The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death
et al., Cellular & Molecular Biology Letters, doi:10.1186/s11658-024-00659-6, Nov 2024
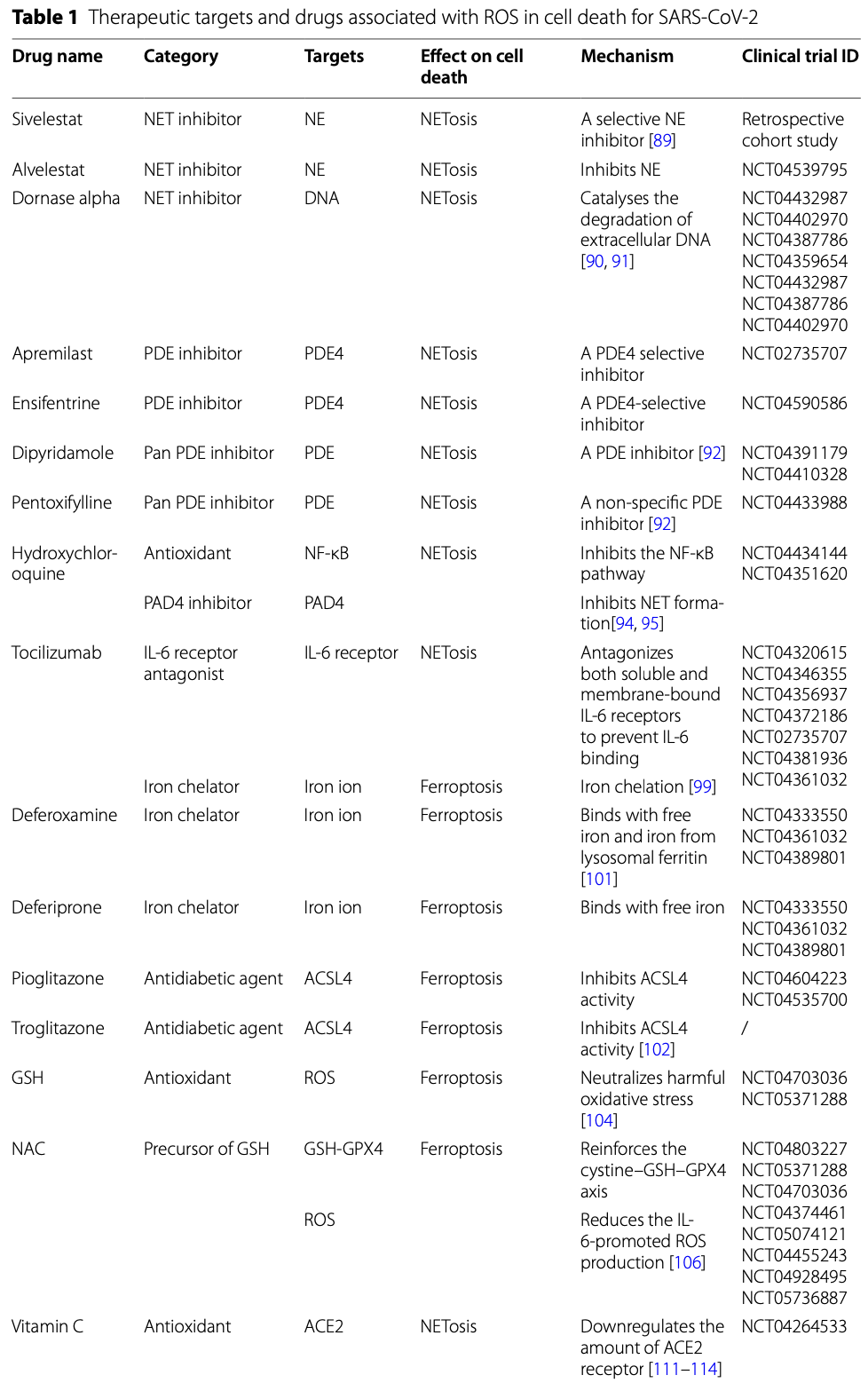
Review of the effects of reactive oxygen species (ROS) on cell death pathways in SARS-CoV-2 infection. SARS-CoV-2 induces oxidative stress and ROS generation which can lead to several types of regulated cell death including NETosis, ferroptosis, apoptosis, pyroptosis, necroptosis, and autophagy. Authors discuss the mechanisms by which ROS promotes each of these cell death pathways. Potential ROS-related therapies for COVID-19 are explored, including NET inhibitors like sivelestat and dornase alpha, the IL-6 receptor antagonist tocilizumab, iron chelators like deferoxamine and deferiprone to inhibit ferroptosis, glutathione and N-acetylcysteine as antioxidants, vitamins C and E, selenium to induce ferroptosis-inhibiting glutathione peroxidase 4, and melatonin as an antioxidant and myeloperoxidase inhibitor. Several ongoing clinical trials are evaluating these ROS-related therapeutic strategies.
1.
Smail et al., Antioxidant and oxidative enzymes, genetic variants, and cofactors as prognostic biomarkers of COVID-19 severity and mortality: a systematic review, Frontiers in Molecular Biosciences, doi:10.3389/fmolb.2025.1700263.
2.
Sarker et al., Selenium as a Nutritional Shield in Viral Defense: A Narrative Review, MDPI AG, doi:10.20944/preprints202502.2251.v1.
3.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
4.
Fazli et al., Possible Link between Gut Microbiota, Diet, and COVID-19 Infection, Journal of Medical Bacteriology, 12:4, jmb.tums.ac.ir/index.php/jmb/article/view/525.
5.
Xie et al., The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death, Cellular & Molecular Biology Letters, doi:10.1186/s11658-024-00659-6.
6.
Kaushal, A., Nutraceuticals and pharmacological to balance the transitional microbiome to extend immunity during COVID-19 and other viral infections, Journal of Translational Medicine, doi:10.1186/s12967-024-05587-9.
7.
Mu et al., Anti-inflammatory and Nutritional Interventions Against SARS-CoV-2: A Comprehensive Review, Journal of Agriculture and Food Research, doi:10.1016/j.jafr.2024.101422.
8.
Maia et al., Selenium—More than Just a Fortuitous Sulfur Substitute in Redox Biology, Molecules, doi:10.3390/molecules29010120.
9.
Yuan et al., The role of cell death in SARS-CoV-2 infection, Signal Transduction and Targeted Therapy, doi:10.1038/s41392-023-01580-8.
10.
Golin et al., Relationship between selenium status, selenoproteins and COVID-19 and other inflammatory diseases: A critical review, Journal of Trace Elements in Medicine and Biology, doi:10.1016/j.jtemb.2022.127099.
11.
Foshati et al., Antioxidants and clinical outcomes of patients with coronavirus disease 2019: A systematic review of observational and interventional studies, Food Science & Nutrition, doi:10.1002/fsn3.3034.
12.
Khatiwada et al., A Mechanistic Link Between Selenium and Coronavirus Disease 2019 (COVID-19), Current Nutrition Reports, doi:10.1007/s13668-021-00354-4.
Xie et al., 8 Nov 2024, peer-reviewed, 8 authors.
Contact: pengtaojiao66@126.com, liuv@henau.edu.cn.
The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death
Cellular & Molecular Biology Letters, doi:10.1186/s11658-024-00659-6
Coronavirus disease 2019 (COVID-19) represents the novel respiratory infectious disorder caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is characterized by rapid spread throughout the world. Reactive oxygen species (ROS) account for cellular metabolic by-products, and excessive ROS accumulation can induce oxidative stress due to insufficient endogenous antioxidant ability. In the case of oxidative stress, ROS production exceeds the cellular antioxidant capacity, thus leading to cell death. SARS-CoV-2 can activate different cell death pathways in the context of infection in host cells, such as neutrophil extracellular trap (NET) osis, ferroptosis, apoptosis, pyroptosis, necroptosis and autophagy, which are closely related to ROS signalling and control. In this review, we comprehensively elucidated the relationship between ROS generation and the death of host cells after SARS-CoV-2 infection, which leads to the development of COVID-19, aiming to provide a reasonable basis for the existing interventions and further development of novel therapies against SARS-CoV-2.
Author contributions W.L. and J.X. wrote the manuscript. Y.C. and W.L. drew the figures and designed the table. S.Y. and P.J. revised the manuscript and figures. Z.M., L.M. and W.L. provided conceptual ideas and revised the manuscript. All the authors have read and approved the final manuscript.
Declarations Ethics approval and consent to participate Not applicable.
Consent for publication Not applicable.
Competing interests The authors declare that they have no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Adhikari, Hashmi, Vijayaraghavan, Haniffa, Beane et al., Intravenous Vitamin C for patients hospitalized with COVID-19: two harmonized randomized clinical trials, JAMA
Alim, Caulfield, Chen, Swarup, Geschwind et al., Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke, Cell
Alraouji, Aboussekhra, Tocilizumab inhibits IL-8 and the proangiogenic potential of triple negative breast cancer cells, Mol Carcinog
Alvarez, Sviderskiy, Terzi, Papagiannakopoulos, Moreira et al., NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis, Nature
Appelberg, Gupta, Akusjärvi, Ambikan, Mikaeloff et al., Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells, Emerg Microbes Infect
Arcanjo, Logullo, Menezes, De, Carvalho Giangiarulo et al., The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19), Sci Rep
Bai, Zhao, Li, Sheng, Li, EV71 virus reduces Nrf2 activation to promote production of reactive oxygen species in infected cells, Gut Pathog
Bartolini, Stabile, Bastianelli, Giustarini, Pierucci et al., SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione, Redox Biol
Bednash, Kagan, Englert, Farkas, Tyurina et al., Syrian hamsters as a model of lung injury with SARS-CoV-2 infection: pathologic, physiologic, and detailed molecular profiling, Transl Res
Bertheloot, Latz, Necroptosis, pyroptosis and apoptosis: an intricate game of cell death, Cell Mol Immunol
Björnsdottir, Welin, Michaëlsson, Osla, Berg et al., Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species, Free Radic Biol Med
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced host response to SARS-CoV-2 drives development of COVID-19, Cell
Bou-Teen, Kaludercic, Weissman, Turan, Maack et al., Mitochondrial ROS and mitochondriatargeted antioxidants in the aged heart, Free Radic Biol Med
Broz, Pelegrín, The gasdermins, a protein family executing cell death and inflammation, Nat Rev Immunol
Camp, Bai, Gonullu, Nayak, Hm, Melatonin interferes with COVID-19 at several distinct ROS-related steps, J Inorg Biochem
Cao, Mu, Necrostatin-1 and necroptosis inhibition: Pathophysiology and therapeutic implications, Pharmacol Res
Carneiro, El-Deiry, Targeting apoptosis in cancer therapy, Nat Rev Clin Oncol
Chandra, Gurjar, Ahmed, Alqahtani, Qamar et al., Exploring potential inhibitor of SARS-CoV2 replicase from FDA approved drugs using insilico drug discovery methods, J Biomol Struct
Chen, Mcmillan-Ward, Kong, Israels, Gibson, Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species, J Cell Sci
Chiang, Korinek, Cheng, Hwang, Targeting neutrophils to treat acute respiratory distress syndrome in Coronavirus disease, Front Pharmacol
Codo, Davanzo, Monteiro, De Souza, Muraro et al., Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis, Cell Metab
Danthi, Viruses and the diversity of cell death, Annu Rev Virol
De Alencar, Moreira, Müller, Chaves, Fukuhara et al., Double-blind, randomized, placebocontrolled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by Coronavirus disease 2019 (COVID-19), Clin Infect Dis
De Pinho, Da Silva, De Castro Côrtes, Da, Sousa et al., Production of MMP-9 and inflammatory cytokines by Trypanosoma cruzi-infected macrophages, Exp Parasitol
Denecker, Vercammen, Declercq, Vandenabeele, Apoptotic and necrotic cell death induced by death domain receptors, Cell Mol Life Sci
Dikic, Elazar, Mechanism and medical implications of mammalian autophagy, Nat Rev Mol Cell Biol
Dixon, Lemberg, Lamprecht, Skouta, Zaitsev et al., Ferroptosis: an iron-dependent form of nonapoptotic cell death, Cell
Elkin, Harris, Loch-Caruso, Trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine induces lipid peroxidation-associated apoptosis via the intrinsic and extrinsic apoptosis pathways in a first-trimester placental cell line, Toxicol Appl Pharmacol
Feng, Pan, Wang, Lei, Yang et al., MERS-CoV nsp1 regulates autophagic flux via mTOR signalling and dysfunctional lysosomes, Emerg Microbes Infect
Ferreira, Soares, De Azevedo-Quintanilha, Dias, Fintelman-Rodrigues et al., SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes, Virology
Forrester, Kikuchi, Hernandes, Xu, Griendling, Reactive oxygen species in metabolic and inflammatory signaling, Circ Res
Habib, Ibrahim, Zaim, Ibrahim, The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators, Biomed Pharmacother
Han, Li, Yang, Bai, Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis, Bioengineered
He, Hara, Núñez, Mechanism and regulation of NLRP3 inflammasome activation, Trends Biochem Sci
He, Zhu, Zhang, Geng, Gong et al., RNF34 functions in immunity and selective mitophagy by targeting MAVS for autophagic degradation, EMBO J
Holliday, Earhart, Alnijoumi, Krvavac, Allen et al., Non-randomized trial of dornase alfa for acute respiratory distress syndrome secondary to COVID-19, Front Immunol
Horowitz, Freeman, Bruzzese, Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases, Respir Med Case Rep
Houslay, Christian, p62 (SQSTM1) forms part of a novel, reversible aggregate containing a specific conformer of the cAMP degrading phosphodiesterase, PDE4A4, Autophagy
Ibrahim, Smith, Lewis, Kon, Goldenberg, Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine, Clin Immunol
Imre, Cell death signalling in virus infection, Cell Signal
Jiang, Stockwell, Ferroptosis: mechanisms, biology and role in disease, Nat Rev Mol Cell Biol
Kieliszek, Lipinski, Selenium supplementation in the prevention of coronavirus infections (COVID-19), Med Hypotheses
Kronstein-Wiedemann, Stadtmüller, Traikov, Georgi, Teichert et al., SARS-CoV-2 infects red blood cell progenitors and dysregulates hemoglobin and iron metabolism, Stem Cell Rev Rep
Kvietys, Fakhoury, Kadan, Yaqinuddin, Al-Mutairy et al., COVID-19: lung-centric immunothrombosis, Front Cell Infect Microbiol
Labarrere, Kassab, Polonikov, Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease, Front Microbiol
Lee, Ghode, Ong, Redox regulation of cell state and fate, Redox Biol
Lennicke, Cochemé, Redox metabolism: ROS as specific molecular regulators of cell signaling and function, Mol Cell
Li, Gao, Precise modulation and use of reactive oxygen species for immunotherapy, Sci Adv
Li, Hou, Ma, Wang, Wang et al., SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy, Cell Mol Immunol
Li, Li, Wang, Yang, Huang et al., SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling, Biochim Biophys Acta Mol Basis Dis
Li, Tan, Miao, Lei, Zhang, ROS and autophagy: interactions and molecular regulatory mechanisms, Cell Mol Neurobiol
Li, Zhang, Guan, Ye, Li et al., SARS-CoV-2 Z-RNA activates the ZBP1-RIPK3 pathway to promote virusinduced inflammatory responses, Cell Res
Li, Zhang, Wang, Gutiérrez-Castrellón, Cell deaths: Involvement in the pathogenesis and intervention therapy of COVID-19, Signal Transduct Target Ther
Liang, Barnett, Initiator cell death event induced by SARS-CoV-2 in the human airway epithelium, Sci Immunol
Liu, Li, Liu, Sun, Chen et al., Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19, Acta Pharm Sin B
Liu, Zhu, Zhang, Li, Peng, Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial, BMJ Open
Lushchak, Free radicals, reactive oxygen species, oxidative stress and its classification, Chem Biol Interact
Maki, Inoue, Ishihara, Hirano, Kondo, Evaluation of appropriate indications for the use of sivelestat sodium in acute respiratory distress syndrome: a retrospective cohort study, Acute Med Surg
Maldonado, Hernandez-Ramírez, Ea, Co, Pimentel-González et al., Pentoxifylline decreases serum LDH levels and increases lymphocyte count in COVID-19 patients: results from an external pilot study, Int Immunopharmacol
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Metzler, Goosmann, Lubojemska, Zychlinsky, Papayannopoulos, A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis, Cell Rep
Mohammed, Fisher, Kraskauskas, Farkas, Brophy et al., Vitamin C: a novel regulator of neutrophil extracellular trap formation, Nutrients
Morais Da Silva, De Lucena, Júnior, De Carvalho, De Oliveira et al., Cell death mechanisms involved in cell injury caused by SARS-CoV-2, Rev Med Virol
Moriyama, Nagai, Maruzuru, Koshiba, Kawaguchi et al., Influenza virus-induced oxidized DNA activates inflammasomes, iScience
Nai, Lorè, Pagani, Lorenzo, Modica et al., Hepcidin levels predict COVID-19 severity and mortality in a cohort of hospitalized Italian patients, Am J Hematol
Nakamura, Kinjo, Arakaki, Miyagi, Tateyama et al., Serum levels of receptor-interacting protein kinase-3 in patients with COVID-19, Crit Care
Narasaraju, Tang, Herrmann, Muller, Chow et al., Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients with COVID-19, Front Pharmacol
Okur, Yalcin, Tastan, Demir, Yurtsever et al., Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection, New Microbes New Infect
Park, Park, Lee, Kim, Seo et al., Bioinspired DNase-I-coated melanin-like nanospheres for modulation of infection-associated NETosis dysregulation, Adv Sci (Weinh)
Pizzimenti, Ciamporcero, Daga, Pettazzoni, Arcaro et al., Interaction of aldehydes derived from lipid peroxidation and membrane proteins, Front Physiol
Porto, Stein, Neutrophil extracellular traps in pulmonary diseases: too much of a good thing?, Front Immunol
Potere, Batticciotto, The role of IL-6 and IL-6 blockade in COVID-19, Expert Rev Clin Immunol
Qian, Tan, Yu, Wang, Zhang, Inactivated sendai virus induces ROS-dependent apoptosis and autophagy in human prostate cancer cells, Biomed Environ Sci
Ratajczak, Bujko, Ciechanowicz, Sielatycka, Cymer et al., SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45(-) precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome, Stem Cell Rev Rep
Redza-Dutordoir, Da, Activation of apoptosis signalling pathways by reactive oxygen species, Biochim Biophys Acta
Renia, Ng, Acquired immunity against SARS-CoV-2 infection and vaccination, EMBO Mol Med
Reshi, Su, Hong, RNA viruses: ROS-mediated cell death, Int J Cell Biol
Rosas, Bräu, Waters, Go, Hunter et al., Tocilizumab in hospitalized patients with severe COVID-19 pneumonia, N Engl J Med
Sander, Fourie, Reactive oxygen species as potential antiviral targets, Rev Med Virol
Scheffel, Scurti, Wyatt, N-acetyl cysteine protects anti-melanoma cytotoxic T cells from exhaustion induced by rapid expansion via the downmodulation of Foxo1 in an Akt-dependent manner, Cancer Immunol Immunother
Schenk, Fulda, Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death, Oncogene
Schulze-Osthoff, Beyaert, Vandevoorde, Haegeman, Fiers, Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF, Embo J
Shakoor, Feehan, Dhaheri, Ali, Platat et al., Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19?, Maturitas
Sharma, Kontodimas, Bosmann, The MAVS immune recognition pathway in viral infection and sepsis, Antioxid Redox Signal
Shi, Zhao, Wang, Shi, Wang et al., Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death, Nature
Shin, Pyo, Jung, Choi, Influenza A virus PB1-F2 is involved in regulation of cellular redox state in alveolar epithelial cells, Biochem Biophys Res Commun
Singh, Karnik, Angiotensin receptors: structure, function, signaling and clinical applications, J Cell Signal
Skendros, Mitsios, Chrysanthopoulou, Mastellos, Metallidis et al., Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis, J Clin Invest
Stockwell, Angeli, Bayir, Bush, Conrad et al., Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease, Cell
Sun, Han, Zhang, Liu, Wang et al., Regulated necrosis in COVID-19: a double-edged sword, Front Immunol
Swanson, Deng, The NLRP3 inflammasome: molecular activation and regulation to therapeutics, Nat Rev Immunol
Sørensen, Borregaard, Neutrophil extracellular traps-the dark side of neutrophils, J Clin Invest
Temraz, Santini, Musallam, Taher, Iron overload and chelation therapy in myelodysplastic syndromes, Crit Rev Oncol Hematol
Thiam, Wong, Wagner, Waterman, Cellular mechanisms of NETosis, Annu Rev Cell Dev Biol
Trepte, Secker, Olivet, Blavier, Kostova et al., AI-guided pipeline for protein-protein interaction drug discovery identifies a SARS-CoV-2 inhibitor, Mol Sys Bio, doi:10.1038/s44320-024-00019-8
Tung, Tsai, Lee, Hsieh, Chen et al., Japanese encephalitis virus induces matrix metalloproteinase-9 in rat brain astrocytes via NF-κB signalling dependent on MAPKs and reactive oxygen species, Br J Pharmacol
Ueland, Holter, Holten, Müller, Lind et al., Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure, J Infection
Vanlangenakker, Berghe, Bogaert, Laukens, Zobel et al., cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production, Cell Death Differ
Vardar Acar, Özgül, The bridge between cell survival and cell death: reactive oxygen species-mediated cellular stress, Excli J
Villalpando-Rodriguez, Gibson, Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat, Oxid Med Cell Longev
Vitale, Pietrocola, Guilbaud, Aaronson, Abrams et al., Apoptotic cell death in disease-current understanding of the NCCD 2023, Cell Death Differ
Vorobjeva, Chernyak, NETosis: molecular mechanisms, role in physiology and pathology, Biochemistry (Mosc)
Wang, Chang, Wang, Hou, Wang, Mitophagy-dependent mitochondrial ROS mediates 2,5-hexanedioneinduced NLRP3 inflammasome activation in BV2 microglia, Neurotoxicology
Wang, Huang, Sun, Stubbs, He et al., SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis, Food Chem Toxicol
Xu, Akinyemi, Chitre, Loeb, Lednicky et al., SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway, Virology
Xu, Kim, Li, Han, Autophagy contributes to caspase-independent macrophage cell death, J Biol Chem
Xu, Li, SARS-CoV-2 promotes RIPK1 activation to facilitate viral propagation, Cell Res
Xu, Xu, Zhang, Yang, Yin et al., Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways, Vet Microbiol
Yang, Wu, Meng, Wang, Younis et al., SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting BOK, Cell Death Differ
Yu, Chen, Tooze, Autophagy pathway: cellular and molecular mechanisms, iScience
Yu, Zhang, Liu, Tang, Peng et al., Pyroptosis: mechanisms and diseases, Signal Transduct Target Ther
Yuan, Ma, Xie, Li, Su et al., The role of cell death in SARS-CoV-2 infection, Signal Transduct Target Ther
Zhang, He, Chen, Su, Yan et al., Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation, Aging
Zhang, Li, Cosme, Gerzanich, Tang et al., Genome-wide characterization of SARS-CoV-2 cytopathogenic proteins in the search of antiviral targets, bioRxiv, doi:10.1128/mbio.00169-22
Zhang, Taylor, Bennett, Saad, Rayman, Association between regional selenium status and reported outcome of COVID-19 cases in China, Am J Clin Nutr
Zhang, Wang, Zhao, Xin, Fan et al., Regulated necrosis, a proinflammatory cell death, potentially counteracts pathogenic infections, Cell Death Dis
Zhang, Zhang, Wang, Guo, Liu et al., Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice, Clin Immunol
Zheng, Duan, He, Wu, Wei et al., Dysregulated dendritic cells in sepsis: functional impairment and regulated cell death, Cell Mol Biol Lett
Zheng, Karki, TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines, Nat Immunol
Zheng, Li, Wang, Liu, Xie, High-content image screening to identify chemical modulators for peroxisome and ferroptosis, Cell Mol Biol Lett
Zhou, Cai, Zhai, Yu, He et al., Necroptosis inhibitors: mechanisms of action and therapeutic potential, Apoptosis
Zuo, Zheng, Huang, He, Zang et al., Vitamin C promotes ACE2 degradation and protects against SARS-CoV-2 infection, EMBO Rep
DOI record:
{
"DOI": "10.1186/s11658-024-00659-6",
"ISSN": [
"1689-1392"
],
"URL": "http://dx.doi.org/10.1186/s11658-024-00659-6",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Coronavirus disease 2019 (COVID-19) represents the novel respiratory infectious disorder caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is characterized by rapid spread throughout the world. Reactive oxygen species (ROS) account for cellular metabolic by-products, and excessive ROS accumulation can induce oxidative stress due to insufficient endogenous antioxidant ability. In the case of oxidative stress, ROS production exceeds the cellular antioxidant capacity, thus leading to cell death. SARS-CoV-2 can activate different cell death pathways in the context of infection in host cells, such as neutrophil extracellular trap (NET)osis, ferroptosis, apoptosis, pyroptosis, necroptosis and autophagy, which are closely related to ROS signalling and control. In this review, we comprehensively elucidated the relationship between ROS generation and the death of host cells after SARS-CoV-2 infection, which leads to the development of COVID-19, aiming to provide a reasonable basis for the existing interventions and further development of novel therapies against SARS-CoV-2.</jats:p>\n <jats:p><jats:bold>Graphical Abstract</jats:bold></jats:p>",
"alternative-id": [
"659"
],
"article-number": "138",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "13 May 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "24 October 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "8 November 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Xie",
"given": "Jiufeng",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yuan",
"given": "Cui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Sen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Zhenling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Wenqing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mao",
"given": "Lin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiao",
"given": "Pengtao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Wei",
"sequence": "additional"
}
],
"container-title": "Cellular & Molecular Biology Letters",
"container-title-short": "Cell Mol Biol Lett",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
11,
8
]
],
"date-time": "2024-11-08T22:10:11Z",
"timestamp": 1731103811000
},
"deposited": {
"date-parts": [
[
2024,
11,
8
]
],
"date-time": "2024-11-08T23:01:13Z",
"timestamp": 1731106873000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"31802164"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001809",
"id-type": "DOI"
}
],
"name": "National Natural Science Foundation of China"
}
],
"indexed": {
"date-parts": [
[
2024,
11,
8
]
],
"date-time": "2024-11-08T23:40:13Z",
"timestamp": 1731109213519,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
11,
8
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
8
]
],
"date-time": "2024-11-08T00:00:00Z",
"timestamp": 1731024000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
8
]
],
"date-time": "2024-11-08T00:00:00Z",
"timestamp": 1731024000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s11658-024-00659-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s11658-024-00659-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s11658-024-00659-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
11,
8
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
8
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41392-022-01043-6",
"author": "X Li",
"doi-asserted-by": "publisher",
"first-page": "186",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "659_CR1",
"unstructured": "Li X, Zhang Z, Wang Z, Gutiérrez-Castrellón P. Cell deaths: Involvement in the pathogenesis and intervention therapy of COVID-19. Signal Transduct Target Ther. 2022;7(1):186.",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.15252/emmm.202216345",
"author": "L Renia",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "EMBO Mol Med",
"key": "659_CR2",
"unstructured": "Renia L, Ng LFP. Acquired immunity against SARS-CoV-2 infection and vaccination. EMBO Mol Med. 2023;15(12): e16345.",
"volume": "15",
"year": "2023"
},
{
"author": "N Vardar Acar",
"first-page": "520",
"journal-title": "Excli J.",
"key": "659_CR3",
"unstructured": "Vardar Acar N, Özgül RK. The bridge between cell survival and cell death: reactive oxygen species-mediated cellular stress. Excli J. 2023;2:520–55.",
"volume": "2",
"year": "2023"
},
{
"DOI": "10.1016/j.redox.2018.11.014",
"author": "BWL Lee",
"doi-asserted-by": "publisher",
"journal-title": "Redox Biol",
"key": "659_CR4",
"unstructured": "Lee BWL, Ghode P, Ong DST. Redox regulation of cell state and fate. Redox Biol. 2019;25: 101056.",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1155/2021/9912436",
"author": "GE Villalpando-Rodriguez",
"doi-asserted-by": "publisher",
"first-page": "9912436",
"journal-title": "Oxid Med Cell Longev",
"key": "659_CR5",
"unstructured": "Villalpando-Rodriguez GE, Gibson SB. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid Med Cell Longev. 2021;2021:9912436.",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1016/j.molcel.2021.08.018",
"author": "C Lennicke",
"doi-asserted-by": "publisher",
"first-page": "3691",
"issue": "18",
"journal-title": "Mol Cell",
"key": "659_CR6",
"unstructured": "Lennicke C, Cochemé HM. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol Cell. 2021;81(18):3691–707.",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1161/CIRCRESAHA.117.311401",
"author": "SJ Forrester",
"doi-asserted-by": "publisher",
"first-page": "877",
"issue": "6",
"journal-title": "Circ Res",
"key": "659_CR7",
"unstructured": "Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902.",
"volume": "122",
"year": "2018"
},
{
"DOI": "10.1126/sciadv.adl0479",
"author": "X Li",
"doi-asserted-by": "publisher",
"first-page": "eadl0479",
"issue": "20",
"journal-title": "Sci Adv.",
"key": "659_CR8",
"unstructured": "Li X, Gao J. Precise modulation and use of reactive oxygen species for immunotherapy. Sci Adv. 2024;10(20):eadl0479.",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1002/rmv.2240",
"author": "WJ Sander",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Rev Med Virol",
"key": "659_CR9",
"unstructured": "Sander WJ, Fourie C. Reactive oxygen species as potential antiviral targets. Rev Med Virol. 2022;32(1): e2240.",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1038/s44320-024-00019-8",
"author": "P Trepte",
"doi-asserted-by": "publisher",
"journal-title": "Mol Sys Bio",
"key": "659_CR10",
"unstructured": "Trepte P, Secker C, Olivet J, Blavier J, Kostova S, Maseko SB, et al. AI-guided pipeline for protein–protein interaction drug discovery identifies a SARS-CoV-2 inhibitor. Mol Sys Bio. 2024. https://doi.org/10.1038/s44320-024-00019-8.",
"year": "2024"
},
{
"DOI": "10.1016/j.cellsig.2020.109772",
"author": "G Imre",
"doi-asserted-by": "publisher",
"journal-title": "Cell Signal",
"key": "659_CR11",
"unstructured": "Imre G. Cell death signalling in virus infection. Cell Signal. 2020;76: 109772.",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1155/2014/467452",
"author": "ML Reshi",
"doi-asserted-by": "publisher",
"journal-title": "Int J Cell Biol",
"key": "659_CR12",
"unstructured": "Reshi ML, Su YC, Hong JR. RNA viruses: ROS-mediated cell death. Int J Cell Biol. 2014;2014: 467452.",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1038/s41423-020-00630-3",
"author": "D Bertheloot",
"doi-asserted-by": "publisher",
"first-page": "1106",
"issue": "5",
"journal-title": "Cell Mol Immunol",
"key": "659_CR13",
"unstructured": "Bertheloot D, Latz E. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–21.",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1038/s41392-023-01580-8",
"author": "C Yuan",
"doi-asserted-by": "publisher",
"first-page": "357",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "659_CR14",
"unstructured": "Yuan C, Ma Z, Xie J, Li W, Su L, Zhang G, et al. The role of cell death in SARS-CoV-2 infection. Signal Transduct Target Ther. 2023;8(1):357.",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1016/j.cbi.2014.10.016",
"author": "VI Lushchak",
"doi-asserted-by": "publisher",
"first-page": "164",
"journal-title": "Chem Biol Interact",
"key": "659_CR15",
"unstructured": "Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–75.",
"volume": "224",
"year": "2014"
},
{
"DOI": "10.1080/22221751.2022.2128434",
"author": "Y Feng",
"doi-asserted-by": "publisher",
"first-page": "2529",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "659_CR16",
"unstructured": "Feng Y, Pan Z, Wang Z, Lei Z, Yang S, Zhao H. MERS-CoV nsp1 regulates autophagic flux via mTOR signalling and dysfunctional lysosomes. Emerg Microbes Infect. 2022;11(1):2529–43.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.bbrc.2015.03.010",
"author": "N Shin",
"doi-asserted-by": "publisher",
"first-page": "699",
"issue": "4",
"journal-title": "Biochem Biophys Res Commun",
"key": "659_CR17",
"unstructured": "Shin N, Pyo CW, Jung KI, Choi SY. Influenza A virus PB1-F2 is involved in regulation of cellular redox state in alveolar epithelial cells. Biochem Biophys Res Commun. 2015;459(4):699–705.",
"volume": "459",
"year": "2015"
},
{
"DOI": "10.1146/annurev-virology-110615-042435",
"author": "P Danthi",
"doi-asserted-by": "publisher",
"first-page": "533",
"issue": "1",
"journal-title": "Annu Rev Virol",
"key": "659_CR18",
"unstructured": "Danthi P. Viruses and the diversity of cell death. Annu Rev Virol. 2016;3(1):533–53.",
"volume": "3",
"year": "2016"
},
{
"DOI": "10.1002/rmv.2292",
"author": "M Morais da Silva",
"doi-asserted-by": "publisher",
"first-page": "e2292",
"issue": "3",
"journal-title": "Rev Med Virol.",
"key": "659_CR19",
"unstructured": "Morais da Silva M, Lira de Lucena AS, Paiva Júnior SSL, Florêncio De Carvalho VM, Santana de Oliveira PS, da Rosa MM, et al. Cell death mechanisms involved in cell injury caused by SARS-CoV-2. Rev Med Virol. 2022;32(3):e2292.",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1146/annurev-cellbio-020520-111016",
"author": "HR Thiam",
"doi-asserted-by": "publisher",
"first-page": "191",
"journal-title": "Annu Rev Cell Dev Biol",
"key": "659_CR20",
"unstructured": "Thiam HR, Wong SL, Wagner DD, Waterman CM. Cellular mechanisms of NETosis. Annu Rev Cell Dev Biol. 2020;36:191–218.",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1172/JCI84538",
"author": "OE Sørensen",
"doi-asserted-by": "publisher",
"first-page": "1612",
"issue": "5",
"journal-title": "J Clin Invest",
"key": "659_CR21",
"unstructured": "Sørensen OE, Borregaard N. Neutrophil extracellular traps—the dark side of neutrophils. J Clin Invest. 2016;126(5):1612–20.",
"volume": "126",
"year": "2016"
},
{
"DOI": "10.3389/fphar.2020.00870",
"author": "T Narasaraju",
"doi-asserted-by": "publisher",
"first-page": "870",
"journal-title": "Front Pharmacol",
"key": "659_CR22",
"unstructured": "Narasaraju T, Tang BM, Herrmann M, Muller S, Chow VTK, Radic M. Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients with COVID-19. Front Pharmacol. 2020;11:870.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-76781-0",
"author": "A Arcanjo",
"doi-asserted-by": "publisher",
"first-page": "19630",
"issue": "1",
"journal-title": "Sci Rep.",
"key": "659_CR23",
"unstructured": "Arcanjo A, Logullo J, Menezes CCB, de Souza Carvalho Giangiarulo TC, Dos Reis MC, de Castro GMM, et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci Rep. 2020;10(1):19630.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.3389/fcimb.2021.679878",
"author": "PR Kvietys",
"doi-asserted-by": "publisher",
"first-page": "679878",
"journal-title": "Front Cell Infect Microbiol.",
"key": "659_CR24",
"unstructured": "Kvietys PR, Fakhoury HMA, Kadan S, Yaqinuddin A, Al-Mutairy E, Al-Kattan K. COVID-19: lung-centric immunothrombosis. Front Cell Infect Microbiol. 2021;11:679878.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1134/S0006297920100065",
"author": "NV Vorobjeva",
"doi-asserted-by": "publisher",
"first-page": "1178",
"issue": "10",
"journal-title": "Biochemistry (Mosc)",
"key": "659_CR25",
"unstructured": "Vorobjeva NV, Chernyak BV. NETosis: molecular mechanisms, role in physiology and pathology. Biochemistry (Mosc). 2020;85(10):1178–90.",
"volume": "85",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2014.06.044",
"author": "KD Metzler",
"doi-asserted-by": "publisher",
"first-page": "883",
"issue": "3",
"journal-title": "Cell Rep",
"key": "659_CR26",
"unstructured": "Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8(3):883–96.",
"volume": "8",
"year": "2014"
},
{
"DOI": "10.1002/advs.202103748",
"author": "HH Park",
"doi-asserted-by": "publisher",
"issue": "19",
"journal-title": "Adv Sci (Weinh)",
"key": "659_CR27",
"unstructured": "Park HH, Park W, Lee YY, Kim H, Seo HS, Choi DW, et al. Bioinspired DNase-I-coated melanin-like nanospheres for modulation of infection-associated NETosis dysregulation. Adv Sci (Weinh). 2021;8(19): e2103748.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2016.00311",
"author": "BN Porto",
"doi-asserted-by": "publisher",
"first-page": "311",
"journal-title": "Front Immunol",
"key": "659_CR28",
"unstructured": "Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front Immunol. 2016;7:311.",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1016/j.cell.2012.03.042",
"author": "SJ Dixon",
"doi-asserted-by": "publisher",
"first-page": "1060",
"issue": "5",
"journal-title": "Cell",
"key": "659_CR29",
"unstructured": "Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72.",
"volume": "149",
"year": "2012"
},
{
"DOI": "10.1038/s41580-020-00324-8",
"author": "X Jiang",
"doi-asserted-by": "publisher",
"first-page": "266",
"issue": "4",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "659_CR30",
"unstructured": "Jiang X, Stockwell BR. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–82.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1186/s11658-024-00544-2",
"author": "D Zheng",
"doi-asserted-by": "publisher",
"first-page": "26",
"issue": "1",
"journal-title": "Cell Mol Biol Lett",
"key": "659_CR31",
"unstructured": "Zheng D, Li F, Wang S, Liu P-S, Xie X. High-content image screening to identify chemical modulators for peroxisome and ferroptosis. Cell Mol Biol Lett. 2024;29(1):26.",
"volume": "29",
"year": "2024"
},
{
"DOI": "10.1016/j.cell.2017.09.021",
"author": "BR Stockwell",
"doi-asserted-by": "publisher",
"first-page": "273",
"issue": "2",
"journal-title": "Cell",
"key": "659_CR32",
"unstructured": "Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–85.",
"volume": "171",
"year": "2017"
},
{
"DOI": "10.1038/nature24637",
"author": "SW Alvarez",
"doi-asserted-by": "publisher",
"first-page": "639",
"issue": "7682",
"journal-title": "Nature",
"key": "659_CR33",
"unstructured": "Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551(7682):639–43.",
"volume": "551",
"year": "2017"
},
{
"DOI": "10.1016/j.trsl.2021.10.007",
"author": "JS Bednash",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Transl Res",
"key": "659_CR34",
"unstructured": "Bednash JS, Kagan VE, Englert JA, Farkas D, Tyurina YY, Tyurin VA, et al. Syrian hamsters as a model of lung injury with SARS-CoV-2 infection: pathologic, physiologic, and detailed molecular profiling. Transl Res. 2022;240:1–16.",
"volume": "240",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"issue": "10229",
"journal-title": "Lancet",
"key": "659_CR35",
"unstructured": "Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1007/s12015-021-10322-8",
"author": "R Kronstein-Wiedemann",
"doi-asserted-by": "publisher",
"first-page": "1809",
"issue": "5",
"journal-title": "Stem Cell Rev Rep",
"key": "659_CR36",
"unstructured": "Kronstein-Wiedemann R, Stadtmüller M, Traikov S, Georgi M, Teichert M, Yosef H, et al. SARS-CoV-2 infects red blood cell progenitors and dysregulates hemoglobin and iron metabolism. Stem Cell Rev Rep. 2022;18(5):1809–21.",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1002/ajh.26027",
"author": "A Nai",
"doi-asserted-by": "publisher",
"first-page": "E32",
"issue": "1",
"journal-title": "Am J Hematol",
"key": "659_CR37",
"unstructured": "Nai A, Lorè NI, Pagani A, De Lorenzo R, Di Modica S, Saliu F, et al. Hepcidin levels predict COVID-19 severity and mortality in a cohort of hospitalized Italian patients. Am J Hematol. 2021;96(1):E32-e35.",
"volume": "96",
"year": "2021"
},
{
"DOI": "10.1016/j.fct.2021.112286",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"journal-title": "Food Chem Toxicol",
"key": "659_CR38",
"unstructured": "Wang Y, Huang J, Sun Y, Stubbs D, He J, Li W, et al. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food Chem Toxicol. 2021;153: 112286.",
"volume": "153",
"year": "2021"
},
{
"DOI": "10.1016/j.redox.2021.102041",
"author": "D Bartolini",
"doi-asserted-by": "publisher",
"journal-title": "Redox Biol",
"key": "659_CR39",
"unstructured": "Bartolini D, Stabile AM, Bastianelli S, Giustarini D, Pierucci S, Busti C, et al. SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione. Redox Biol. 2021;45: 102041.",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1038/s41571-020-0341-y",
"author": "BA Carneiro",
"doi-asserted-by": "publisher",
"first-page": "395",
"issue": "7",
"journal-title": "Nat Rev Clin Oncol",
"key": "659_CR40",
"unstructured": "Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395–417.",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1007/PL00000863",
"author": "G Denecker",
"doi-asserted-by": "publisher",
"first-page": "356",
"issue": "3",
"journal-title": "Cell Mol Life Sci",
"key": "659_CR41",
"unstructured": "Denecker G, Vercammen D, Declercq W, Vandenabeele P. Apoptotic and necrotic cell death induced by death domain receptors. Cell Mol Life Sci. 2001;58(3):356–70.",
"volume": "58",
"year": "2001"
},
{
"DOI": "10.1038/s41418-023-01153-w",
"author": "I Vitale",
"doi-asserted-by": "publisher",
"first-page": "1097",
"issue": "5",
"journal-title": "Cell Death Differ",
"key": "659_CR42",
"unstructured": "Vitale I, Pietrocola F, Guilbaud E, Aaronson SA, Abrams JM, Adam D, et al. Apoptotic cell death in disease—current understanding of the NCCD 2023. Cell Death Differ. 2023;30(5):1097–154.",
"volume": "30",
"year": "2023"
},
{
"DOI": "10.3389/fphys.2013.00242",
"author": "S Pizzimenti",
"doi-asserted-by": "publisher",
"first-page": "242",
"journal-title": "Front Physiol",
"key": "659_CR43",
"unstructured": "Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242.",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1016/j.taap.2017.11.006",
"author": "ER Elkin",
"doi-asserted-by": "publisher",
"first-page": "30",
"journal-title": "Toxicol Appl Pharmacol",
"key": "659_CR44",
"unstructured": "Elkin ER, Harris SM, Loch-Caruso R. Trichloroethylene metabolite S-(1,2-dichlorovinyl)-l-cysteine induces lipid peroxidation-associated apoptosis via the intrinsic and extrinsic apoptosis pathways in a first-trimester placental cell line. Toxicol Appl Pharmacol. 2018;338:30–42.",
"volume": "338",
"year": "2018"
},
{
"DOI": "10.1016/j.freeradbiomed.2021.02.043",
"author": "D Bou-Teen",
"doi-asserted-by": "publisher",
"first-page": "109",
"journal-title": "Free Radic Biol Med",
"key": "659_CR45",
"unstructured": "Bou-Teen D, Kaludercic N, Weissman D, Turan B, Maack C, Di Lisa F, et al. Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic Biol Med. 2021;167:109–24.",
"volume": "167",
"year": "2021"
},
{
"DOI": "10.1186/s13099-020-00361-w",
"author": "Z Bai",
"doi-asserted-by": "publisher",
"first-page": "22",
"journal-title": "Gut Pathog",
"key": "659_CR46",
"unstructured": "Bai Z, Zhao X, Li C, Sheng C, Li H. EV71 virus reduces Nrf2 activation to promote production of reactive oxygen species in infected cells. Gut Pathog. 2020;12:22.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1111/j.1476-5381.2010.00982.x",
"author": "WH Tung",
"doi-asserted-by": "publisher",
"first-page": "1566",
"issue": "7",
"journal-title": "Br J Pharmacol",
"key": "659_CR47",
"unstructured": "Tung WH, Tsai HW, Lee IT, Hsieh HL, Chen WJ, Chen YL, et al. Japanese encephalitis virus induces matrix metalloproteinase-9 in rat brain astrocytes via NF-κB signalling dependent on MAPKs and reactive oxygen species. Br J Pharmacol. 2010;161(7):1566–83.",
"volume": "161",
"year": "2010"
},
{
"author": "M Qian",
"first-page": "280",
"issue": "4",
"journal-title": "Biomed Environ Sci",
"key": "659_CR48",
"unstructured": "Qian M, Tan HM, Yu N, Wang T, Zhang Q. Inactivated sendai virus induces ROS-dependent apoptosis and autophagy in human prostate cancer cells. Biomed Environ Sci. 2018;31(4):280–9.",
"volume": "31",
"year": "2018"
},
{
"DOI": "10.1016/j.vetmic.2019.03.028",
"author": "X Xu",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Vet Microbiol.",
"key": "659_CR49",
"unstructured": "Xu X, Xu Y, Zhang Q, Yang F, Yin Z, Wang L, et al. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet Microbiol. 2019;232:1–12.",
"volume": "232",
"year": "2019"
},
{
"DOI": "10.1016/j.bbadis.2021.166260",
"author": "F Li",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "Biochim Biophys Acta Mol Basis Dis",
"key": "659_CR50",
"unstructured": "Li F, Li J, Wang PH, Yang N, Huang J, Ou J, et al. SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim Biophys Acta Mol Basis Dis. 2021;1867(12): 166260.",
"volume": "1867",
"year": "2021"
},
{
"DOI": "10.1038/s41418-022-00928-x",
"author": "Y Yang",
"doi-asserted-by": "publisher",
"first-page": "1395",
"issue": "7",
"journal-title": "Cell Death Differ.",
"key": "659_CR51",
"unstructured": "Yang Y, Wu Y, Meng X, Wang Z, Younis M, Liu Y, et al. SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting BOK. Cell Death Differ. 2022;29(7):1395–408.",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1038/nature15514",
"author": "J Shi",
"doi-asserted-by": "publisher",
"first-page": "660",
"issue": "7575",
"journal-title": "Nature",
"key": "659_CR52",
"unstructured": "Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5.",
"volume": "526",
"year": "2015"
},
{
"DOI": "10.1038/s41392-021-00507-5",
"author": "P Yu",
"doi-asserted-by": "publisher",
"first-page": "128",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "659_CR53",
"unstructured": "Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41577-019-0228-2",
"author": "P Broz",
"doi-asserted-by": "publisher",
"first-page": "143",
"issue": "3",
"journal-title": "Nat Rev Immunol",
"key": "659_CR54",
"unstructured": "Broz P, Pelegrín P. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143–57.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1038/s41577-019-0165-0",
"author": "KV Swanson",
"doi-asserted-by": "publisher",
"first-page": "477",
"issue": "8",
"journal-title": "Nat Rev Immunol",
"key": "659_CR55",
"unstructured": "Swanson KV, Deng M. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89.",
"volume": "19",
"year": "2019"
},
{
"author": "KD Singh",
"first-page": "111",
"issue": "2",
"journal-title": "J Cell Signal.",
"key": "659_CR56",
"unstructured": "Singh KD, Karnik SS. Angiotensin receptors: structure, function, signaling and clinical applications. J Cell Signal. 2016;1(2):111.",
"volume": "1",
"year": "2016"
},
{
"DOI": "10.1007/s12015-020-10010-z",
"author": "MZ Ratajczak",
"doi-asserted-by": "publisher",
"first-page": "266",
"issue": "1",
"journal-title": "Stem Cell Rev Rep",
"key": "659_CR57",
"unstructured": "Ratajczak MZ, Bujko K, Ciechanowicz A, Sielatycka K, Cymer M, Marlicz W, et al. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45(−) precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. Stem Cell Rev Rep. 2021;17(1):266–77.",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/j.tibs.2016.09.002",
"author": "Y He",
"doi-asserted-by": "publisher",
"first-page": "1012",
"issue": "12",
"journal-title": "Trends Biochem Sci",
"key": "659_CR58",
"unstructured": "He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41(12):1012–21.",
"volume": "41",
"year": "2016"
},
{
"DOI": "10.1016/j.virol.2022.01.003",
"author": "H Xu",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "Virology",
"key": "659_CR59",
"unstructured": "Xu H, Akinyemi IA, Chitre SA, Loeb JC, Lednicky JA, McIntosh MT, et al. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology. 2022;568:13–22.",
"volume": "568",
"year": "2022"
},
{
"author": "AC Ferreira",
"first-page": "43",
"issue": "1",
"journal-title": "Virology",
"key": "659_CR60",
"unstructured": "Ferreira AC, Soares VC, de Azevedo-Quintanilha IG, Dias S, Fintelman-Rodrigues N, Sacramento CQ, et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Virology. 2021;7(1):43.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1038/s41590-021-00937-x",
"author": "M Zheng",
"doi-asserted-by": "publisher",
"first-page": "829",
"issue": "7",
"journal-title": "Nat Immunol",
"key": "659_CR61",
"unstructured": "Zheng M, Karki R. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol. 2021;22(7):829–38.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.isci.2020.101270",
"author": "M Moriyama",
"doi-asserted-by": "publisher",
"first-page": "101270",
"issue": "7",
"journal-title": "iScience.",
"key": "659_CR62",
"unstructured": "Moriyama M, Nagai M, Maruzuru Y, Koshiba T, Kawaguchi Y, Ichinohe T. Influenza virus-induced oxidized DNA activates inflammasomes. iScience. 2020;23(7):101270.",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1038/s41580-018-0003-4",
"author": "I Dikic",
"doi-asserted-by": "publisher",
"first-page": "349",
"issue": "6",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "659_CR63",
"unstructured": "Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–64.",
"volume": "19",
"year": "2018"
},
{
"author": "L Yu",
"first-page": "207",
"issue": "2",
"journal-title": "iScience.",
"key": "659_CR64",
"unstructured": "Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. iScience. 2018;14(2):207–15.",
"volume": "14",
"year": "2018"
},
{
"DOI": "10.4161/auto.6.8.13479",
"author": "MD Houslay",
"doi-asserted-by": "publisher",
"first-page": "1198",
"issue": "8",
"journal-title": "Autophagy",
"key": "659_CR65",
"unstructured": "Houslay MD, Christian F. p62 (SQSTM1) forms part of a novel, reversible aggregate containing a specific conformer of the cAMP degrading phosphodiesterase, PDE4A4. Autophagy. 2010;6(8):1198–200.",
"volume": "6",
"year": "2010"
},
{
"DOI": "10.1074/jbc.M513377200",
"author": "Y Xu",
"doi-asserted-by": "publisher",
"first-page": "19179",
"issue": "28",
"journal-title": "J Biol Chem",
"key": "659_CR66",
"unstructured": "Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem. 2006;281(28):19179–87.",
"volume": "281",
"year": "2006"
},
{
"DOI": "10.1242/jcs.011163",
"author": "Y Chen",
"doi-asserted-by": "publisher",
"first-page": "4155",
"issue": "Pt 23",
"journal-title": "J Cell Sci",
"key": "659_CR67",
"unstructured": "Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120(Pt 23):4155–66.",
"volume": "120",
"year": "2007"
},
{
"DOI": "10.1080/22221751.2020.1799723",
"author": "S Appelberg",
"doi-asserted-by": "publisher",
"first-page": "1748",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "659_CR68",
"unstructured": "Appelberg S, Gupta S, Svensson Akusjärvi S, Ambikan AT, Mikaeloff F, Saccon E, et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg Microbes Infect. 2020;9(1):1748–60.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1007/s10571-015-0166-x",
"author": "L Li",
"doi-asserted-by": "publisher",
"first-page": "615",
"issue": "5",
"journal-title": "Cell Mol Neurobiol",
"key": "659_CR69",
"unstructured": "Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 2015;35(5):615–21.",
"volume": "35",
"year": "2015"
},
{
"DOI": "10.1038/s41423-021-00807-4",
"author": "X Li",
"doi-asserted-by": "publisher",
"first-page": "67",
"issue": "1",
"journal-title": "Cell Mol Immunol",
"key": "659_CR70",
"unstructured": "Li X, Hou P, Ma W, Wang X, Wang H, Yu Z, et al. SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy. Cell Mol Immunol. 2022;19(1):67–78.",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.15252/embj.2018100978",
"author": "X He",
"doi-asserted-by": "publisher",
"issue": "14",
"journal-title": "EMBO J",
"key": "659_CR71",
"unstructured": "He X, Zhu Y, Zhang Y, Geng Y, Gong J, Geng J, et al. RNF34 functions in immunity and selective mitophagy by targeting MAVS for autophagic degradation. EMBO J. 2019;38(14): e100978.",
"volume": "38",
"year": "2019"
},
{
"DOI": "10.1089/ars.2021.0167",
"author": "A Sharma",
"doi-asserted-by": "publisher",
"first-page": "1376",
"issue": "16",
"journal-title": "Antioxid Redox Signal",
"key": "659_CR72",
"unstructured": "Sharma A, Kontodimas K, Bosmann M. The MAVS immune recognition pathway in viral infection and sepsis. Antioxid Redox Signal. 2021;35(16):1376–92.",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.neuro.2023.09.008",
"author": "W Wang",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Neurotoxicology",
"key": "659_CR73",
"unstructured": "Wang W, Chang R, Wang Y, Hou L, Wang Q. Mitophagy-dependent mitochondrial ROS mediates 2,5-hexanedione-induced NLRP3 inflammasome activation in BV2 microglia. Neurotoxicology. 2023;99:50–8.",
"volume": "99",
"year": "2023"
},
{
"DOI": "10.1038/s41419-022-05066-3",
"author": "G Zhang",
"doi-asserted-by": "publisher",
"first-page": "637",
"issue": "7",
"journal-title": "Cell Death Dis",
"key": "659_CR74",
"unstructured": "Zhang G, Wang J, Zhao Z, Xin T, Fan X, Shen Q, et al. Regulated necrosis, a proinflammatory cell death, potentially counteracts pathogenic infections. Cell Death Dis. 2022;13(7):637.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1007/s10495-023-01905-6",
"author": "Y Zhou",
"doi-asserted-by": "publisher",
"first-page": "22",
"issue": "1–2",
"journal-title": "Apoptosis",
"key": "659_CR75",
"unstructured": "Zhou Y, Cai Z, Zhai Y, Yu J, He Q, He Y, et al. Necroptosis inhibitors: mechanisms of action and therapeutic potential. Apoptosis. 2024;29(1–2):22–44.",
"volume": "29",
"year": "2024"
},
{
"DOI": "10.1038/s41422-021-00578-7",
"author": "G Xu",
"doi-asserted-by": "publisher",
"first-page": "1230",
"issue": "12",
"journal-title": "Cell Res",
"key": "659_CR76",
"unstructured": "Xu G, Li Y. SARS-CoV-2 promotes RIPK1 activation to facilitate viral propagation. Cell Res. 2021;31(12):1230–43.",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-03209-6",
"author": "H Nakamura",
"doi-asserted-by": "publisher",
"first-page": "484",
"issue": "1",
"journal-title": "Crit Care",
"key": "659_CR77",
"unstructured": "Nakamura H, Kinjo T, Arakaki W, Miyagi K, Tateyama M, Fujita J. Serum levels of receptor-interacting protein kinase-3 in patients with COVID-19. Crit Care. 2020;24(1):484.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1038/s41422-022-00775-y",
"author": "S Li",
"doi-asserted-by": "publisher",
"first-page": "201",
"issue": "3",
"journal-title": "Cell Res",
"key": "659_CR78",
"unstructured": "Li S, Zhang Y, Guan Z, Ye M, Li H, You M, et al. SARS-CoV-2 Z-RNA activates the ZBP1-RIPK3 pathway to promote virus-induced inflammatory responses. Cell Res. 2023;33(3):201–14.",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1016/j.bbamcr.2016.09.012",
"author": "M Redza-Dutordoir",
"doi-asserted-by": "publisher",
"first-page": "2977",
"issue": "12",
"journal-title": "Biochim Biophys Acta",
"key": "659_CR79",
"unstructured": "Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–92.",
"volume": "1863",
"year": "2016"
},
{
"DOI": "10.1038/onc.2015.35",
"author": "B Schenk",
"doi-asserted-by": "publisher",
"first-page": "5796",
"issue": "47",
"journal-title": "Oncogene",
"key": "659_CR80",
"unstructured": "Schenk B, Fulda S. Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death. Oncogene. 2015;34(47):5796–806.",
"volume": "34",
"year": "2015"
},
{
"DOI": "10.1002/j.1460-2075.1993.tb05978.x",
"author": "K Schulze-Osthoff",
"doi-asserted-by": "publisher",
"first-page": "3095",
"issue": "8",
"journal-title": "Embo J",
"key": "659_CR81",
"unstructured": "Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. Embo J. 1993;12(8):3095–104.",
"volume": "12",
"year": "1993"
},
{
"DOI": "10.1038/cdd.2010.138",
"author": "N Vanlangenakker",
"doi-asserted-by": "publisher",
"first-page": "656",
"issue": "4",
"journal-title": "Cell Death Differ",
"key": "659_CR82",
"unstructured": "Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, Deshayes K, et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18(4):656–65.",
"volume": "18",
"year": "2011"
},
{
"DOI": "10.3389/fimmu.2022.917141",
"author": "C Sun",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "659_CR83",
"unstructured": "Sun C, Han Y, Zhang R, Liu S, Wang J, Zhang Y, et al. Regulated necrosis in COVID-19: a double-edged sword. Front Immunol. 2022;13: 917141.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.phrs.2020.105297",
"author": "L Cao",
"doi-asserted-by": "publisher",
"journal-title": "Pharmacol Res",
"key": "659_CR84",
"unstructured": "Cao L, Mu W. Necrostatin-1 and necroptosis inhibition: Pathophysiology and therapeutic implications. Pharmacol Res. 2021;163: 105297.",
"volume": "163",
"year": "2021"
},
{
"DOI": "10.1126/sciimmunol.adn0178",
"author": "K Liang",
"doi-asserted-by": "publisher",
"first-page": "eadn0178",
"issue": "97",
"journal-title": "Sci Immunol.",
"key": "659_CR85",
"unstructured": "Liang K, Barnett KC. Initiator cell death event induced by SARS-CoV-2 in the human airway epithelium. Sci Immunol. 2024;9(97):eadn0178.",
"volume": "9",
"year": "2024"
},
{
"DOI": "10.1128/mbio.00169-22",
"author": "J Zhang",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv.",
"key": "659_CR86",
"unstructured": "Zhang J, Li Q, Cruz Cosme RS, Gerzanich V, Tang Q, Simard JM, et al. Genome-wide characterization of SARS-CoV-2 cytopathogenic proteins in the search of antiviral targets. bioRxiv. 2021. https://doi.org/10.1128/mbio.00169-22.",
"year": "2021"
},
{
"DOI": "10.1016/j.cmet.2020.07.007",
"author": "AC Codo",
"doi-asserted-by": "publisher",
"first-page": "437",
"issue": "3",
"journal-title": "Cell Metab",
"key": "659_CR87",
"unstructured": "Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32(3):437-446.e5.",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2020.572009",
"author": "CC Chiang",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "659_CR88",
"unstructured": "Chiang CC, Korinek M, Cheng WJ, Hwang TL. Targeting neutrophils to treat acute respiratory distress syndrome in Coronavirus disease. Front Pharmacol. 2020;11: 572009.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1002/ams2.471",
"author": "C Maki",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Acute Med Surg",
"key": "659_CR89",
"unstructured": "Maki C, Inoue Y, Ishihara T, Hirano Y, Kondo Y. Evaluation of appropriate indications for the use of sivelestat sodium in acute respiratory distress syndrome: a retrospective cohort study. Acute Med Surg. 2020;7(1): e471.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.nmni.2020.100756",
"author": "HK Okur",
"doi-asserted-by": "publisher",
"journal-title": "New Microbes New Infect",
"key": "659_CR90",
"unstructured": "Okur HK, Yalcin K, Tastan C, Demir S, Yurtsever B, Karakus GS, et al. Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection. New Microbes New Infect. 2020;37: 100756.",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.714833",
"author": "ZM Holliday",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "659_CR91",
"unstructured": "Holliday ZM, Earhart AP, Alnijoumi MM, Krvavac A, Allen LH, Schrum AG. Non-randomized trial of dornase alfa for acute respiratory distress syndrome secondary to COVID-19. Front Immunol. 2021;12: 714833.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.apsb.2020.04.008",
"author": "X Liu",
"doi-asserted-by": "publisher",
"first-page": "1205",
"issue": "7",
"journal-title": "Acta Pharm Sin B",
"key": "659_CR92",
"unstructured": "Liu X, Li Z, Liu S, Sun J, Chen Z, Jiang M, et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B. 2020;10(7):1205–15.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2020.107209",
"author": "V Maldonado",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "659_CR93",
"unstructured": "Maldonado V, Hernandez-Ramírez C, Oliva-Pérez EA, Sánchez-Martínez CO, Pimentel-González JF, Molina-Sánchez JR, et al. Pentoxifylline decreases serum LDH levels and increases lymphocyte count in COVID-19 patients: results from an external pilot study. Int Immunopharmacol. 2021;90: 107209.",
"volume": "90",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2020.108461",
"author": "S Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Clin Immunol",
"key": "659_CR94",
"unstructured": "Zhang S, Zhang Q, Wang F, Guo X, Liu T, Zhao Y, et al. Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice. Clin Immunol. 2020;216: 108461.",
"volume": "216",
"year": "2020"
},
{
"DOI": "10.1172/JCI141374",
"author": "P Skendros",
"doi-asserted-by": "publisher",
"first-page": "6151",
"issue": "11",
"journal-title": "J Clin Invest",
"key": "659_CR95",
"unstructured": "Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130(11):6151–7.",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1080/1744666X.2021.1919086",
"author": "N Potere",
"doi-asserted-by": "publisher",
"first-page": "601",
"issue": "6",
"journal-title": "Expert Rev Clin Immunol",
"key": "659_CR96",
"unstructured": "Potere N, Batticciotto A. The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev Clin Immunol. 2021;17(6):601–18.",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1002/mc.23270",
"author": "NN Alraouji",
"doi-asserted-by": "publisher",
"first-page": "51",
"issue": "1",
"journal-title": "Mol Carcinog",
"key": "659_CR97",
"unstructured": "Alraouji NN, Aboussekhra A. Tocilizumab inhibits IL-8 and the proangiogenic potential of triple negative breast cancer cells. Mol Carcinog. 2021;60(1):51–9.",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028700",
"author": "IO Rosas",
"doi-asserted-by": "publisher",
"first-page": "1503",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "659_CR98",
"unstructured": "Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021;384(16):1503–16.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2021.111228",
"author": "HM Habib",
"doi-asserted-by": "publisher",
"journal-title": "Biomed Pharmacother",
"key": "659_CR99",
"unstructured": "Habib HM, Ibrahim S, Zaim A, Ibrahim WH. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021;136: 111228.",
"volume": "136",
"year": "2021"
},
{
"DOI": "10.1016/j.maturitas.2020.08.003",
"author": "H Shakoor",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Maturitas",
"key": "659_CR100",
"unstructured": "Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9.",
"volume": "143",
"year": "2021"
},
{
"DOI": "10.1016/j.critrevonc.2014.01.006",
"author": "S Temraz",
"doi-asserted-by": "publisher",
"first-page": "64",
"issue": "1",
"journal-title": "Crit Rev Oncol Hematol",
"key": "659_CR101",
"unstructured": "Temraz S, Santini V, Musallam K, Taher A. Iron overload and chelation therapy in myelodysplastic syndromes. Crit Rev Oncol Hematol. 2014;91(1):64–73.",
"volume": "91",
"year": "2014"
},
{
"DOI": "10.1080/07391102.2020.1871416",
"author": "A Chandra",
"doi-asserted-by": "publisher",
"first-page": "5507",
"issue": "12",
"journal-title": "J Biomol Struct",
"key": "659_CR102",
"unstructured": "Chandra A, Gurjar V, Ahmed MZ, Alqahtani AS, Qamar I, Singh N. Exploring potential inhibitor of SARS-CoV2 replicase from FDA approved drugs using insilico drug discovery methods. J Biomol Struct. 2022;40(12):5507–14.",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.3389/fmicb.2022.979719",
"author": "CA Labarrere",
"doi-asserted-by": "publisher",
"journal-title": "Front Microbiol",
"key": "659_CR103",
"unstructured": "Labarrere CA, Kassab GS. Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease. Front Microbiol. 2022;13: 979719.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1021/acsinfecdis.0c00288",
"author": "A Polonikov",
"doi-asserted-by": "publisher",
"first-page": "1558",
"issue": "7",
"journal-title": "ACS Infect Dis",
"key": "659_CR104",
"unstructured": "Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect Dis. 2020;6(7):1558–62.",
"volume": "6",
"year": "2020"
},
{
"author": "RI Horowitz",
"journal-title": "Respir Med Case Rep",
"key": "659_CR105",
"unstructured": "Horowitz RI, Freeman PR, Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Respir Med Case Rep. 2020;30: 101063.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1080/21655979.2021.1964158",
"author": "F Han",
"doi-asserted-by": "publisher",
"first-page": "5279",
"issue": "1",
"journal-title": "Bioengineered",
"key": "659_CR106",
"unstructured": "Han F, Li S, Yang Y, Bai Z. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered. 2021;12(1):5279–88.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1007/s00262-018-2120-5",
"author": "MJ Scheffel",
"doi-asserted-by": "publisher",
"first-page": "691",
"issue": "4",
"journal-title": "Cancer Immunol Immunother",
"key": "659_CR107",
"unstructured": "Scheffel MJ, Scurti G, Wyatt MM. N-acetyl cysteine protects anti-melanoma cytotoxic T cells from exhaustion induced by rapid expansion via the downmodulation of Foxo1 in an Akt-dependent manner. Cancer Immunol Immunother. 2018;67(4):691–702.",
"volume": "67",
"year": "2018"
},
{
"DOI": "10.1016/j.clim.2020.108544",
"author": "H Ibrahim",
"doi-asserted-by": "publisher",
"journal-title": "Clin Immunol",
"key": "659_CR108",
"unstructured": "Ibrahim H, Perl A, Smith D, Lewis T, Kon Z, Goldenberg R, et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol. 2020;219: 108544.",
"volume": "219",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1443",
"author": "JCG de Alencar",
"doi-asserted-by": "publisher",
"first-page": "e736",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "659_CR109",
"unstructured": "de Alencar JCG, Moreira CL, Müller AD, Chaves CE, Fukuhara MA, da Silva EA, et al. Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by Coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2021;72(11):e736–41.",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-039519",
"author": "F Liu",
"doi-asserted-by": "publisher",
"issue": "7",
"journal-title": "BMJ Open",
"key": "659_CR110",
"unstructured": "Liu F, Zhu Y, Zhang J, Li Y, Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10(7): e039519.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1001/jama.2023.21407",
"author": "NKJ Adhikari",
"doi-asserted-by": "publisher",
"first-page": "1745",
"issue": "18",
"journal-title": "JAMA",
"key": "659_CR111",
"unstructured": "Adhikari NKJ, Hashmi M, Tirupakuzhi Vijayaraghavan BK, Haniffa R, Beane A, Webb SA, et al. Intravenous Vitamin C for patients hospitalized with COVID-19: two harmonized randomized clinical trials. JAMA. 2023;330(18):1745–59.",
"volume": "330",
"year": "2023"
},
{
"DOI": "10.15252/embr.202256374",
"author": "Y Zuo",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "EMBO Rep",
"key": "659_CR112",
"unstructured": "Zuo Y, Zheng Z, Huang Y, He J, Zang L, Ren T, et al. Vitamin C promotes ACE2 degradation and protects against SARS-CoV-2 infection. EMBO Rep. 2023;24(4): e56374.",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"author": "D Blanco-Melo",
"doi-asserted-by": "publisher",
"first-page": "1036",
"issue": "5",
"journal-title": "Cell",
"key": "659_CR113",
"unstructured": "Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036-1045.e9.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.3390/nu5083131",
"author": "BM Mohammed",
"doi-asserted-by": "publisher",
"first-page": "3131",
"issue": "8",
"journal-title": "Nutrients",
"key": "659_CR114",
"unstructured": "Mohammed BM, Fisher BJ, Kraskauskas D, Farkas D, Brophy DF, Fowler AA 3rd, et al. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5(8):3131–51.",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.1093/ajcn/nqaa095",
"author": "J Zhang",
"doi-asserted-by": "publisher",
"first-page": "1297",
"issue": "6",
"journal-title": "Am J Clin Nutr",
"key": "659_CR115",
"unstructured": "Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111(6):1297–9.",
"volume": "111",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109878",
"author": "M Kieliszek",
"doi-asserted-by": "publisher",
"journal-title": "Med Hypotheses",
"key": "659_CR116",
"unstructured": "Kieliszek M, Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med Hypotheses. 2020;143: 109878.",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2019.03.032",
"author": "I Alim",
"doi-asserted-by": "publisher",
"first-page": "1262",
"issue": "5",
"journal-title": "Cell",
"key": "659_CR117",
"unstructured": "Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177(5):1262-1279.e25.",
"volume": "177",
"year": "2019"
},
{
"DOI": "10.1016/j.freeradbiomed.2015.10.398",
"author": "H Björnsdottir",
"doi-asserted-by": "publisher",
"first-page": "1024",
"journal-title": "Free Radic Biol Med",
"key": "659_CR118",
"unstructured": "Björnsdottir H, Welin A, Michaëlsson E, Osla V, Berg S, Christenson K, et al. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic Biol Med. 2015;89:1024–35.",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.1016/j.jinorgbio.2021.111546",
"author": "OG Camp",
"doi-asserted-by": "publisher",
"journal-title": "J Inorg Biochem",
"key": "659_CR119",
"unstructured": "Camp OG, Bai D, Gonullu DC, Nayak N, Abu-Soud HM. Melatonin interferes with COVID-19 at several distinct ROS-related steps. J Inorg Biochem. 2021;223: 111546.",
"volume": "223",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2020.06.061",
"author": "T Ueland",
"doi-asserted-by": "publisher",
"first-page": "e41",
"issue": "3",
"journal-title": "J Infection",
"key": "659_CR120",
"unstructured": "Ueland T, Holter JC, Holten AR, Müller KE, Lind A, Bekken GK, et al. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J Infection. 2020;81(3):e41–3.",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1016/j.exppara.2014.09.003",
"author": "RT de Pinho",
"doi-asserted-by": "publisher",
"first-page": "72",
"journal-title": "Exp Parasitol.",
"key": "659_CR121",
"unstructured": "de Pinho RT, da Silva WS, de Castro Côrtes LM, da Silva Vasconcelos Sousa P, de Araujo Soares RO, Alves CR. Production of MMP-9 and inflammatory cytokines by Trypanosoma cruzi-infected macrophages. Exp Parasitol. 2014;147:72–80.",
"volume": "147",
"year": "2014"
},
{
"DOI": "10.18632/aging.102472",
"author": "Y Zhang",
"doi-asserted-by": "publisher",
"first-page": "10499",
"issue": "22",
"journal-title": "Aging (Albany NY)",
"key": "659_CR122",
"unstructured": "Zhang Y, He F, Chen Z, Su Q, Yan M, Zhang Q, et al. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging (Albany NY). 2019;11(22):10499–512.",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1186/s11658-024-00602-9",
"author": "LY Zheng",
"doi-asserted-by": "publisher",
"first-page": "81",
"issue": "1",
"journal-title": "Cell Mol Biol Lett.",
"key": "659_CR123",
"unstructured": "Zheng LY, Duan Y, He PY, Wu MY, Wei ST, Du XH, et al. Dysregulated dendritic cells in sepsis: functional impairment and regulated cell death. Cell Mol Biol Lett. 2024;29(1):81.",
"volume": "29",
"year": "2024"
}
],
"reference-count": 123,
"references-count": 123,
"relation": {},
"resource": {
"primary": {
"URL": "https://cmbl.biomedcentral.com/articles/10.1186/s11658-024-00659-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "29"
}
xie8