",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.jtemb.2022.127099_bib84",
"volume": "182",
"year": "2000"
},
{
"DOI": "10.1007/s12026-019-9064-5",
"article-title": "Potential roles of neutrophils in maintaining the health and productivity of dairy cows during various physiological and physiopathological conditions: a review",
"author": "Alhussien",
"doi-asserted-by": "crossref",
"first-page": "21",
"issue": "1",
"journal-title": "Immunol. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib85",
"volume": "67",
"year": "2019"
},
{
"DOI": "10.1007/s00018-020-03591-y",
"article-title": "The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens",
"author": "Ulfig",
"doi-asserted-by": "crossref",
"first-page": "385",
"issue": "2",
"journal-title": "Cell. Mol. Life Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib86",
"volume": "78",
"year": "2021"
},
{
"DOI": "10.3390/cells11132025",
"article-title": "Integrin regulators in neutrophils",
"author": "Pulikkot",
"doi-asserted-by": "crossref",
"first-page": "2025",
"issue": "13",
"journal-title": "Cells",
"key": "10.1016/j.jtemb.2022.127099_bib87",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1042/cs1000543",
"article-title": "Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase",
"author": "Miller",
"doi-asserted-by": "crossref",
"first-page": "543",
"issue": "5",
"journal-title": "Clin. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib88",
"volume": "100",
"year": "2001"
},
{
"DOI": "10.1016/S0021-9150(01)00672-4",
"article-title": "Inhibition of TNF-alpha induced ICAM-1, VCAM-1 and E-selectin expression by selenium",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "381",
"issue": "2",
"journal-title": "Atherosclerosis",
"key": "10.1016/j.jtemb.2022.127099_bib89",
"volume": "161",
"year": "2002"
},
{
"DOI": "10.1016/S0891-5849(99)00251-8",
"article-title": "Altered eicosanoid biosynthesis in selenium deficient endothelial cells",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "381",
"issue": "3",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib90",
"volume": "28",
"year": "2000"
},
{
"DOI": "10.1007/s12011-017-1145-5",
"article-title": "Inflammatory response occurs in veins of broiler chickens treated with a selenium deficiency diet",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "361",
"issue": "2",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib91",
"volume": "183",
"year": "2018"
},
{
"DOI": "10.1093/jn/133.5.1457S",
"article-title": "Selenium in the immune system",
"author": "Arthur",
"doi-asserted-by": "crossref",
"first-page": "1457S",
"issue": "5 Suppl 1",
"journal-title": "J. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib92",
"volume": "133",
"year": "2003"
},
{
"DOI": "10.1016/S0891-5849(02)00775-X",
"article-title": "Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency",
"author": "Zamamiri-Davis",
"doi-asserted-by": "crossref",
"first-page": "890",
"issue": "9",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib93",
"volume": "32",
"year": "2002"
},
{
"DOI": "10.1074/jbc.M703075200",
"article-title": "The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages",
"author": "Vunta",
"doi-asserted-by": "crossref",
"first-page": "17964",
"issue": "25",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib94",
"volume": "282",
"year": "2007"
},
{
"DOI": "10.1016/j.trsl.2019.01.004",
"article-title": "Selenium and selenoproteins: from endothelial cytoprotection to clinical outcomes",
"author": "Junior",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Transl. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib95",
"volume": "208",
"year": "2019"
},
{
"DOI": "10.1016/j.smallrumres.2009.12.042",
"article-title": "The importance of selenium and the effects of its deficiency in animal health",
"author": "Hefnawy",
"doi-asserted-by": "crossref",
"first-page": "185",
"issue": "2–3",
"journal-title": "Small Rumin. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib96",
"volume": "89",
"year": "2010"
},
{
"DOI": "10.4049/jimmunol.1002878",
"article-title": "Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses",
"author": "Verma",
"doi-asserted-by": "crossref",
"first-page": "2127",
"issue": "4",
"journal-title": "J. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib97",
"volume": "186",
"year": "2011"
},
{
"DOI": "10.1056/NEJMcibr1402199",
"article-title": "Keshan disease, selenium deficiency, and the selenoproteome",
"author": "Loscalzo",
"doi-asserted-by": "crossref",
"first-page": "1756",
"issue": "18",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib98",
"volume": "370",
"year": "2014"
},
{
"DOI": "10.1007/s12011-018-1396-9",
"article-title": "Selenium deficiency affects immune function by influencing selenoprotein and cytokine expression in chicken spleen",
"author": "Khoso",
"doi-asserted-by": "crossref",
"first-page": "506",
"issue": "2",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib99",
"volume": "187",
"year": "2019"
},
{
"DOI": "10.1007/s12011-011-9108-8",
"article-title": "The relationship between serum selenium concentration and neutrophil function in peripheral blood",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "396",
"issue": "1–3",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib100",
"volume": "144",
"year": "2011"
},
{
"DOI": "10.1042/BJ20101762",
"article-title": "Selenium-containing amino acids are targets for myeloperoxidase-derived hypothiocyanous acid: determination of absolute rate constants and implications for biological damage",
"author": "Skaf",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "Biochem. J.",
"key": "10.1016/j.jtemb.2022.127099_bib101",
"volume": "441",
"year": "2012"
},
{
"DOI": "10.1038/s41467-022-28385-7",
"article-title": "Selenophosphate synthetase 1 deficiency exacerbates osteoarthritis by dysregulating redox homeostasis",
"author": "Kang",
"doi-asserted-by": "crossref",
"first-page": "779",
"issue": "1",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.jtemb.2022.127099_bib102",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.jnutbio.2022.109001",
"article-title": "MiR-1656 targets GPX4 to trigger pyroptosis in broilers kidney tissues by activating NLRP3 inflammasome under Se deficiency",
"author": "Gu",
"doi-asserted-by": "crossref",
"journal-title": "J. Nutr. Biochem.",
"key": "10.1016/j.jtemb.2022.127099_bib103",
"volume": "105",
"year": "2022"
},
{
"DOI": "10.4049/jimmunol.180.9.6168",
"article-title": "Alternatively activated myeloid cells limit pathogenicity associated with African trypanosomiasis through the IL-10 inducible gene selenoprotein p",
"author": "Bosschaerts",
"doi-asserted-by": "crossref",
"first-page": "6168",
"journal-title": "J. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib104",
"volume": "180",
"year": "2008"
},
{
"DOI": "10.1172/JCI76099",
"article-title": "Selenoprotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage",
"author": "Barrett",
"doi-asserted-by": "crossref",
"first-page": "2646",
"journal-title": "J. Clin. Investig.",
"key": "10.1016/j.jtemb.2022.127099_bib105",
"volume": "125",
"year": "2015"
},
{
"DOI": "10.2460/ajvr.1990.51.02.269",
"article-title": "Phagocytosis, bactericidal activity, and oxidative metabolism of milk neutrophils from dairy cows fed selenium-supplemented and selenium-deficient diets",
"author": "Grasso",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "2",
"journal-title": "Am. J. Vet. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib106",
"volume": "51",
"year": "1990"
},
{
"DOI": "10.1007/BF02788958",
"article-title": "Selenium and cellular immunity. Evidence that selenoproteins may be encoded in the +1 reading frame overlapping the human CD4, CD8, and HLA-DR genes",
"author": "Taylor",
"doi-asserted-by": "crossref",
"first-page": "85",
"issue": "2–3",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib107",
"volume": "49",
"year": "1995"
},
{
"DOI": "10.3390/ijms222011060",
"article-title": "The trace element selenium is important for redox signaling in phorbol ester-differentiated THP-1 macrophages",
"author": "Wolfram",
"doi-asserted-by": "crossref",
"first-page": "11060",
"issue": "20",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib108",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1074/jbc.M802559200",
"article-title": "Selenoproteins mediate T cell immunity through an antioxidant mechanism",
"author": "Shrimali",
"doi-asserted-by": "crossref",
"first-page": "20181",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib109",
"volume": "283",
"year": "2008"
},
{
"DOI": "10.1186/1471-2172-10-57",
"article-title": "Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression",
"author": "Carlson",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "BMC Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib110",
"volume": "10",
"year": "2009"
},
{
"article-title": "Effects of selenium on immunity and aging",
"author": "McKenzie",
"first-page": "257",
"key": "10.1016/j.jtemb.2022.127099_bib111",
"series-title": "Selenium",
"year": "2001"
},
{
"DOI": "10.1002/mnfr.200700330",
"article-title": "The influence of selenium on immune responses",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "1273",
"issue": "11",
"journal-title": "Mol. Nutr. Food Res.",
"key": "10.1016/j.jtemb.2022.127099_bib112",
"volume": "52",
"year": "2008"
},
{
"DOI": "10.1055/s-0029-1220724",
"article-title": "Selenium and inflammation: underlying anti-inflammatory mechanisms",
"author": "Duntas",
"doi-asserted-by": "crossref",
"first-page": "443",
"issue": "06",
"journal-title": "Horm. Metab. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib113",
"volume": "41",
"year": "2009"
},
{
"article-title": "Selenium attenuates pro-inflammatory gene expression in macrophages",
"author": "Vunta",
"first-page": "1316",
"issue": "11",
"journal-title": "Mol. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib114",
"volume": "52",
"year": "2008"
},
{
"article-title": "Review paper. The role of selected microelements: selenium, zinc, chromium and iron in immune system",
"author": "Terpiłowska",
"first-page": "303",
"issue": "4",
"journal-title": "Cent. Eur. J. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib115",
"volume": "36",
"year": "2011"
},
{
"DOI": "10.1016/j.bbrc.2010.03.083",
"article-title": "Thioredoxin and thioredoxin reductase: current research with special reference to human disease",
"author": "Holmgren",
"doi-asserted-by": "crossref",
"first-page": "120",
"issue": "1",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.jtemb.2022.127099_bib116",
"volume": "396",
"year": "2010"
},
{
"DOI": "10.3390/nu7085297",
"article-title": "Dietary selenium levels affect selenoprotein expression and support the interferon-γ and IL-6 immune response pathways in mice",
"author": "Tsuji",
"doi-asserted-by": "crossref",
"first-page": "6529",
"issue": "8",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib117",
"volume": "7",
"year": "2015"
},
{
"DOI": "10.3390/nu12103007",
"article-title": "The relevance of selenium status in rheumatoid arthritis",
"author": "Turrubiates-Hernández",
"doi-asserted-by": "crossref",
"first-page": "3007",
"issue": "10",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib118",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/S0891-5849(00)00183-0",
"article-title": "Reactive oxygen species and proinflammatory cytokine signaling in endothelial cells: effect of selenium supplementation",
"author": "Tolando",
"doi-asserted-by": "crossref",
"first-page": "979",
"issue": "6",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib119",
"volume": "28",
"year": "2000"
},
{
"DOI": "10.1017/jns.2013.17",
"article-title": "Regulation of inflammation by selenium and selenoproteins: impact on eicosanoid biosynthesis",
"author": "Mattmiller",
"doi-asserted-by": "crossref",
"journal-title": "J. Nutr. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib120",
"volume": "2",
"year": "2013"
},
{
"DOI": "10.3109/10715762.2010.498478",
"article-title": "Pathological aspects of lipid peroxidation",
"author": "Negre-Salvayre",
"doi-asserted-by": "crossref",
"first-page": "1125",
"issue": "10",
"journal-title": "Free Radic. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib121",
"volume": "44",
"year": "2010"
},
{
"DOI": "10.1016/j.jnutbio.2011.06.011",
"article-title": "Marginal selenium deficiency down-regulates inflammation-related genes in splenic leukocytes of the mouse",
"author": "Kipp",
"doi-asserted-by": "crossref",
"first-page": "1170",
"issue": "9",
"journal-title": "J. Nutr. Biochem.",
"key": "10.1016/j.jtemb.2022.127099_bib122",
"volume": "23",
"year": "2012"
},
{
"DOI": "10.1152/physrev.00039.2013",
"article-title": "Selenoproteins: molecular pathways and physiological roles",
"author": "Labunskyy",
"doi-asserted-by": "crossref",
"first-page": "739",
"issue": "3",
"journal-title": "Physiol. Rev.",
"key": "10.1016/j.jtemb.2022.127099_bib123",
"volume": "94",
"year": "2014"
},
{
"DOI": "10.1042/BCJ20170920",
"article-title": "Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease",
"author": "Addinsall",
"doi-asserted-by": "crossref",
"first-page": "1037",
"issue": "6",
"journal-title": "Biochem. J.",
"key": "10.1016/j.jtemb.2022.127099_bib124",
"volume": "475",
"year": "2018"
},
{
"DOI": "10.1186/1743-422X-5-66",
"article-title": "In vitro effects of selenium deficiency on West Nile virus replication and cytopathogenicity",
"author": "Verma",
"doi-asserted-by": "crossref",
"first-page": "66",
"journal-title": "Virol. J.",
"key": "10.1016/j.jtemb.2022.127099_bib125",
"volume": "5",
"year": "2008"
},
{
"DOI": "10.1126/science.aam7928",
"article-title": "Inflammation and metabolism in tissue repair and regeneration",
"author": "Eming",
"doi-asserted-by": "crossref",
"first-page": "1026",
"issue": "6342",
"journal-title": "Science",
"key": "10.1016/j.jtemb.2022.127099_bib126",
"volume": "356",
"year": "2017"
},
{
"DOI": "10.1016/0306-9877(84)90129-4",
"article-title": "Can dietary selenium reduce leukotriene production",
"author": "McCarty",
"doi-asserted-by": "crossref",
"first-page": "45",
"issue": "1",
"journal-title": "Med. Hypotheses",
"key": "10.1016/j.jtemb.2022.127099_bib127",
"volume": "13",
"year": "1984"
},
{
"DOI": "10.1016/S0021-9258(18)80071-0",
"article-title": "The role of selenium-dependent and selenium-independent glutathione peroxidases in the formation of prostaglandin F2α",
"author": "Hong",
"doi-asserted-by": "crossref",
"first-page": "13793",
"issue": "23",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib128",
"volume": "264",
"year": "1989"
},
{
"DOI": "10.1016/0262-1746(84)90100-8",
"article-title": "Biological consequences of fatty acid oxygenase reaction mechanisms",
"author": "Lands",
"doi-asserted-by": "crossref",
"first-page": "35",
"journal-title": "Prostaglandins Leukot. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib129",
"volume": "13",
"year": "1984"
},
{
"DOI": "10.1074/jbc.273.4.1990",
"article-title": "Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase",
"author": "Imai",
"doi-asserted-by": "crossref",
"first-page": "1990",
"issue": "4",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib130",
"volume": "273",
"year": "1998"
},
{
"DOI": "10.1016/0049-0172(91)90031-T",
"article-title": "Selenium in rheumatic diseases",
"author": "Peretz",
"doi-asserted-by": "crossref",
"first-page": "305",
"issue": "5",
"journal-title": "Semin. Arthritis Rheum.",
"key": "10.1016/j.jtemb.2022.127099_bib131",
"volume": "20",
"year": "1991"
},
{
"article-title": "Selenium and the selenium-dependent glutathione peroxidase in rheumatoid arthritis",
"author": "Tarp",
"first-page": "264",
"issue": "3",
"journal-title": "Dan. Med. Bull.",
"key": "10.1016/j.jtemb.2022.127099_bib132",
"volume": "41",
"year": "1994"
},
{
"DOI": "10.3390/antiox7030036",
"article-title": "Selenium and selenoproteins in gut inflammation: a review",
"author": "Nettleford",
"doi-asserted-by": "crossref",
"first-page": "6",
"issue": "3",
"journal-title": "Antioxidants",
"key": "10.1016/j.jtemb.2022.127099_bib133",
"volume": "7",
"year": "2018"
},
{
"article-title": "Selenium, glutathione peroxidase, peroxides and platelet functions",
"author": "Vitoux",
"first-page": "181",
"issue": "5",
"journal-title": "Ann.de Biol. Clin.",
"key": "10.1016/j.jtemb.2022.127099_bib134",
"volume": "54",
"year": "1996"
},
{
"DOI": "10.1074/jbc.M900392200",
"article-title": "Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses",
"author": "Handy",
"doi-asserted-by": "crossref",
"first-page": "11913",
"issue": "18",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib135",
"volume": "284",
"year": "2009"
},
{
"DOI": "10.1007/s12011-014-0076-7",
"article-title": "Selenium downregulates oxidative stress-induced activation of leukotriene pathway in experimental rats with diabetic cardiac hypertrophy",
"author": "Dhanya",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib136",
"volume": "161",
"year": "2014"
},
{
"DOI": "10.1055/s-0038-1647308",
"article-title": "Selenium dependent glutathione peroxidase: a physiological regulatory system for platelet function",
"author": "Perona",
"doi-asserted-by": "crossref",
"first-page": "312",
"issue": "2",
"journal-title": "Thromb. Haemost.",
"key": "10.1016/j.jtemb.2022.127099_bib137",
"volume": "64",
"year": "1990"
},
{
"article-title": "Glutathione peroxidases and redox-regulated transcription factors",
"author": "Brigelius-Flohé",
"first-page": "1329",
"issue": "10–11",
"journal-title": "Biol. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib138",
"volume": "387",
"year": "2006"
},
{
"DOI": "10.1007/s12011-015-0536-8",
"article-title": "Selenium deficiency influences the mRNA expression of selenoproteins and cytokines in chicken erythrocytes",
"author": "Luan",
"doi-asserted-by": "crossref",
"first-page": "427",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib139",
"volume": "171",
"year": "2016"
},
{
"DOI": "10.1093/jn/nxac016",
"article-title": "Multi-Omics profiling reveals Se deficiency-induced redox imbalance, metabolic reprogramming, and inflammation in pig muscle",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1207",
"issue": "5",
"journal-title": "J. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib140",
"volume": "152",
"year": "2022"
},
{
"article-title": "Selenium deficiency induces spleen pathological changes in pigs by decreasing selenoprotein expression, evoking oxidative stress, and activating inflammation and apoptosis",
"author": "Li",
"issue": "65",
"journal-title": "J. Anim. Sci. Biotechnol.",
"key": "10.1016/j.jtemb.2022.127099_bib141",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.fct.2021.112286",
"article-title": "SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, ER stress and DNA synthesis",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "Food Chem. Toxicol.",
"key": "10.1016/j.jtemb.2022.127099_bib142",
"volume": "153",
"year": "2021"
},
{
"article-title": "Thyroid hormone deiodinases: physiology and clinical disorders",
"author": "Hernandez",
"first-page": "416",
"issue": "4",
"journal-title": "Endocrinol. Metab.",
"key": "10.1016/j.jtemb.2022.127099_bib143",
"volume": "15",
"year": "2003"
},
{
"DOI": "10.1530/JOE-12-0204",
"article-title": "Iodothyronine deiodinase structure and function: from ascidians to humans",
"author": "Darras",
"doi-asserted-by": "crossref",
"first-page": "189",
"issue": "2",
"journal-title": "J. Endocrinol.",
"key": "10.1016/j.jtemb.2022.127099_bib144",
"volume": "215",
"year": "2012"
},
{
"DOI": "10.1016/j.ceca.2017.05.001",
"article-title": "Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis",
"author": "Pitts",
"doi-asserted-by": "crossref",
"first-page": "76",
"journal-title": "Cell Calcium",
"key": "10.1016/j.jtemb.2022.127099_bib145",
"volume": "70",
"year": "2018"
},
{
"DOI": "10.3390/ijms222111906",
"article-title": "Selenoprotein DIO2 is a regulator of mitochondrial function, morphology and UPRmt in human cardiomyocytes",
"author": "Bomer",
"doi-asserted-by": "crossref",
"first-page": "11906",
"issue": "21",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib146",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.joca.2012.02.006",
"article-title": "DIO2 modifies inflammatory responses in chondrocytes",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "440",
"issue": "5",
"journal-title": "Osteoarthr. Cartil.",
"key": "10.1016/j.jtemb.2022.127099_bib147",
"volume": "20",
"year": "2012"
},
{
"DOI": "10.1677/JOE-09-0448",
"article-title": "Regulation of thyroid hormone activation via the liver X-receptor/retinoid X-receptor pathway",
"author": "Christoffolete",
"doi-asserted-by": "crossref",
"first-page": "179",
"issue": "2",
"journal-title": "J. Endocrinol.",
"key": "10.1016/j.jtemb.2022.127099_bib148",
"volume": "205",
"year": "2010"
},
{
"DOI": "10.1007/s12011-015-0282-y",
"article-title": "Selenium deficiency downregulates selenoproteins and suppresses immune function in chicken thymus",
"author": "Khoso",
"doi-asserted-by": "crossref",
"first-page": "48",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib149",
"volume": "167",
"year": "2015"
},
{
"article-title": "Neutrophil extracellular traps: formation and involvement in disease progression",
"author": "Kumar",
"first-page": "208",
"issue": "3",
"journal-title": "Iran. J. Allergy Asthma Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib150",
"volume": "17",
"year": "2018"
},
{
"DOI": "10.1016/j.micres.2020.126644",
"article-title": "Avoiding the trap: mechanisms developed by pathogens to scape neutrophil extracellular traps",
"author": "Ríos-López",
"doi-asserted-by": "crossref",
"journal-title": "Microbiol. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib151",
"volume": "243",
"year": "2021"
},
{
"DOI": "10.1007/s12016-020-08804-7",
"article-title": "A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics",
"author": "Mutua",
"doi-asserted-by": "crossref",
"first-page": "194",
"issue": "2",
"journal-title": "Clin. Rev. Allergy Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib152",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1007/s12011-020-02477-1",
"article-title": "Effect of copper, zinc and selenium on the formation of bovine neutrophil extracellular traps",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "3312",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib153",
"volume": "199",
"year": "2020"
},
{
"DOI": "10.1016/j.redox.2021.102003",
"article-title": "Roles of selenoprotein S in reactive oxygen species-dependent neutrophil extracellular trap formation induced by selenium-deficient arteritis",
"author": "Chi",
"doi-asserted-by": "crossref",
"journal-title": "Redox Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib154",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2017.00081",
"article-title": "Neutrophil extracellular traps and its implications in inflammation: an overview",
"author": "Delgado-Rizo",
"doi-asserted-by": "crossref",
"first-page": "81",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib155",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1111/resp.12730",
"article-title": "Neutrophil extracellular traps are associated with inflammation in chronic airway disease",
"author": "Wright",
"doi-asserted-by": "crossref",
"first-page": "467",
"issue": "3",
"journal-title": "Respirology",
"key": "10.1016/j.jtemb.2022.127099_bib156",
"volume": "21",
"year": "2016"
},
{
"article-title": "NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and beyond",
"author": "Zhu",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib157",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1084/jem.20201129",
"article-title": "SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology",
"author": "Veras",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "J. Exp. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib158",
"volume": "217",
"year": "2020"
},
{
"article-title": "Neutrophil extracellular traps in COVID-19",
"author": "Zuo",
"issue": "11",
"journal-title": "JCI Insight",
"key": "10.1016/j.jtemb.2022.127099_bib159",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.110475",
"article-title": "Impairment in selenocysteine synthesis as a candidate mechanism of inducible coagulopathy in COVID-19 patients",
"author": "Vavougios",
"doi-asserted-by": "crossref",
"journal-title": "Med. Hypotheses",
"key": "10.1016/j.jtemb.2022.127099_bib160",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1016/j.clnu.2013.01.005",
"article-title": "Erythrocyte selenium concentration as a marker of selenium status",
"author": "Stefanowicz",
"doi-asserted-by": "crossref",
"first-page": "837",
"issue": "5",
"journal-title": "Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib161",
"volume": "32",
"year": "2013"
},
{
"DOI": "10.1007/s00246-004-0659-8",
"article-title": "The impact of cardiopulmonary bypass on selenium status, thyroid function, and oxidative defense in children",
"author": "Holzer",
"doi-asserted-by": "crossref",
"first-page": "522",
"journal-title": "Pediatr. Cardiol.",
"key": "10.1016/j.jtemb.2022.127099_bib162",
"volume": "25",
"year": "2004"
},
{
"DOI": "10.1016/j.exger.2020.111219",
"article-title": "Reduced levels of plasma selenium are associated with increased inflammation and cardiovascular disease in an Italian elderly population",
"author": "Giacconi",
"doi-asserted-by": "crossref",
"journal-title": "Exp. Gerontol.",
"key": "10.1016/j.jtemb.2022.127099_bib163",
"volume": "145",
"year": "2021"
},
{
"DOI": "10.1017/S0007114516002208",
"article-title": "Selenium status in neonates with connatal infection",
"author": "Wiehe",
"doi-asserted-by": "crossref",
"first-page": "504",
"journal-title": "Br. J. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib164",
"volume": "116",
"year": "2016"
},
{
"DOI": "10.3390/nu12061894",
"article-title": "Selenium and copper as biomarkers for pulmonary arterial hypertension in systemic sclerosis",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "1894",
"issue": "6",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib165",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2013.10.012",
"article-title": "Assessment of plasma and red trace element concentrations, disease severity, and outcome in patients with critical illness",
"author": "Stefanowicz",
"doi-asserted-by": "crossref",
"first-page": "214",
"issue": "2",
"journal-title": "J. Crit. Care",
"key": "10.1016/j.jtemb.2022.127099_bib166",
"volume": "29",
"year": "2014"
},
{
"DOI": "10.1093/bja/aev073",
"article-title": "Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation",
"author": "Mertens",
"doi-asserted-by": "crossref",
"first-page": "990",
"issue": "6",
"journal-title": "BJA: Br. J. Anaesth.",
"key": "10.1016/j.jtemb.2022.127099_bib167",
"volume": "114",
"year": "2015"
},
{
"DOI": "10.1186/cc5960",
"article-title": "Effects of high doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study",
"author": "Forceville",
"doi-asserted-by": "crossref",
"first-page": "R73",
"issue": "4",
"journal-title": "Crit. Care",
"key": "10.1016/j.jtemb.2022.127099_bib168",
"volume": "11",
"year": "2007"
},
{
"DOI": "10.1007/s00134-008-1356-5",
"article-title": "Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill?",
"author": "Manzanares",
"doi-asserted-by": "crossref",
"first-page": "882",
"journal-title": "Intensive Care Med.",
"key": "10.1016/j.jtemb.2022.127099_bib169",
"volume": "35",
"year": "2008"
},
{
"DOI": "10.17925/USE.2015.11.02.97",
"article-title": "Selenium and inflammation-potential use and perspectives",
"author": "Duntas",
"doi-asserted-by": "crossref",
"first-page": "97",
"issue": "2",
"journal-title": "US Endocrinol.",
"key": "10.1016/j.jtemb.2022.127099_bib170",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1016/j.freeradbiomed.2018.02.030",
"article-title": "Selenium, selenoprotein P, and Alzheimer’s disease: is there a link",
"author": "Solovyev",
"doi-asserted-by": "crossref",
"first-page": "124",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib171",
"volume": "127",
"year": "2018"
},
{
"DOI": "10.1007/s12011-020-02444-w",
"article-title": "Zinc and Selenium in Inflammatory Bowel Disease: Trace Elements with Key Roles?",
"author": "Vaghari-Tabari",
"doi-asserted-by": "crossref",
"first-page": "3190",
"issue": "9",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib172",
"volume": "199",
"year": "2021"
},
{
"DOI": "10.1016/j.prostaglandins.2005.06.006",
"article-title": "Transforming growth factor beta(1) and prostaglandin E2 concentrations are associated with bone formation markers in ulcerative colitis patients",
"author": "Wiercinska-Drapalo",
"doi-asserted-by": "crossref",
"first-page": "160",
"issue": "1–4",
"journal-title": "Prostaglandins Other Lipid Mediat.",
"key": "10.1016/j.jtemb.2022.127099_bib173",
"volume": "78",
"year": "2005"
},
{
"DOI": "10.1038/sj.onc.1203237",
"article-title": "The Rel/NR-κB signal transduction pathway introduction",
"author": "Gilmore",
"doi-asserted-by": "crossref",
"first-page": "6842",
"issue": "49",
"journal-title": "Oncogene",
"key": "10.1016/j.jtemb.2022.127099_bib174",
"volume": "18",
"year": "1999"
},
{
"DOI": "10.1016/j.jnutbio.2014.02.005",
"article-title": "Reduced macrophage selenoprotein expression alters oxidized lipid metabolite biosynthesis from arachidonic and linoleic acid",
"author": "Mattmiller",
"doi-asserted-by": "crossref",
"first-page": "647",
"issue": "6",
"journal-title": "J. Nutr. Biochem.",
"key": "10.1016/j.jtemb.2022.127099_bib175",
"volume": "25",
"year": "2014"
},
{
"DOI": "10.12669/pjms.305.5214",
"article-title": "Effects of B-lymphocyte dysfunction on the serum copper, selenium and zinc levels of rheumatoid arthritis patients",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1064",
"issue": "5",
"journal-title": "Pak. J. Med. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib176",
"volume": "30",
"year": "2014"
},
{
"DOI": "10.1093/ajcn/80.2.299",
"article-title": "Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients",
"author": "Kalantar-Zadeh",
"doi-asserted-by": "crossref",
"first-page": "299",
"issue": "2",
"journal-title": "Am. J. Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib177",
"volume": "80",
"year": "2004"
},
{
"DOI": "10.1681/ASN.2006080926",
"article-title": "Inflammatory syndrome in patients on hemodialysis",
"author": "Jofré",
"doi-asserted-by": "crossref",
"first-page": "S274",
"issue": "12 Suppl 3",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "10.1016/j.jtemb.2022.127099_bib178",
"volume": "17",
"year": "2006"
},
{
"DOI": "10.1016/j.biopha.2006.12.007",
"article-title": "Oxidative stress and delta-ALA-D activity in chronic renal failure patients",
"author": "da Silva",
"doi-asserted-by": "crossref",
"first-page": "180",
"issue": "2–3",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.jtemb.2022.127099_bib179",
"volume": "61",
"year": "2007"
},
{
"DOI": "10.1016/j.jtemb.2020.126580",
"article-title": "Trace element imbalances in patients undergoing chronic hemodialysis therapy - report of an observational study in a cohort of Portuguese patients",
"author": "Almeida",
"doi-asserted-by": "crossref",
"journal-title": "J. Trace Elem. Med. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib180",
"volume": "62",
"year": "2020"
},
{
"DOI": "10.1016/j.jtemb.2021.126899",
"article-title": "Prevalence and correlates of low plasma selenium concentrations in peritoneal dialysis patients",
"author": "Beligaswatta",
"doi-asserted-by": "crossref",
"journal-title": "J. Trace Elem. Med. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib181",
"volume": "69",
"year": "2022"
},
{
"DOI": "10.2174/1573397114666181016112342",
"article-title": "Selenium and autoimmune diseases: a review article",
"author": "Sahebari",
"doi-asserted-by": "crossref",
"first-page": "123",
"issue": "2",
"journal-title": "Curr. Rheumatol. Rev.",
"key": "10.1016/j.jtemb.2022.127099_bib182",
"volume": "15",
"year": "2019"
},
{
"DOI": "10.1001/jama.2016.0287",
"article-title": "The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)",
"author": "Singer",
"doi-asserted-by": "crossref",
"first-page": "801",
"issue": "8",
"journal-title": "JAMA",
"key": "10.1016/j.jtemb.2022.127099_bib183",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.1017/S0954422418000124",
"article-title": "A review of micronutrients in sepsis: the role of thiamine, L-carnitine, vitamin C, selenium and vitamin D",
"author": "Belsky",
"doi-asserted-by": "crossref",
"first-page": "281",
"issue": "2",
"journal-title": "Nutr. Res. Rev.",
"key": "10.1016/j.jtemb.2022.127099_bib184",
"volume": "31",
"year": "2018"
},
{
"DOI": "10.1155/2017/5985209",
"article-title": "Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies",
"author": "Mantzarlis",
"doi-asserted-by": "crossref",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "10.1016/j.jtemb.2022.127099_bib185",
"volume": "2017",
"year": "2017"
},
{
"DOI": "10.1097/MD.0000000000014733",
"article-title": "A meta-analysis of randomized controlled trials: efficacy of selenium treatment for sepsis",
"author": "Li",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "Medicine",
"key": "10.1016/j.jtemb.2022.127099_bib186",
"volume": "98",
"year": "2019"
},
{
"DOI": "10.1097/CCM.0b013e31828a24c6",
"article-title": "The effect of selenium therapy on mortality in patients with sepsis syndrome: a systematic review and meta-analysis of randomized controlled trials",
"author": "Alhazzani",
"doi-asserted-by": "crossref",
"first-page": "1555",
"issue": "6",
"journal-title": "Crit. Care Med.",
"key": "10.1016/j.jtemb.2022.127099_bib187",
"volume": "41",
"year": "2013"
},
{
"DOI": "10.1093/tropej/fmv096",
"article-title": "Selenium supplementation for prevention of late-onset sepsis in very low birth weight preterm neonates",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "185",
"issue": "3",
"journal-title": "J. Trop. Pediatr.",
"key": "10.1016/j.jtemb.2022.127099_bib188",
"volume": "62",
"year": "2016"
},
{
"DOI": "10.1385/BTER:75:1-3:107",
"article-title": "Leukocyte selenium, zinc, and copper concentrations in preeclamptic and normotensive pregnant women",
"author": "Mahomed",
"doi-asserted-by": "crossref",
"first-page": "107",
"issue": "1–3",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib189",
"volume": "75",
"year": "2000"
},
{
"DOI": "10.1111/j.1753-4887.2010.00337.x",
"article-title": "Role of selenium in HIV infection",
"author": "Stone",
"doi-asserted-by": "crossref",
"first-page": "671",
"issue": "11",
"journal-title": "Nutr. Rev.",
"key": "10.1016/j.jtemb.2022.127099_bib190",
"volume": "68",
"year": "2010"
},
{
"DOI": "10.3390/nu6115061",
"article-title": "Pre-antiretroviral therapy serum selenium concentrations predict WHO stages 3, 4 or death but not virologic failure post-antiretroviral therapy",
"author": "Shivakoti",
"doi-asserted-by": "crossref",
"first-page": "5061",
"issue": "11",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib191",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.1001/jamainternmed.2016.2514",
"article-title": "Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial",
"author": "Bloos",
"doi-asserted-by": "crossref",
"first-page": "1266",
"issue": "9",
"journal-title": "JAMA Intern. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib192",
"volume": "176",
"year": "2016"
},
{
"DOI": "10.5603/ARM.a2022.0018",
"article-title": "Therapeutic use of intravenous selenium in respiratory and immunological diseases: a narrative review",
"author": "Oliveira",
"doi-asserted-by": "crossref",
"first-page": "134",
"journal-title": "Adv. Respir. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib193",
"volume": "90",
"year": "2022"
},
{
"DOI": "10.1265/jjh.56.641",
"article-title": "Keshan disease--a review from the aspect of history and etiology",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "641",
"issue": "4",
"journal-title": "Nihon Eiseigaku Zasshi",
"key": "10.1016/j.jtemb.2022.127099_bib194",
"volume": "56",
"year": "2002"
},
{
"DOI": "10.1016/j.tips.2011.09.001",
"article-title": "Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy",
"author": "Vlahos",
"doi-asserted-by": "crossref",
"first-page": "3",
"issue": "1",
"journal-title": "Trends Pharmacol. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib195",
"volume": "33",
"year": "2012"
},
{
"DOI": "10.1016/j.redox.2020.101764",
"article-title": "Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker",
"author": "Heller",
"doi-asserted-by": "crossref",
"journal-title": "Redox Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib196",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2020.108545",
"article-title": "Micronutrients as immunomodulatory tools for COVID-19 management",
"author": "Gasmi",
"doi-asserted-by": "crossref",
"journal-title": "Clin. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib197",
"volume": "220",
"year": "2020"
},
{
"DOI": "10.1007/s12291-021-00961-6",
"article-title": "Trace elements as immunoregulators in SARS-CoV-2 and other viral infections",
"author": "Dharmalingam",
"doi-asserted-by": "crossref",
"first-page": "416",
"issue": "4",
"journal-title": "Indian J. Clin. Biochem.",
"key": "10.1016/j.jtemb.2022.127099_bib198",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1093/ajcn/nqaa177",
"article-title": "A role for selenium-dependent GPX1 in SARS-CoV-2 virulence",
"author": "Seale",
"doi-asserted-by": "crossref",
"first-page": "447",
"issue": "2",
"journal-title": "Am. J. Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib199",
"volume": "112",
"year": "2020"
},
{
"DOI": "10.3390/ijms23010005",
"article-title": "Historical roles of selenium and selenoproteins in health and development: the good, the bad and the ugly",
"author": "Tsuji",
"doi-asserted-by": "crossref",
"first-page": "5",
"issue": "1",
"journal-title": "Int. J. Mol. Sci.",
"key": "10.1016/j.jtemb.2022.127099_bib200",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1016/j.etap.2022.103937",
"article-title": "Can iron, zinc, copper and selenium status be a prognostic determinant in COVID-19 patients",
"author": "Engin",
"doi-asserted-by": "crossref",
"journal-title": "Environ. Toxicol. Pharmacol.",
"key": "10.1016/j.jtemb.2022.127099_bib201",
"volume": "95",
"year": "2022"
},
{
"DOI": "10.1096/fasebj.12.12.1143",
"article-title": "Glutathione peroxidase protects mice from viral-induced myocarditis",
"author": "Beck",
"doi-asserted-by": "crossref",
"first-page": "1143",
"issue": "12",
"journal-title": "FASEB J.",
"key": "10.1016/j.jtemb.2022.127099_bib202",
"volume": "12",
"year": "1998"
},
{
"DOI": "10.1093/jn/133.5.1463S",
"article-title": "Selenium deficiency and viral infection",
"author": "Beck",
"doi-asserted-by": "crossref",
"first-page": "1463S",
"issue": "5",
"journal-title": "The Journal of Nutrition",
"key": "10.1016/j.jtemb.2022.127099_bib203",
"volume": "133",
"year": "2003"
},
{
"DOI": "10.1165/rcmb.2011-0345OC",
"article-title": "Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation",
"author": "Yatmaz",
"doi-asserted-by": "crossref",
"first-page": "17",
"issue": "1",
"journal-title": "Am. J. Respir. Cell Mol. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib204",
"volume": "48",
"year": "2013"
},
{
"DOI": "10.1097/QAD.0000000000000673",
"article-title": "Effect of selenium supplementation on CD4+ T=cell recovery, viral suppression and morbidity of HIV-infected patients in Rwanda: a randomized controlled trial",
"author": "Kamwesiga",
"doi-asserted-by": "crossref",
"first-page": "1045",
"issue": "9",
"journal-title": "AIDS",
"key": "10.1016/j.jtemb.2022.127099_bib205",
"volume": "29",
"year": "2015"
},
{
"DOI": "10.1258/0007142991902592",
"article-title": "Selenium and viral virulence",
"author": "Levander",
"doi-asserted-by": "crossref",
"first-page": "528",
"issue": "3",
"journal-title": "Br. Med. Bull.",
"key": "10.1016/j.jtemb.2022.127099_bib206",
"volume": "55",
"year": "1999"
},
{
"article-title": "Selenium in biology: facts and medical perspectives",
"author": "Köhrle",
"first-page": "849",
"issue": "9–10",
"journal-title": "Biol. Chem.",
"key": "10.1016/j.jtemb.2022.127099_bib207",
"volume": "381",
"year": "2000"
},
{
"DOI": "10.1089/thy.2005.15.841",
"article-title": "Selenium and the control of thyroid hormone metabolism",
"author": "Köhrle",
"doi-asserted-by": "crossref",
"first-page": "841",
"issue": "8",
"journal-title": "Thyroid",
"key": "10.1016/j.jtemb.2022.127099_bib208",
"volume": "15",
"year": "2005"
},
{
"DOI": "10.1089/ars.2007.1528",
"article-title": "From selenium to selenoproteins: synthesis, identity, and their role in human health",
"author": "Papp",
"doi-asserted-by": "crossref",
"first-page": "775",
"issue": "7",
"journal-title": "Antioxid. Redox Signal.",
"key": "10.1016/j.jtemb.2022.127099_bib209",
"volume": "9",
"year": "2007"
},
{
"DOI": "10.1089/ars.2010.3275",
"article-title": "Selenium in human health and disease",
"author": "Fairweather-Tait",
"doi-asserted-by": "crossref",
"first-page": "1337",
"issue": "7",
"journal-title": "Antioxid. Redox Signal.",
"key": "10.1016/j.jtemb.2022.127099_bib210",
"volume": "14",
"year": "2011"
},
{
"DOI": "10.1002/ejhf.1644",
"article-title": "Selenium and outcome in heart failure",
"author": "Bomer",
"doi-asserted-by": "crossref",
"first-page": "1415",
"issue": "8",
"journal-title": "Eur. J. Heart Fail.",
"key": "10.1016/j.jtemb.2022.127099_bib211",
"volume": "22",
"year": "2020"
},
{
"article-title": "Dietary and serum selenium in coronary heart disease and all-cause mortality: an international perspective",
"author": "Xie",
"first-page": "827",
"issue": "4",
"journal-title": "Asia Pac. J. Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib212",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.4081/idr.2010.e18",
"article-title": "Selenium deficiency and HIV infection",
"author": "Di-Bella",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Infect. Dis. Rep.",
"key": "10.1016/j.jtemb.2022.127099_bib213",
"volume": "2",
"year": "2010"
},
{
"article-title": "An original discovery: selenium deficiency and Keshan disease (an endemic heart disease)",
"author": "Chen",
"first-page": "320",
"issue": "3",
"journal-title": "Asia Pac. J. Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib214",
"volume": "21",
"year": "2012"
},
{
"DOI": "10.1016/B978-0-444-53488-0.00018-3",
"article-title": "Enterovirus/picornavirus infections",
"author": "Jubelt",
"doi-asserted-by": "crossref",
"first-page": "379",
"journal-title": "Handb. Clin. Neurol.",
"key": "10.1016/j.jtemb.2022.127099_bib215",
"volume": "123",
"year": "2014"
},
{
"DOI": "10.1038/nm0595-433",
"article-title": "Rapid genomic evolution of a non-virulent Coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates",
"author": "Beck",
"doi-asserted-by": "crossref",
"first-page": "433",
"journal-title": "Nat. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib216",
"volume": "1",
"year": "1995"
},
{
"DOI": "10.1016/j.tim.2004.07.007",
"article-title": "Host nutritional status: the neglected virulence factor",
"author": "Beck",
"doi-asserted-by": "crossref",
"first-page": "417",
"issue": "9",
"journal-title": "Trends Microbiol.",
"key": "10.1016/j.jtemb.2022.127099_bib217",
"volume": "12",
"year": "2004"
},
{
"DOI": "10.1101/cshperspect.a038398",
"article-title": "Structure and function of the influenza virus transcription and replication machinery",
"author": "Fodor",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "Cold Spring Harb. Perspect. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib218",
"volume": "10",
"year": "2020"
},
{
"article-title": "Overview of influenza viruses",
"author": "Pleschaka",
"first-page": "1",
"journal-title": "Curr. Top. Microbiol. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib219",
"volume": "370",
"year": "2013"
},
{
"DOI": "10.1016/S2468-2667(19)30163-X",
"article-title": "Influenza-associated excess respiratory mortality in China, 2010–15: a population-based study",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "e473",
"issue": "9",
"journal-title": "Lancet Public Health",
"key": "10.1016/j.jtemb.2022.127099_bib220",
"volume": "4",
"year": "2019"
},
{
"DOI": "10.1016/0009-2797(94)90040-X",
"article-title": "Selenium in the maintenance and therapy of HIV-infected patients",
"author": "Schrauzer",
"doi-asserted-by": "crossref",
"first-page": "199",
"issue": "2–3",
"journal-title": "Chem. Biol. Interact.",
"key": "10.1016/j.jtemb.2022.127099_bib221",
"volume": "91",
"year": "1994"
},
{
"DOI": "10.3945/an.114.007575",
"article-title": "Dietary selenium in adjuvant therapy of viral and bacterial infections",
"author": "Steinbrenner",
"doi-asserted-by": "crossref",
"first-page": "73",
"issue": "1",
"journal-title": "Adv. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib222",
"volume": "6",
"year": "2015"
},
{
"DOI": "10.1016/0009-9120(91)90601-A",
"article-title": "Serum selenium concentration and disease progress in patients with HIV infection",
"author": "Cirelli",
"doi-asserted-by": "crossref",
"first-page": "211",
"issue": "2",
"journal-title": "Clin. Biochem.",
"key": "10.1016/j.jtemb.2022.127099_bib223",
"volume": "24",
"year": "1991"
},
{
"DOI": "10.1186/1471-2334-8-165",
"article-title": "Nutritional status and serum zinc and selenium levels in Iranian HIV infected individuals",
"author": "Khalili",
"doi-asserted-by": "crossref",
"first-page": "165",
"issue": "1",
"journal-title": "BMC Infect. Dis.",
"key": "10.1016/j.jtemb.2022.127099_bib224",
"volume": "8",
"year": "2008"
},
{
"DOI": "10.1097/00042560-199708150-00007",
"article-title": "High risk of HIV-related mortality is associated with selenium deficiency",
"author": "Baum",
"doi-asserted-by": "crossref",
"first-page": "370",
"issue": "5",
"journal-title": "JAIDS J. Acquir. Immune Defic. Syndr.",
"key": "10.1016/j.jtemb.2022.127099_bib225",
"volume": "15",
"year": "1997"
},
{
"DOI": "10.1097/00042560-199904150-00015",
"article-title": "Mortality risk in selenium-deficient HIV-positive children",
"author": "Campa",
"doi-asserted-by": "crossref",
"first-page": "508",
"issue": "5",
"journal-title": "J. Acquir. Immune Defic. Syndr. Hum. retrovirol.",
"key": "10.1016/j.jtemb.2022.127099_bib226",
"volume": "20",
"year": "1999"
},
{
"DOI": "10.1001/archinte.167.2.148",
"article-title": "Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial",
"author": "Hurwitz",
"doi-asserted-by": "crossref",
"first-page": "148",
"issue": "2",
"journal-title": "Arch. Intern. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib227",
"volume": "167",
"year": "2007"
},
{
"DOI": "10.1038/nrmicro3066",
"article-title": "Uncovering the mysteries of hantavirus infections",
"author": "Vaheri",
"doi-asserted-by": "crossref",
"first-page": "539",
"journal-title": "Nat. Rev. Microbiol.",
"key": "10.1016/j.jtemb.2022.127099_bib228",
"volume": "11",
"year": "2013"
},
{
"DOI": "10.1111/1440-1681.13403",
"article-title": "Hantavirus diseases pathophysiology, their diagnostic strategies and therapeutic approaches: A review",
"author": "Munir",
"doi-asserted-by": "crossref",
"first-page": "20",
"issue": "1",
"journal-title": "Clin. Exp. Pharmacol. Physiol.",
"key": "10.1016/j.jtemb.2022.127099_bib229",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.3390/v7010333",
"article-title": "The association between Hantavirus infection and selenium deficiency in Mainland China",
"author": "Fang",
"doi-asserted-by": "crossref",
"first-page": "333",
"issue": "1",
"journal-title": "Viruses",
"key": "10.1016/j.jtemb.2022.127099_bib230",
"volume": "7",
"year": "2015"
},
{
"DOI": "10.3389/fnut.2020.00164",
"article-title": "Selenium and RNA virus interactions: Potential implications for SARS-CoV-2 infection (COVID-19",
"author": "Hiffler",
"doi-asserted-by": "crossref",
"first-page": "164",
"journal-title": "Front. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib231",
"volume": "7",
"year": "2020"
},
{
"article-title": "Selenium and viral infection: are there lessons for COVID-19",
"author": "Bermano",
"first-page": "1",
"journal-title": "Br. J. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib232",
"year": "2020"
},
{
"DOI": "10.3390/nu12082358",
"article-title": "Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19",
"author": "Alexander",
"doi-asserted-by": "crossref",
"first-page": "2358",
"issue": "8",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib233",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/molecules25225346",
"article-title": "Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19",
"author": "Bae",
"doi-asserted-by": "crossref",
"first-page": "5346",
"issue": "22",
"journal-title": "Molecules",
"key": "10.1016/j.jtemb.2022.127099_bib234",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.3389/fnut.2020.00143",
"article-title": "Understanding selenium and glutathione as antiviral factors in COVID-19: does the viral Mpro protease target host selenoproteins and glutathione synthesis",
"author": "Taylor",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "Front. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib235",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.08.018",
"article-title": "Nutritional status of patients with COVID-19",
"author": "Im",
"doi-asserted-by": "crossref",
"first-page": "390",
"journal-title": "Int. J. Infect. Dis.",
"key": "10.1016/j.jtemb.2022.127099_bib236",
"volume": "100",
"year": "2020"
},
{
"DOI": "10.1016/j.dsx.2020.04.015",
"article-title": "Enhancing immunity in viral infections, with special emphasis on COVID-19: a review",
"author": "Jayawardena",
"doi-asserted-by": "crossref",
"first-page": "367",
"issue": "4",
"journal-title": "Diabetes Metab. Syndr.",
"key": "10.1016/j.jtemb.2022.127099_bib237",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.maturitas.2020.08.003",
"article-title": "Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19",
"author": "Shakoor",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Maturitas",
"key": "10.1016/j.jtemb.2022.127099_bib238",
"volume": "143",
"year": "2021"
},
{
"DOI": "10.1007/s13668-021-00354-4",
"article-title": "A mechanistic link between selenium and coronavirus disease 2019 (COVID-19",
"author": "Khatiwada",
"doi-asserted-by": "crossref",
"first-page": "125",
"issue": "2",
"journal-title": "Curr. Nutr. Rep.",
"key": "10.1016/j.jtemb.2022.127099_bib239",
"volume": "10",
"year": "2021"
},
{
"article-title": "Micronutrient level is negatively correlated with the neutrophil-lymphocyte ratio in patients with severe COVI-19",
"author": "Zhou",
"first-page": "8",
"journal-title": "Int. J. Clin. Pract.",
"key": "10.1016/j.jtemb.2022.127099_bib240",
"volume": "6498794",
"year": "2022"
},
{
"DOI": "10.3390/nu13103304",
"article-title": "Course and survival of COVID-19 patients with comorbidities in relation to the trace element status at hospital admission",
"author": "Du Laing",
"doi-asserted-by": "crossref",
"first-page": "3304",
"issue": "10",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib241",
"volume": "13",
"year": "2021"
},
{
"article-title": "Correlation between micronutrients plasma concentration and disease severity in COVID-19 patients",
"author": "Alkattan",
"first-page": "21",
"issue": "1",
"journal-title": "Alex. J. Med.",
"key": "10.1016/j.jtemb.2022.127099_bib242",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06617-3",
"article-title": "The correlation between serum selenium, zinc, and COVID-19 severity: an observational study",
"author": "Jahromi",
"doi-asserted-by": "crossref",
"first-page": "899",
"issue": "1",
"journal-title": "BMC Infect. Dis.",
"key": "10.1016/j.jtemb.2022.127099_bib243",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.nut.2020.111053",
"article-title": "An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: the case for adequate selenium status",
"author": "Majeed",
"doi-asserted-by": "crossref",
"journal-title": "Nutrition",
"key": "10.1016/j.jtemb.2022.127099_bib244",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.3390/metabo11040244",
"article-title": "Serum zinc, cooper and other biometals are associated with COVID-19 severity markers",
"author": "Skalny",
"doi-asserted-by": "crossref",
"first-page": "244",
"issue": "4",
"journal-title": "Metabolites",
"key": "10.1016/j.jtemb.2022.127099_bib245",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1007/s12011-021-02797-w",
"article-title": "Decreased serum selenium levels of COVID-19 patients in comparison with healthy individuals",
"author": "Younesian",
"doi-asserted-by": "crossref",
"first-page": "1562",
"issue": "4",
"journal-title": "Biol. Trace Elem.",
"key": "10.1016/j.jtemb.2022.127099_bib246",
"volume": "200",
"year": "2022"
},
{
"DOI": "10.1016/j.envres.2021.110984",
"article-title": "Selenium (Se) plays a key role in the biological effects of some viruses: implications for COVID-19",
"author": "Liu",
"doi-asserted-by": "crossref",
"journal-title": "Environ. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib247",
"volume": "196",
"year": "2021"
},
{
"DOI": "10.1093/ajcn/60.4.510",
"article-title": "Selenium status, fatty acids, vitamins A and E, and aging: the novel study",
"author": "Olivieri",
"doi-asserted-by": "crossref",
"first-page": "510",
"issue": "4",
"journal-title": "Am. J. Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib248",
"volume": "60",
"year": "1994"
},
{
"DOI": "10.1097/01.ede.0000248202.83695.4e",
"article-title": "Plasma selenium over time and cognitive decline in the elderly",
"author": "Akbaraly",
"doi-asserted-by": "crossref",
"first-page": "52",
"issue": "1",
"journal-title": "Epidemiology",
"key": "10.1016/j.jtemb.2022.127099_bib249",
"volume": "18",
"year": "2007"
},
{
"DOI": "10.1007/s40520-018-1086-7",
"article-title": "Selenium, aging and aging-related diseases",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "1035",
"journal-title": "Aging Clin. Exp. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib250",
"volume": "31",
"year": "2019"
},
{
"article-title": "Selenium status of the Australian population: effect of age, gender and cardiovascular disease",
"author": "Lymbury",
"issue": "1",
"journal-title": "Biol. Trace Elem. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib251",
"volume": "126",
"year": "2008"
},
{
"DOI": "10.3390/nu13061898",
"article-title": "Relation of serum copper status to survival in COVID-19",
"author": "Hackler",
"doi-asserted-by": "crossref",
"first-page": "1898",
"issue": "6",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib252",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1080/1744666X.2022.2085561",
"article-title": "Is post-COVID syndrome and autoimmune disease?",
"author": "Anaya",
"doi-asserted-by": "crossref",
"first-page": "653",
"issue": "7",
"journal-title": "Expert Rev. Clin. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib253",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1038/s41590-021-00993-3",
"article-title": "Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1107",
"journal-title": "Nat. Immunol.",
"key": "10.1016/j.jtemb.2022.127099_bib254",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1002/ajh.26384",
"article-title": "Late-onset hematological complications post COVID-19: an emerging medical problem for the hematologist",
"author": "Korompoki",
"doi-asserted-by": "crossref",
"first-page": "119",
"journal-title": "Am. J. Hematol.",
"key": "10.1016/j.jtemb.2022.127099_bib255",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1136/bcr-2021-242596",
"article-title": "Delayed, transient and self-resolving neutropenia following CPVOD-19 pneumonia",
"author": "Mank",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Case Rep.",
"key": "10.1016/j.jtemb.2022.127099_bib256",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1002/ccr3.3369",
"article-title": "Can COVID-19 cause severe neutropenia?",
"author": "López-Pereira",
"doi-asserted-by": "crossref",
"first-page": "3348",
"journal-title": "Clin. Case Rep.",
"key": "10.1016/j.jtemb.2022.127099_bib257",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1111/acel.13230",
"article-title": "COVID‐19 is an emergent disease of aging",
"author": "Santesmasses",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "Aging Cell",
"key": "10.1016/j.jtemb.2022.127099_bib258",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1038/ejcn.2015.92",
"article-title": "Relatively high mortality risk in elderly Swedish subjects with low selenium status",
"author": "Alehagen",
"doi-asserted-by": "crossref",
"first-page": "91",
"issue": "1",
"journal-title": "Eur. J. Clin. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib259",
"volume": "70",
"year": "2016"
},
{
"DOI": "10.3390/biom11101478",
"article-title": "Impact of selenium on biomarkers and clinical aspects related to ageing. a review",
"author": "Alehagen",
"doi-asserted-by": "crossref",
"first-page": "1478",
"issue": "10",
"journal-title": "Biomolecules",
"key": "10.1016/j.jtemb.2022.127099_bib260",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3390/nu13041344",
"article-title": "Dietary supplementation with selenium and coenzyme Q10 prevents increase in plasma d-dimer while lowering cardiovascular mortality in an Elderly SwedisH Population",
"author": "Alehagen",
"doi-asserted-by": "crossref",
"first-page": "1344",
"issue": "4",
"journal-title": "Nutrients",
"key": "10.1016/j.jtemb.2022.127099_bib261",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41569-018-0064-2",
"article-title": "Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty",
"author": "Ferrucci",
"doi-asserted-by": "crossref",
"first-page": "505",
"issue": "9",
"journal-title": "Nat. Rev. Cardiol.",
"key": "10.1016/j.jtemb.2022.127099_bib262",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1007/s00394-020-02384-0",
"article-title": "Sex-dependent association between selenium status and cognitive performance in older adults",
"author": "Cardoso",
"doi-asserted-by": "crossref",
"first-page": "1153",
"issue": "2",
"journal-title": "Eur. J. Nutr.",
"key": "10.1016/j.jtemb.2022.127099_bib263",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.1016/j.numecd.2010.09.005",
"article-title": "Gender differences in copper, zinc and selenium status in diabetic-free metabolic syndrome European population–The IMMIDIET study",
"author": "Arnaud",
"doi-asserted-by": "crossref",
"first-page": "517",
"issue": "6",
"journal-title": "Nutr. Metab. Cardiovasc. Dis.",
"key": "10.1016/j.jtemb.2022.127099_bib264",
"volume": "22",
"year": "2012"
},
{
"DOI": "10.1016/j.mehy.2020.109878",
"article-title": "Selenium supplementation in the prevention of coronavirus infections (COVID-19",
"author": "Kieliszek",
"doi-asserted-by": "crossref",
"journal-title": "Med. Hypotheses",
"key": "10.1016/j.jtemb.2022.127099_bib265",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1073/pnas.0308096101",
"article-title": "Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase",
"author": "McClung",
"doi-asserted-by": "crossref",
"first-page": "8852",
"issue": "24",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "10.1016/j.jtemb.2022.127099_bib266",
"volume": "101",
"year": "2004"
},
{
"DOI": "10.1016/j.cmet.2010.09.015",
"article-title": "A liver-derived secretory protein, selenoprotein P, causes insulin resistance",
"author": "Misu",
"doi-asserted-by": "crossref",
"first-page": "483",
"issue": "5",
"journal-title": "Cell Metab.",
"key": "10.1016/j.jtemb.2022.127099_bib267",
"volume": "12",
"year": "2010"
},
{
"DOI": "10.1038/s41467-017-01863-z",
"article-title": "Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models",
"author": "Mita",
"doi-asserted-by": "crossref",
"first-page": "1658",
"issue": "1",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.jtemb.2022.127099_bib268",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1038/s41598-018-35067-2",
"article-title": "Serum selenoprotein P, but not selenium, predicts future hyperglycemia in a general Japanese population",
"author": "Oo",
"doi-asserted-by": "crossref",
"first-page": "16727",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.jtemb.2022.127099_bib269",
"volume": "8",
"year": "2018"
},
{
"article-title": "Selenoprotein P as a significant regulator of pancreatic beta cell function",
"author": "Saito",
"first-page": "119",
"issue": "2",
"journal-title": "J. Biochem.",
"key": "10.1016/j.jtemb.2022.127099_bib270",
"volume": "167",
"year": "2020"
},
{
"DOI": "10.3390/biom10040658",
"article-title": "Selenium and selenoproteins in adipose tissue physiology and obesity",
"author": "Tinkov",
"doi-asserted-by": "crossref",
"first-page": "658",
"issue": "4",
"journal-title": "Biomolecules",
"key": "10.1016/j.jtemb.2022.127099_bib271",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1089/ars.2020.8087",
"article-title": "Hepatokine selenoprotein P-mediated reductive stress causes resistance to intracellular signal transduction",
"author": "Takamura",
"doi-asserted-by": "crossref",
"first-page": "517",
"issue": "7",
"journal-title": "Antioxid. Redox Signal.",
"key": "10.1016/j.jtemb.2022.127099_bib272",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1016/j.envres.2022.113092",
"article-title": "Safety of selenium exposure and limitations of selenoprotein maximization: molecular and epidemiologic perspectives",
"author": "Vinceti",
"doi-asserted-by": "crossref",
"journal-title": "Environ. Res.",
"key": "10.1016/j.jtemb.2022.127099_bib273",
"volume": "211",
"year": "2022"
},
{
"DOI": "10.1016/j.jtemb.2022.126956",
"article-title": "Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies",
"author": "Balboni",
"doi-asserted-by": "crossref",
"journal-title": "J. Trace Elem. Med. Biol.",
"key": "10.1016/j.jtemb.2022.127099_bib274",
"volume": "71",
"year": "2022"
}
],
"reference-count": 274,
"references-count": 274,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0946672X22001791"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Relationship between selenium status, selenoproteins and COVID-19 and other inflammatory diseases: A critical review",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "75"
}