Washing Illness Away: A Systematic Review of the Impact of Nasal Irrigation and Spray on COVID‐19
et al., The Laryngoscope, doi:10.1002/lary.31761, PROSPERO CRD42024500158, Sep 2024
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
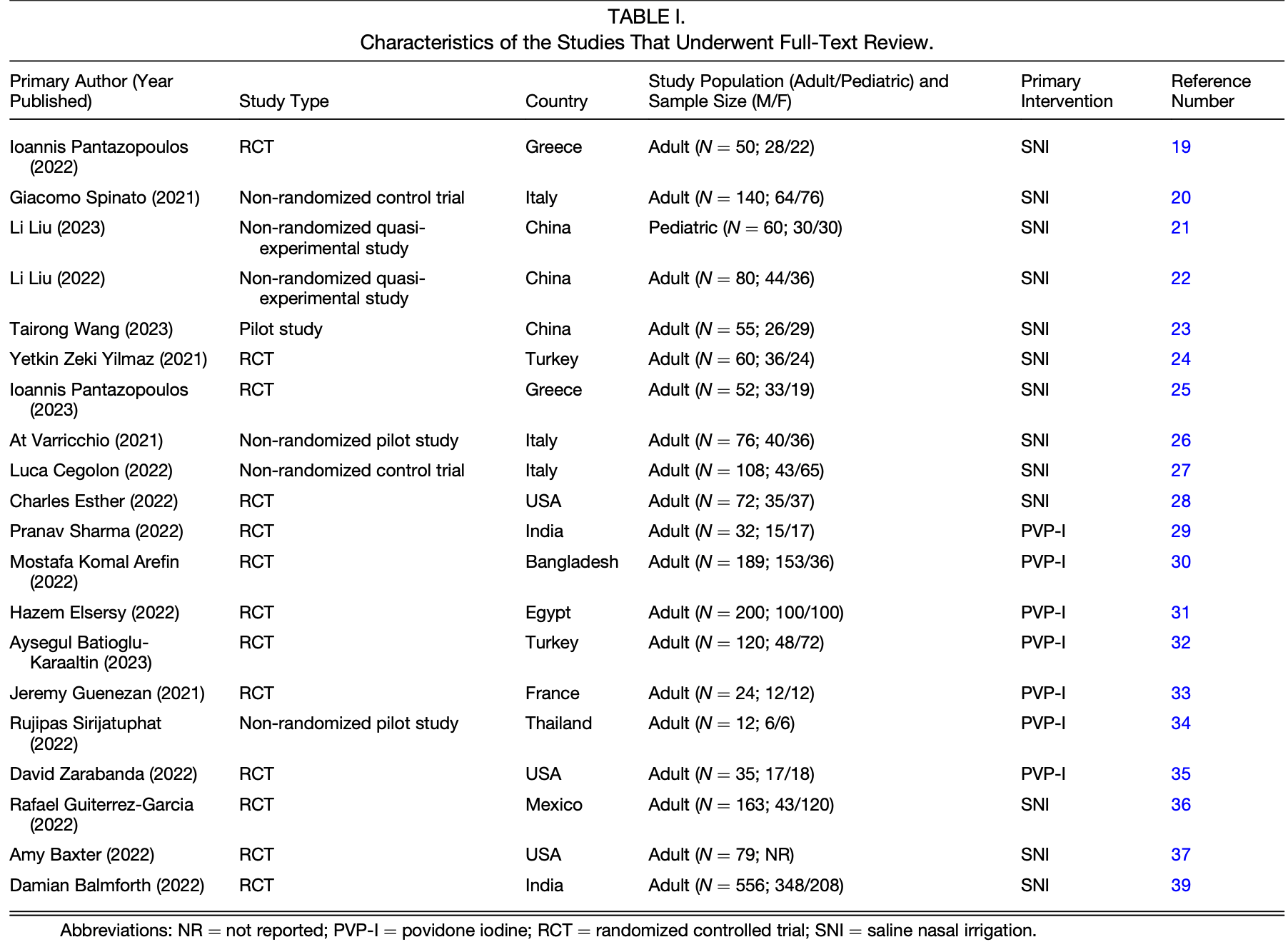
Review of the efficacy of nasal irrigation with saline, povidone-iodine (PVP-I), and intranasal corticosteroids (INCS) at reducing SARS-CoV-2 nasopharyngeal viral load (NVL) and transmissibility. Saline nasal irrigation (SNI) showed the greatest efficacy in 9 out of 10 studies, with benefits manifesting as an earlier time to a negative COVID-19 test ranging from 2-11 days and greater proportional decline in NVL during early time points. Both isotonic and hypertonic saline were effective, with most studies using saline alone without additives. Results for PVP-I were mixed and heterogenous across 7 studies, with 4 showing greater viral clearance and 3 showing no impact. No studies were found for INCS. Four studies also found that SNI and PVP-I reduced COVID-19 transmission.
Review covers NaCl and povidone-iodine.
1.
Angjelova et al., Effects of Antiseptic Formulations on Oral Microbiota and Related Systemic Diseases: A Scoping Review, Antibiotics, doi:10.3390/antibiotics14080815.
2.
Thangamani et al., Rinsing away the threat: Antiviral mouthwashes and their efficacy, World Academy of Sciences Journal, doi:10.3892/wasj.2025.366.
3.
Gunawan et al., The Role of Poly Vinyl Pyrrolidone Iodine (PVP-I) in Preventing Cross-Infection during Dental Procedures: A Systematic Review in the COVID-19 Context, Journal of International Society of Preventive and Community Dentistry, doi:10.4103/jispcd.jispcd_78_24.
4.
Okeke et al., Antiseptics: An expeditious third force in the prevention and management of coronavirus diseases, Current Research in Microbial Sciences, doi:10.1016/j.crmicr.2024.100293.
5.
Gandhi et al., Washing Illness Away: A Systematic Review of the Impact of Nasal Irrigation and Spray on COVID‐19, The Laryngoscope, doi:10.1002/lary.31761.
6.
Brito-Reia et al., Antiviral Mechanism and Clinical Benefits of Mouthwash Active Against SARS-CoV-2, Current Oral Health Reports, doi:10.1007/s40496-024-00368-1.
7.
Donzelli, A., Neglected Effective Early Therapies against COVID-19: Focus on Functional Foods and Related Active Substances. A Review, MDPI AG, doi:10.20944/preprints202312.1178.v1.
8.
Chavda et al., Nasal sprays for treating COVID-19: a scientific note, Pharmacological Reports, doi:10.1007/s43440-023-00463-7.
9.
Ting et al., The In Vitro Virucidal Effects of Mouthwashes on SARS-CoV-2, International Journal of Translational Medicine, doi:10.3390/ijtm2030030.
10.
Lim et al., Repurposing povidone-iodine to reduce the risk of SARS-CoV-2 infection and transmission: a narrative review, Annals of Medicine, doi:10.1080/07853890.2022.2076902.
Gandhi et al., 13 Sep 2024, Canada, peer-reviewed, 3 authors, trial PROSPERO CRD42024500158.
Contact: leigh.sowerby@sjhc.london.on.ca.
Washing Illness Away: A Systematic Review of the Impact of Nasal Irrigation and Spray on COVID‐19
The Laryngoscope, doi:10.1002/lary.31761
Objective: Nasal irrigation is a common treatment for sinonasal disorders; however, it is unknown if it can reduce SARS-CoV-2 nasopharyngeal viral load (NVL). This systematic review investigated the efficacy of nasal irrigation with saline, povidone iodine (PVP-I), and intranasal corticosteroids (INCS) at reducing SARS-CoV-2 NVL and transmissibility.
References
Abdullah, Periasamy, Ismail, Nasal irrigation as treatment in Sinonasal symptoms relief: a review of its efficacy and clinical applications, Indian J Otolaryngol Head Neck Surg, doi:10.1007/s12070-017-1070-0
Achilles, Mösges, Nasal saline irrigations for the symptoms of acute and chronic Rhinosinusitis, Curr Allergy Asthma Rep, doi:10.1007/s11882-013-0339-y
Anderson, Sivalingam, Kang, Povidone-iodine demonstrates rapid in vitro Virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease, Infect Dis Ther, doi:10.1007/s40121-020-00316-3
Balmforth, Swales, Silpa, Evaluating the efficacy and safety of a novel prophylactic nasal spray in preventing SARS-CoV-2 infection: a multi-centre, double-blind, placebo-controlled, randomized trial, J Clin Virol, doi:10.1016/j.jcv.2022.105248
Bastier, Lechot, Bordenave, Durand, De Gabory, Nasal irrigation: from empiricism to evidence-based medicine. A review, Eur Ann Otorhinolaryngol Head Neck Dis, doi:10.1016/j.anorl.2015.08.001
Batioglu-Karaaltin, Yigit, Cakan, Effect of the povidone iodine, hypertonic alkaline solution and saline nasal lavage on nasopharyngeal viral load in COVID-19, Clin Otolaryngol, doi:10.1111/coa.14056
Baxter, Schwartz, Johnson, Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients, Ear Nose Throat J, doi:10.1177/01455613221123737
Cannon, Westover, Bleher, Sanchez-Gonzalez, Ferrer, In vitro analysis of the anti-viral potential of nasal spray constituents against SARS-CoV-2, bioRxiv, doi:10.1101/2020.12.02.408575
Cegolon, Mastrangelo, Emanuelli, Camerotto, Spinato et al., Early negativization of SARS-CoV-2 infection by nasal spray of seawater plus additives: the RENAISSANCE open-label controlled clinical trial, Pharmaceutics, doi:10.3390/pharmaceutics14112502
Cnockaert, Vecellio, Dubus, Jamar, Reychler, A large-volume low-pressure nasal irrigation delivers drug into the nasal cavity? An in vivo study, Drug Deliv Transl Res, doi:10.1007/s13346-023-01455-z
De Gabory, Kérimian, Baux, Boisson, Bordenave, Computational fluid dynamics simulation to compare large volume irrigation and continuous spraying during nasal irrigation, Int Forum Allergy Rhinol, doi:10.1002/alr.22458
Elsersy, Zahran, Elbakry, Combined nasal, oropharyngeal povidone iodine plus Glycyrrhizic acid sprays, accelerate clinical and laboratory recovery and reduces household transmission of SARS-CoV-2: a randomized placebo-controlled clinical trial, Front Med, doi:10.3389/fmed.2022.863917
Esther Cr, Kimura, Mikami, Pharmacokinetic-based failure of a detergent virucidal for severe acute respiratory syndromecoronavirus-2 (SARS-CoV-2) nasal infections: a preclinical study and randomized controlled trial, Int Forum Allergy Rhinol, doi:10.1002/alr.22975
Frank, Brown, Capriotti, Westover, Pelletier et al., In vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053
Go, Pandav, Sanchez-Gonzalez, Ferrer, Potential role of xylitol plus grapefruit seed extract nasal spray solution in COVID-19: case series, Cureus, doi:10.7759/cureus.11315
Guenezan, Garcia, Strasters, Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.5490
Gutiérrez-García, De, Cerda-Ángeles, Cabrera-Licona, Delgado-Enciso et al., Nasopharyngeal and oropharyngeal rinses with neutral electrolyzed water prevents COVID-19 in front-line health professionals: a randomized, open-label, controlled trial in a general hospital in Mexico City, Biomed Rep, doi:10.3892/br.2021.1494
Hastie, Amogan, Looney, Mehta, Association between SARS-CoV-2 viral load and patient symptoms and clinical outcomes using droplet digital PCR, Viruses, doi:10.3390/v15020446
Higgins, Altman, Gøtzsche, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ, doi:10.1136/bmj.d5928
Hou, Okuda, Edwards, SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell, doi:10.1016/j.cell.2020.05.042
Huijghebaert, Hoste, Vanham, Essentials in saline pharmacology for nasal or respiratory hygiene in times of COVID-19, Eur J Clin Pharmacol, doi:10.1007/s00228-021-03102-3
Jajou, Mutsaers-Van Oudheusden, Verweij, Rietveld, Murk, SARS-CoV-2 transmitters have more than three times higher viral loads than non-transmitterspractical use of viral load for disease control, J Clin Virol, doi:10.1016/j.jcv.2022.105131
Kamal Arefin, Banu, Uddin, Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License randomized clinical trial, Indian J Otolaryngol Head Neck Surg, doi:10.1007/s12070-022-03106-0
Kimura, Freeman, Wessinger, Interim analysis of an open-label randomized controlled trial evaluating nasal irrigations in nonhospitalized patients with coronavirus disease 2019, Int Forum Allergy Rhinol, doi:10.1002/alr.22703
Lei, Xu, Xiao, Wu, Shu, Household transmission of COVID-19-a systematic review and meta-analysis, J Infect, doi:10.1016/j.jinf.2020.08.033
Lipworth, Chan, Carr, Corticosteroid protection against COVID-19: begin with the nose, J Allergy Clin Immunol Pract, doi:10.1016/j.jaip.2021.08.025
Liu, Pan, Li, Tan, Yang, Efficacy of nasal irrigation with hypertonic saline on chronic rhinosinusitis: systematic review and meta-analysis, Braz J Otorhinolaryngol, doi:10.1016/j.bjorl.2020.03.008
Liu, Wang, Xie, Su, Wang, Effect of nasal irrigation in children with omicron variant of COVID-19 infection, Ear Nose Throat J, doi:10.1177/01455613231172337
Liu, Xie, Li, Su, Zhu, Effect of nasal irrigation in adults infected with omicron variant of COVID-19: a quasi-experimental study, Front Public Health, doi:10.3389/fpubh.2022.1046112
Marc, Kerioui, Blanquart, Quantifying the relationship between SARS-CoV-2 viral load and infectiousness, eLife, doi:10.7554/eLife.69302
Page, Mckenzie, Bossuyt, The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ, doi:10.1136/bmj.n71
Panta, Chatti, Andhavarapu, Do saline water gargling and nasal irrigation confer protection against COVID-19?, Explore, doi:10.1016/j.explore.2020.09.010
Pantazopoulos, Chalkias, Mavrovounis, Nasopharyngeal wash with normal saline decreases SARS-CoV-2 viral load: a randomized pilot controlled trial, Can Respir J, doi:10.1155/2022/8794127
Pantazopoulos, Chalkias, Miziou, A hypertonic seawater nasal irrigation solution containing algal and herbal natural ingredients reduces viral load and SARS-CoV-2 detection time in the nasal cavity, J Pers Med, doi:10.3390/jpm13071093
Parasher, COVID-19: current understanding of its pathophysiology, clinical presentation and treatment, Postgrad Med J, doi:10.1136/postgradmedj-2020-138577
Peters, Sajuthi, Deford, COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids, Am J Respir Crit Care Med, doi:10.1164/rccm.202003-0821OC
Pujadas, Chaudhry, Mcbride, SARS-CoV-2 viral load predicts COVID-19 mortality, Lancet Respir
Rabago, Zgierska, Saline nasal irrigation for upper respiratory conditions, Am Fam Physician
Ramalingam, Graham, Dove, Morrice, Sheikh, A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold, Sci Rep, doi:10.1038/s41598-018-37703-3
Rocha, Fagre, Latham, A novel glucocorticoid and androgen receptor modulator reduces viral entry and innate immune inflammatory responses in the Syrian hamster model of SARS-CoV-2 infection, Front Immunol, doi:10.3389/fimmu.2022.811430
Salli, Lehtinen, Tiihonen, Ouwehand, Xylitol's health benefits beyond dental health: a comprehensive review, Nutrients, doi:10.3390/nu11081813
Sharma, Singh, Singh, Takhelchangbam, Kumar et al., Effect of 0.5% povidone-iodine on the nasopharyngeal and oropharyngeal viral loads in patients with COVID-19: a double-blind placebo-controlled randomized clinical trial, J Family Med Prim Care, doi:10.4103/jfmpc.jfmpc_446_22
Sirijatuphat, Leelarasamee, Puangpet, Thitithanyanont, A pilot study of 0.4% povidone-iodine nasal spray to eradicate SARS-CoV-2 in the nasopharynx, Infect Drug Resist, doi:10.2147/IDR.S391630
Soler, De Mendoza, Cuello, Intranasal xylitol for the treatment of COVID-19 in the outpatient setting: a pilot study, Cureus, doi:10.7759/cureus.27182
Spinato, Fabbris, Costantini, The effect of isotonic saline nasal lavages in improving symptoms in SARS-CoV-2 infection: a case-control study, Front Neurol, doi:10.3389/fneur.2021.794471
Strauss, Jawhari, Attaway, Intranasal corticosteroids are associated with better outcomes in coronavirus disease 2019, J Allergy Clin Immunol Pract, doi:10.1016/j.jaip.2021.08.007
Sungnak, Huang, Bécavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med, doi:10.1038/s41591-020-0868-6
Triggle, Bansal, Ding, A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic, Front Immunol, doi:10.3389/fimmu.2021.631139
Varricchio, Brunese, Varricchio, Ciprandi, Viral shedding in symptomatic patients with mild COVID-19: an experience with nebulized nasal treatment, J Biol Regul Homeost Agents, doi:10.23812/21-137-L
Wang, Zhang, Zhang, Efficacy of nasal irrigation and oral rinse with sodium bicarbonate solution on virus clearance for COVID-19 patients, Front Public Health, doi:10.3389/fpubh.2023.1145669
Yilmaz, Yilmaz, Ozdemir, Effects of hypertonic alkaline nasal irrigation on COVID-19, Laryngoscope Investig Otolaryngol, doi:10.1002/lio2.686
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet, doi:10.1016/S0140-6736(21)01744-X
Zarabanda, Vukkadala, Phillips, The effect of povidone-iodine nasal spray on nasopharyngeal SARS-CoV-2 viral load: a randomized control trial, Laryngoscope, doi:10.1002/lary.29935
DOI record:
{
"DOI": "10.1002/lary.31761",
"ISSN": [
"0023-852X",
"1531-4995"
],
"URL": "http://dx.doi.org/10.1002/lary.31761",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>Nasal irrigation is a common treatment for sinonasal disorders; however, it is unknown if it can reduce SARS‐CoV‐2 nasopharyngeal viral load (NVL). This systematic review investigated the efficacy of nasal irrigation with saline, povidone iodine (PVP‐I), and intranasal corticosteroids (INCS) at reducing SARS‐CoV‐2 NVL and transmissibility.</jats:p></jats:sec><jats:sec><jats:title>Data Sources</jats:title><jats:p>Databases including Embase, MEDLINE, Web of Science, and ClinicalTrials.gov.</jats:p></jats:sec><jats:sec><jats:title>Review Methods</jats:title><jats:p>A systematic review was completed with pre‐defined search criteria using keywords related to nasal irrigation and COVID‐19 from 1946 through January 2024. This review followed PRISMA reporting guidelines and was registered on PROSPERO. Only in‐vivo studies testing nasal irrigation with either saline, PVP‐I, or INCS for reducing NVL were included.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Nine out of ten studies on saline‐based solutions reported positive effects in reducing NVL, with benefits noted in earlier time to negative nasopharyngeal PCR and a greater decline in NVL during early study time points, compared with controls. Isotonic and hypertonic saline mediums were found to be effective with three studies demonstrating enhanced efficacy with additives. Four out of seven studies on PVP‐I showed a positive effect on reducing NVL, but results were heterogenous. Four studies demonstrated reduction of transmission with saline or PVP‐I. No studies were found on INCS.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Saline nasal irrigation showed the best efficacy in reducing SARS‐CoV‐2 NVL. Additives to saline may have a clinical benefit, but further studies are needed to elucidate their isolated impacts on NVL. Data on PVP‐I is inconclusive and further studies are warranted to determine the ideal concentration for irrigation. <jats:italic>Laryngoscope</jats:italic>, 2024</jats:p></jats:sec>",
"alternative-id": [
"10.1002/lary.31761"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-06-11"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2024-08-28"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2024-09-13"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4159-8670",
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery, Schulich School of Medicine and Dentistry Western University London Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Gandhi",
"given": "Karan",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0009-0001-5580-6920",
"affiliation": [
{
"name": "Schulich School of Medicine and Dentistry Western University London Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Paczkowski",
"given": "Freeman",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5825-2759",
"affiliation": [
{
"name": "Department of Otolaryngology‐Head and Neck Surgery, Schulich School of Medicine and Dentistry Western University London Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Sowerby",
"given": "Leigh",
"sequence": "additional"
}
],
"container-title": "The Laryngoscope",
"container-title-short": "The Laryngoscope",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
9,
13
]
],
"date-time": "2024-09-13T13:43:45Z",
"timestamp": 1726235025000
},
"deposited": {
"date-parts": [
[
2024,
9,
13
]
],
"date-time": "2024-09-13T13:43:50Z",
"timestamp": 1726235030000
},
"indexed": {
"date-parts": [
[
2024,
9,
14
]
],
"date-time": "2024-09-14T00:27:46Z",
"timestamp": 1726273666736
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
9,
13
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
13
]
],
"date-time": "2024-09-13T00:00:00Z",
"timestamp": 1726185600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/lary.31761",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2024,
9,
13
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
13
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "e_1_2_11_2_1",
"unstructured": "Coronavirus (COVID‐19) | IPAC Canada. Accessed June 9 2024.https://ipac-canada.org/coronavirus-resources"
},
{
"DOI": "10.3389/fimmu.2021.631139",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1136/postgradmedj-2020-138577",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"DOI": "10.7554/eLife.69302",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_1"
},
{
"DOI": "10.1016/j.jcv.2022.105131",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.3390/v15020446",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.1016/j.anorl.2015.08.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.1007/s12070-017-1070-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1038/s41598-018-37703-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.1016/j.explore.2020.09.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_1"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"DOI": "10.3389/fimmu.2022.811430",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_1"
},
{
"DOI": "10.1001/jamaoto.2020.3053",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"DOI": "10.1007/s40121-020-00316-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.1136/bmj.n71",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1136/bmj.d5928",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.1155/2022/8794127",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.3389/fneur.2021.794471",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1177/01455613231172337",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"DOI": "10.3389/fpubh.2022.1046112",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_1"
},
{
"DOI": "10.3389/fpubh.2023.1145669",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1002/lio2.686",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"DOI": "10.3390/jpm13071093",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_1"
},
{
"DOI": "10.23812/21-137-L",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_27_1"
},
{
"DOI": "10.3390/pharmaceutics14112502",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_28_1"
},
{
"DOI": "10.1002/alr.22975",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_29_1"
},
{
"DOI": "10.4103/jfmpc.jfmpc_446_22",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_1"
},
{
"DOI": "10.1007/s12070-022-03106-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_31_1"
},
{
"DOI": "10.3389/fmed.2022.863917",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_32_1"
},
{
"DOI": "10.1111/coa.14056",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_33_1"
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_34_1"
},
{
"DOI": "10.2147/IDR.S391630",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_35_1"
},
{
"DOI": "10.1002/lary.29935",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"DOI": "10.3892/br.2021.1494",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_37_1"
},
{
"DOI": "10.1177/01455613221123737",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_38_1"
},
{
"DOI": "10.1016/j.jinf.2020.08.033",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_39_1"
},
{
"DOI": "10.1016/j.jcv.2022.105248",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_40_1"
},
{
"DOI": "10.1007/s00228-021-03102-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_41_1"
},
{
"article-title": "Saline nasal irrigation for upper respiratory conditions",
"author": "Rabago D",
"first-page": "1117",
"issue": "10",
"journal-title": "Am Fam Physician",
"key": "e_1_2_11_42_1",
"volume": "80",
"year": "2009"
},
{
"DOI": "10.1016/j.bjorl.2020.03.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_43_1"
},
{
"DOI": "10.1002/alr.22703",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_44_1"
},
{
"DOI": "10.1016/S2213-2600(20)30354-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_45_1"
},
{
"DOI": "10.1007/s11882-013-0339-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_46_1"
},
{
"DOI": "10.1007/s13346-023-01455-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_47_1"
},
{
"DOI": "10.1002/alr.22458",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_48_1"
},
{
"DOI": "10.3390/nu11081813",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_49_1"
},
{
"DOI": "10.1101/2020.12.02.408575",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_50_1"
},
{
"DOI": "10.7759/cureus.27182",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_51_1"
},
{
"DOI": "10.7759/cureus.11315",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_52_1"
},
{
"DOI": "10.1016/j.jaip.2021.08.025",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_53_1"
},
{
"DOI": "10.1164/rccm.202003-0821OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_54_1"
},
{
"DOI": "10.1016/j.jaip.2021.08.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_55_1"
}
],
"reference-count": 54,
"references-count": 54,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/lary.31761"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Washing Illness Away: A Systematic Review of the Impact of Nasal Irrigation and Spray on <scp>COVID</scp>‐19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
gandhi