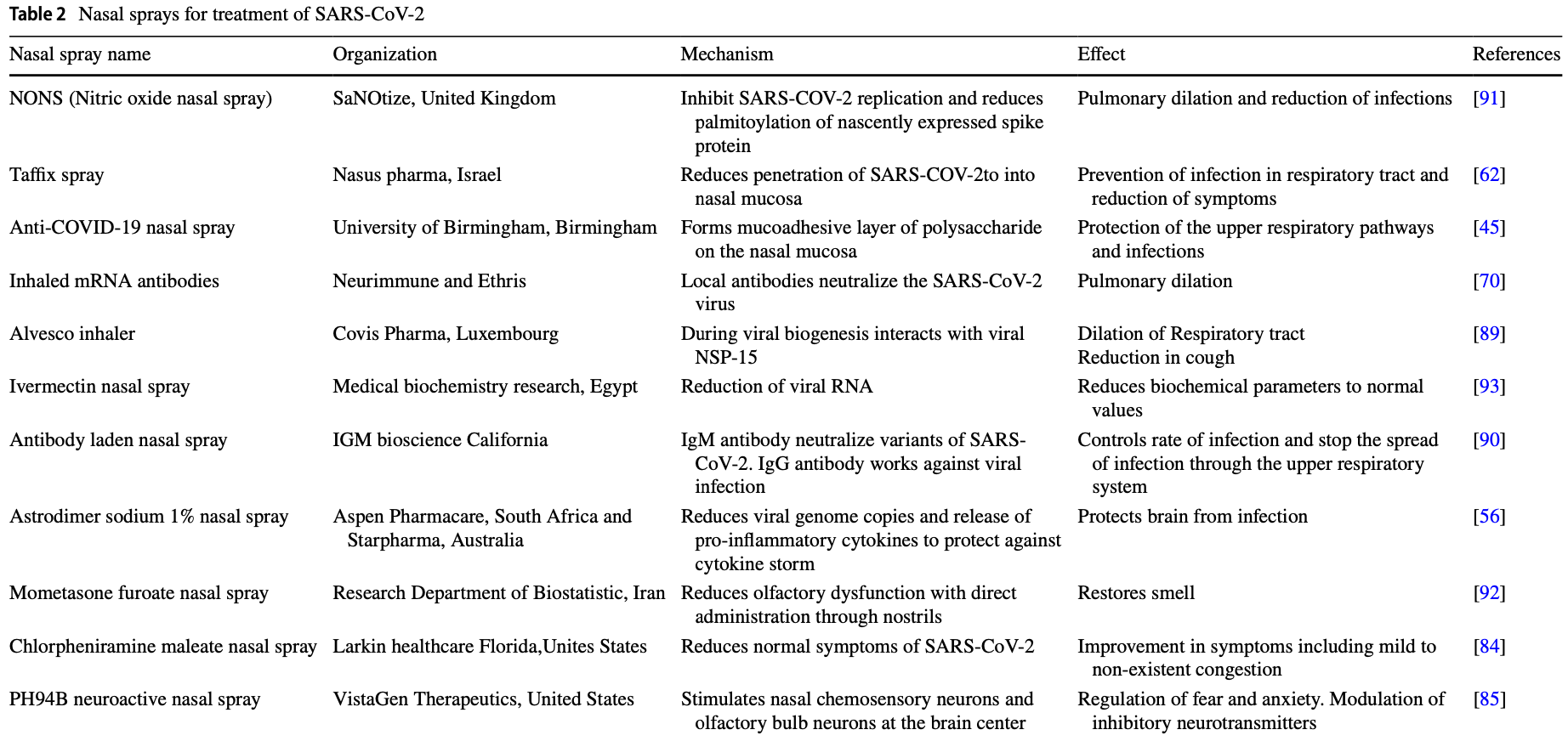
Nasal sprays for treating COVID-19: a scientific note
et al., Pharmacological Reports, doi:10.1007/s43440-023-00463-7, Feb 2023
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of nasal sprays for treatment of COVID-19. Authors note that the nasal epithelium is typically the primary site of SARS-CoV-2 infection, and there may be significant advantages for treatments via the nasal route.
Review covers povidone-iodine and iota-carrageenan.
1.
Angjelova et al., Effects of Antiseptic Formulations on Oral Microbiota and Related Systemic Diseases: A Scoping Review, Antibiotics, doi:10.3390/antibiotics14080815.
2.
Thangamani et al., Rinsing away the threat: Antiviral mouthwashes and their efficacy, World Academy of Sciences Journal, doi:10.3892/wasj.2025.366.
3.
Gunawan et al., The Role of Poly Vinyl Pyrrolidone Iodine (PVP-I) in Preventing Cross-Infection during Dental Procedures: A Systematic Review in the COVID-19 Context, Journal of International Society of Preventive and Community Dentistry, doi:10.4103/jispcd.jispcd_78_24.
4.
Okeke et al., Antiseptics: An expeditious third force in the prevention and management of coronavirus diseases, Current Research in Microbial Sciences, doi:10.1016/j.crmicr.2024.100293.
5.
Gandhi et al., Washing Illness Away: A Systematic Review of the Impact of Nasal Irrigation and Spray on COVID‐19, The Laryngoscope, doi:10.1002/lary.31761.
6.
Brito-Reia et al., Antiviral Mechanism and Clinical Benefits of Mouthwash Active Against SARS-CoV-2, Current Oral Health Reports, doi:10.1007/s40496-024-00368-1.
7.
Donzelli, A., Neglected Effective Early Therapies against COVID-19: Focus on Functional Foods and Related Active Substances. A Review, MDPI AG, doi:10.20944/preprints202312.1178.v1.
8.
Chavda et al., Nasal sprays for treating COVID-19: a scientific note, Pharmacological Reports, doi:10.1007/s43440-023-00463-7.
9.
Ting et al., The In Vitro Virucidal Effects of Mouthwashes on SARS-CoV-2, International Journal of Translational Medicine, doi:10.3390/ijtm2030030.
10.
Lim et al., Repurposing povidone-iodine to reduce the risk of SARS-CoV-2 infection and transmission: a narrative review, Annals of Medicine, doi:10.1080/07853890.2022.2076902.
Chavda et al., 27 Feb 2023, peer-reviewed, 5 authors.
Contact: vivek.chavda@lmcp.ac.in, vivek7chavda@gmail.com.
Nasal sprays for treating COVID-19: a scientific note
Pharmacological Reports, doi:10.1007/s43440-023-00463-7
Clinical management of COVID-19 has been a daunting task. Due to the lack of specific treatment, vaccines have been regarded as the first line of defence. Innate responses and cell-mediated systemic immunity, including serum antibodies, have been the primary focus of practically all studies of the immune response to COVID-19. However, owing to the difficulties encountered by the conventional route, alternative routes for prophylaxis and therapy became the need of the hour. The first site invaded by SARS-CoV-2 is the upper respiratory tract. Nasal vaccines are already in different stages of development. Apart from prophylactic purposes, mucosal immunity can be exploited for therapeutic purposes too. The nasal route for drug delivery offers many advantages over the conventional route. Besides offering a needle-free delivery, they can be selfadministered. They present less logistical burden as there is no need for refrigeration. The present article focuses on various aspects of nasal spray for eliminating COVID-19.
Keywords SARS-CoV-2 • Immunity • Nasal route • Nasal vaccine • Nasal spray • Clinical trials • Devices Abbreviations ACE2 Angiotensin converting enzyme 2 CD4 Clusters of differentiation 4 cGMP Guanosine mono phosphate COVID-19 Coronavirus disease-2019 HCoV Human coronavirus HPMC Hydroxypropyl methyl cellulose IgG Immunoglobulin G IL-2 Interleukin-2 MERS-CoV Middle East respiratory syndrome coronavirus mRNA Messenger ribonucleic acid NC Nasal cycle NONS Nitric oxide nasal spray RNA Ribonucleic acid SARS-CoV-2 Severe acute respiratory syndrome coronavirus disease 2 URTI Upper respiratory tract infection The Post-COVID Era -Advances and Challenges in Pharmacology * Vivek P. Chavda
Author contributions VPC (VPC) prepared the manuscript's backbone and wrote the original draft of the manuscript along with other coauthors. All authors have read and approved the final version of the manuscript.
Declarations
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
References
Alam, Ahmed, Ali, Sarker, Kabir et al., Challenges to COVID-19 vaccine supply chain: implications for sustainable development goals, Int J Prod Econ, doi:10.1016/j.ijpe.2021.108193
Aref, Bazeed, Hassan, Hassan, Hassan, Clinical, biochemical and molecular evaluations of ivermectin mucoadhesive nanosuspension nasal spray in reducing upper respiratory symptoms of mild COVID-19, IJN, doi:10.2147/IJN.S313093
Ayenigbara, Adegboro, Ayenigbara, Adeleke, Olofintuyi, The challenges to a successful COVID-19 vaccination programme in Africa, Germs
Bansal, Jonsson, Taylor, Figueroa, Dugour et al., Iota-carrageenan and xylitol inhibit SARS-CoV-2 in Vero cell culture, PLoS ONE, doi:10.1371/journal.pone.0259943
Bellussi, Cambi, Passali, Functional maturation of nasal mucosa: role of secretory immunoglobulin A (SIgA), doi:10.1186/2049-6958-8-46
Berkenfeld, Lamprecht, Mcconville, Devices for dry powder drug delivery to the lung, AAPS PharmSciTech, doi:10.1208/s12249-015-0317-x
Birkhoff, Leitz, Marx, Advantages of intranasal vaccination and considerations on device selection, Indian J Pharm Sci
Burton, Clarkson, Goulao, Glenny, Mcbain et al., Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them, Coch Database Syst Rev, doi:10.1002/14651858.CD013627.pub2
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Castellarnau, Heery, Seta, Luscombe, Kinghorn et al., Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial, Sci Rep, doi:10.1038/s41598-022-14601-3
Chakraverty, Swiss and German Team to Develop Inhaled mRNA Coronavirus Treatment
Chavda, Vora, Pandya, Patravale, Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management, Drug Discovery Today, doi:10.1016/j.drudis.2021.07.021
Ciotti, Ciccozzi, Terrinoni, Jiang, Wang et al., The COVID-19 pandemic, Crit Rev Clin Lab Sci, doi:10.1080/10408363.2020.1783198
Coonrod, The role of extracellular bactericidal factors in pulmonary host defense, Semin Respir Infect
Corr, Gahan, Hill, M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis, FEMS Immunol Med Microbiol
Czop, Mcgowan, Center, Opsonin-independent phagocytosis by human alveolar macrophages: augmentation by human plasma fibronectin, Am Rev Respir Dis
Dai, Song, Transforming COVID-19 vaccines into vaccination : challenges and opportunities for management scientists, Health Care Manag Sci, doi:10.1007/s10729-021-09563-3
De Apostólico, Lunardelli, Coirada, Boscardin, Rosa, Adjuvants: classification, modus operandi, and licensing, J Immunol Res, doi:10.1155/2016/1459394
Defrancesco, COVID-19 antibodies on trial, Nat Biotechnol
Dhama, Dhawan, Tiwari, Emran, Mitra et al., COVID-19 intranasal vaccines: current progress, advantages, prospects, and challenges, Hum Vaccin Immunother, doi:10.1080/21645515.2022.2045853
Djupesland, Skretting, Winderen, Holand, Bi-directional nasal delivery of aerosols can prevent lung deposition, J Aerosol Med, doi:10.1089/jam.2004.17.249
Dr, Buller, Neurimmune and Ethris Sign Collaboration Agreement to Rapidly Develop Inhaled mRNA-based Antibody Therapy for the Treatment of Covid-19
Durbin, Letter: acid secretion by gastric mucous membrane, Am J Physiol, doi:10.1152/ajplegacy.1975.229.6.1726
Ehrhart, Parker, Weidner, Dabney, Scott et al., Coronary vascular and myocardial responses to carotid body stimulation in the dog, Am J Physiol, doi:10.1152/ajplegacy.1975.229.3.754
Errecalde, Lifschitz, Vecchioli, Ceballos, Errecalde et al., Safety and pharmacokinetic assessments of a novel ivermectin nasal spray formulation in a pig model, J Pharm Sci, doi:10.1016/j.xphs.2021.01.017
Fehr, Perlman, Maier, Bickerton, Britton, An overview of their replication and pathogenesis; genomic organization, Methods Mol Biol
Fong, Dey, Chaki, An Introduction to COVID-19, Artif Intell Cor Outbreak, doi:10.1007/978-981-15-5936-5_1
Forbes, Bommer, Goole, Hellfritzsch, Kruijf et al., A consensus research agenda for optimising nasal drug delivery, Expert Opin Drug Deliv, doi:10.1080/17425247.2020.1714589
Frank, Capriotti, Brown, Tessema, Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era, Ear Nose Throat J, doi:10.1177/0145561320932318
Fujimura, Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans, Virchows Arch
Glenn, Goldman, Task delegation to physician extenders-some comparisons, Am J Public Health, doi:10.2105/ajph.66.1.64
Hanif, Jawad, Eccles, The nasal cycle in health and disease, Clin Otolaryngol Allied Sci
Harris, Review: clinical opportunities provided by the nasal administration of peptides, J Drug Target, doi:10.3109/10611869308996066
Hassan, Kafai, Dmitriev, Fox, Smith et al., A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2, Cell
Hasöksüz, Kilic, Saraç, Coronaviruses and sars-cov-2, Turkish Journal of Medical Sciences
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Hosseinpoor, Kabiri, Haghi, Soltani, Rezaei et al., Intranasal corticosteroid treatment on recovery of long-term olfactory dysfunction due to COVID-19, Laryngoscope, doi:10.1002/lary.30353
Hou, Okuda, Edwards, Martinez, Asakura et al., SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell
Huart, Philpott, Konstantinidis, Altundag, Whitcroft et al., Comparison of COVID-19 and common cold chemosensory dysfunction, Rhin, doi:10.4193/Rhin20.251
Illum, Nasal drug delivery-possibilities, problems and solutions, J Control Release, doi:10.1016/S0168-3659(02)00363-2
Illum, Nasal drug delivery: recent developments and future prospects, J Control Release, doi:10.1016/j.jconrel.2012.01.024
Iwabuchi, Kurakami, Takahashi, Kato, Morishima, Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases, J Infect Chemother, doi:10.1016/j.jiac.2020.04.007
Iwasaki, Exploiting mucosal immunity for antiviral vaccines, Annu Rev Immunol
Jansen, Russell, Chernick, Respiratory effects of H+ and dinitrophenol injections into the brain stem subarachnoid space of fetal lambs, Can J Physiol Pharmacol, doi:10.1139/y75-101
Jonsson, Musher, Goree, Clinton, Human alveolar lining material and antibacterial defenses, Am Rev Respir Dis
Kanowitz, Batra, Citardi, Topical budesonide via mucosal atomization device in refractory postoperative chronic rhinosinusitis, Otolaryngol Head Neck Surg, doi:10.1016/j.otohns.2008.03.009
Kashkooli, Rozema, Espejo-Ramirez, Lasko, Fagotto, Ectoderm to mesoderm transition by down-regulation of actomyosin contractility, PLoS Biol, doi:10.1371/journal.pbio.3001060
Kashte, Gulbake, El-Amin Iii, Gupta, COVID-19 vaccines: rapid development, implications, challenges and future prospects, Hum Cell, doi:10.1007/s13577-021-00512-4
Kasiri, Rouhani, Salehifar, Ghazaeian, Fallah, Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: a randomized, double blind clinical trial, Int Immunopharmacol, doi:10.1016/j.intimp.2021.107871
Keller, Merkel, Popp, Intranasal drug delivery: opportunities and toxicologic challenges during drug development, Drug Deliv and Transl Res, doi:10.1007/s13346-020-00891-5
Kilian, Reinholdt, Mortensen, Sørensen, Perturbation of mucosal immune defence mechanisms by bacterial IgA proteases, Bull Eur Physiopathol Respir
King, Silva-Sanchez, Peel, Botta, Dickson et al., Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects mice from lethal challenge, Vaccines, doi:10.3390/vaccines9080881
Kiyono, Fukuyama, NALT-versus Peyer's-patch-mediated mucosal immunity, Nat Rev Immunol
Kolhe, Shah, Rathore, Sterile product development: formulation, process, quality and regulatory considerations
Ku, Xie, Davidson, Ye, Su et al., Molecular determinants and mechanism for antibody cocktail preventing SARS-CoV-2 escape, Nat Commun, doi:10.1038/s41467-020-20789-7
Ku, Xie, Hinton, Liu, Ye et al., Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants, Nature
Kundoor, Dalby, Effect of formulation-and administrationrelated variables on deposition pattern of nasal spray pumps evaluated using a nasal cast, Pharm Res, doi:10.1007/s11095-011-0417-6
Kurono, Fujiyoshi, Mogi, Secretory IgA and bacterial adherence to nasal mucosal cells, Ann Otol Rhinol Laryngol, doi:10.1177/000348948909800407
Lan, Ge, Yu, Shan, Zhou et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature
Lehrer, Rheinstein, Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2, Vivo, doi:10.21873/invivo.12134
Li, Wu, Nie, Zhang, Hao et al., The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity, Cell
Liebowitz, Nicolini, Rosenthal, Hanover, Monti, Effect of an acute intranasal aerosol dose of PH94B on social and performance anxiety in women with social anxiety disorder, AJP, doi:10.1176/appi.ajp.2014.12101342
Lin, Yue, Yang, Yang, Pan et al., Nasal spray of neutralizing monoclonal antibody 35B5 confers potential prophylaxis against severe acute respiratory syndrome coronavirus 2 variants of concern: a small-scale clinical trial, Clin Infect Dis, doi:10.1093/cid/ciac448
Lo, Cruz, Efficacy of Carragelose® nasal spray impregnated versus mupirocin ointment impregnated nasal packs on mucosal healing after endoscopic sinus surgery: a double-blind, non-randomized, right-left side comparison. Philipp J Otolaryngol, Head Neck Surg
Lobaina, Nasal route for vaccine and drug delivery: features and current opportunities, Int J Pharmaceut, doi:10.1016/j.ijpharm.2019.118813
Lu, Zhao, Li, Niu, Yang et al., Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, The Lancet
Marino, Aptar Pharma's Nasal Unidose Device Approved by US FDA
Matsuyama, Kawase, Nao, Shirato, Ujike et al., The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells, J Virol, doi:10.1128/JVI.01648-20
Meister, Todt, Brüggemann, Steinmann, Banava et al., Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2, J Hosp Infect, doi:10.1016/j.jhin.2021.10.019
Mekler, On the problem of oncogene of tumour viruses, Acta Virol
Michael, Liebowitz, Salman, Nicolini, Effect of an Acute Intranasal Aerosol Dose of PH94B on Social and Performance Anxiety in Women With Social Anxiety Disorder, Am J Psychiatry
Mitchell, Berlinski, Canisius, Cipolla, Dolovich et al., Urgent appeal from international society for aerosols in medicine (ISAM) during COVID-19: clinical decision makers and governmental agencies should consider the inhaled route of administration: a statement from the ISAM regulatory and standardization issues networking group, J Aerosol Med Pulm Drug Deliv, doi:10.1089/jamp.2020.1622
Moakes, Davies, Stamataki, Grover, Formulation of a composite nasal spray enabling enhanced surface coverage and prophylaxis of SARS-COV-2, Adv Mater, doi:10.1002/adma.202008304
Momper, Green, Park, Burckart, Snyder, Ethical considerations for pediatric placebo-controlled trials: FDA outcomes and perspectives, Ther Innov Regul Sci, doi:10.1007/s43441-020-00214-3
Moreira, Matos, Paula, Santana, Mata et al., Corrigendum: nasal administration of anti-CD3 monoclonal antibody (foralumab) reduces lung inflammation and blood inflammatory biomarkers in mild to moderate COVID-19 patients: a pilot study, Front Immunol, doi:10.3389/fimmu.2021.815812
Mygind, Dahl, Anatomy, physiology and function of the nasal cavities in health and disease, Adv Drug Deliv Rev
Navarro, Tendal, Tingay, Vasilunas, Anderson et al., Clinical care of children and adolescents with COVID-19: recommendations from the national COVID-19 clinical evidence taskforce, Med J Aust, doi:10.5694/mja2.51305
Papadopoulos, Guibas, Rhinitis subtypes, endotypes, and definitions, Immunol Aller Clinics
Paull, Luscombe, Castellarnau, Heery, Bobardt et al., Protective effects of astodrimer sodium 1% nasal spray formulation against SARS-CoV-2 nasal challenge in K18-hACE2 mice, Viruses, doi:10.3390/v13081656
Peiris, Coronaviruses, Medical Microbiology, doi:10.1016/B978-0-7020-4089-4.00072-X
Pendolino, Lund, Nardello, Ottaviano, The nasal cycle: a comprehensive review, Rhinol Onl
Pereira, Critchley, The COVID 19 novel coronavirus pandemic 2020: seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times?, J Appl Phycol, doi:10.1007/s10811-020-02143-y
Pharma, A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Efficacy of Ciclesonide Metered-Dose Inhaler in Non-hospitalized Patients 12 Years of Age and Older With Symptomatic COVID-19 Infection
Pharma, Covis Pharma, Initiates Phase 3 Clinical Trial of Alvesco (Ciclesonide) Inhaler for the Treatment of COVID-19
Pilicheva, Boyuklieva, Can the nasal cavity help tackle COVID-19?, Pharmaceutics
Prieschl-Grassauer, Nasal spray works against COVID-19 variants
Pujadas, Chaudhry, Mcbride, Richter, Zhao et al., SARS-CoV-2 viral load predicts COVID-19 mortality, Lancet Respir Med
Regev-Shoshani, Vimalanathan, Mcmullin, Road, Gay et al., Gaseous nitric oxide reduces influenza infectivity in vitro, Nitric Oxide, doi:10.1016/j.niox.2013.03.007
Reynolds, Kazmierowski, Newball, Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages, J Clin Investig
Ricciardolo, Bertolini, Carriero, Högman, Nitric oxide's physiologic effects and potential as a therapeutic agent against COVID-19, J Breath Res, doi:10.1088/1752-7163/abc302
Shmuel, Dalia, Tair, Low pH Hypromellose (Taffix) nasal powder spray could reduce SARS-CoV-2 infection rate post mass-gathering event at a highly endemic community: an observational prospective open label user survey, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2021.1908127
Siddiqui, Khan, Proposed intranasal route for drug administration in the management of central nervous system manifestations of COVID-19, ACS Chem Neurosci
Silen, Machen, Forte, Acid-base balance in amphibian gastric mucosa, Am J Physiol, doi:10.1152/ajplegacy.1975.229.3.721
Smith, Perelman, Hinchcliffe, Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines, Hum Vaccin Immunother, doi:10.4161/hv.27449
Suman, Nasal drug delivery, Expert Opin Biol Ther
Sun, Mccroskery, Liu, Leist, Liu et al., A newcastle disease virus (NDV) expressing a membraneanchored spike as a cost-effective inactivated SARS-CoV-2 vaccine, Vaccines, doi:10.3390/vaccines8040771
Tagoe, Sheikh, Morton, Nonvignon, Sarker et al., barriers, and potential solutions, doi:10.3389/fpubh.2021.709127
Therapeutics, PH94B in the treatment of adjustment disorder with anxiety
Torres, Go, Chohan, Gc, Sanchez-Gonzalez et al., Chlorpheniramine maleate nasal spray in COVID-19 patients: Case Series, Review, doi:10.21203/rs.3.rs-138252/v1
Touitou, Natsheh, Duchi, Buspirone nanovesicular nasal system for non-hormonal hot flushes treatment, Pharmaceutics, doi:10.3390/pharmaceutics10030082
University, A Phase I Double-blind, Randomized, Placebo-controlled Study to Evaluate Safety of Hypromellosebased Nasal Spray
Vaira, Hopkins, Sandison, Manca, Machouchas et al., Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia, J Laryngol Otol, doi:10.1017/S0022215120002455
Walker, Darowski, Morris, Wraith, Anaesthesia and mucopolysaccharidoses: a review of airway problems in children, Anaesthesia, doi:10.1111/j.1365-2044.1994.tb04360.x
Wang, Lorenzi, Muecksch, Finkin, Viant et al., Enhanced SARS-CoV-2 neutralization by dimeric IgA, Sci Translat Med
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Westover, Ferrer, Vazquez, Bethencourt-Mirabal, Go, Vitro Virucidal Effect of Intranasally Delivered Chlorpheniramine Maleate Compound Against Severe Acute Respiratory Syndrome Coronavirus 2, Cureus, doi:10.7759/cureus.10501
Winchester, John, Jabbar, John, Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection, J Infect, doi:10.1016/j.jinf.2021.05.009
Winkler, Bailey, Kafai, Nair, Mccune et al., SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function, Nat Immunol, doi:10.1038/s41590-020-0778-2
Wolfe, Braude, Intranasal medication delivery for children: a brief review and update, Pediatrics, doi:10.1542/peds.2010-0616
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science
Xi, Lei, Zouzas, April, Nasally inhaled therapeutics and vaccination for COVID-19: developments and challenges, MedComm, doi:10.1002/mco2.101
Xiang, Fu, Ye, Zheng, Zhu et al., Effect of Lactobacillus gasseri PA3 on gut microbiota in an in vitro colonic simulation, Food Sci Nutr, doi:10.1002/fsn3.1236
Xu, Xia, Pu, Wang, Li et al., The antihistamine drugs carbinoxamine maleate and chlorpheniramine maleate exhibit potent antiviral activity against a broad spectrum of influenza viruses, Front Microbiol, doi:10.3389/fmicb.2018.02643
Yigit, Ozkaya-Parlakay, Senel, Evaluation of COVID-19 vaccine refusal in parents, Pediatr Infect Dis J, doi:10.1097/INF.0000000000003042
Yonker, Shen, Kinane, Lessons unfolding from pediatric cases of COVID-19 disease caused by SARS-CoV-2 infection, Pediatr Pulmonol, doi:10.1002/ppul.24748
Yoshimura, Suzuki, Calcium-stimulated adenosine triphosphatase in the microsomal fraction of tooth germ from porcine fetus, Biochim Biophys Acta, doi:10.1016/0005-2744(75)90218-1
Zhang, Yang, Xiang, Cui, Liu et al., Intranasal administration of SARS-CoV-2 neutralizing human antibody prevents infection in mice, Bioengineering, doi:10.1101/2020.12.08.416677
Zuercher, Coffin, Thurnheer, Fundova, Cebra, Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses, J Immunol
Åkerström, Gunalan, Keng, Tan, Mirazimi, Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected, Virology, doi:10.1016/j.virol.2009.09.007
DOI record:
{
"DOI": "10.1007/s43440-023-00463-7",
"ISSN": [
"1734-1140",
"2299-5684"
],
"URL": "http://dx.doi.org/10.1007/s43440-023-00463-7",
"alternative-id": [
"463"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "30 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "6 February 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "7 February 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "27 February 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7701-8597",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chavda",
"given": "Vivek P.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Baviskar",
"given": "Kajal P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaghela",
"given": "Dixa A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raut",
"given": "Shilpa S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bedse",
"given": "Anjali P.",
"sequence": "additional"
}
],
"container-title": "Pharmacological Reports",
"container-title-short": "Pharmacol. Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
2,
27
]
],
"date-time": "2023-02-27T12:03:12Z",
"timestamp": 1677499392000
},
"deposited": {
"date-parts": [
[
2023,
2,
27
]
],
"date-time": "2023-02-27T13:05:46Z",
"timestamp": 1677503146000
},
"indexed": {
"date-parts": [
[
2023,
2,
28
]
],
"date-time": "2023-02-28T05:30:07Z",
"timestamp": 1677562207700
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
2,
27
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
27
]
],
"date-time": "2023-02-27T00:00:00Z",
"timestamp": 1677456000000
}
},
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
27
]
],
"date-time": "2023-02-27T00:00:00Z",
"timestamp": 1677456000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s43440-023-00463-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s43440-023-00463-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s43440-023-00463-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2023,
2,
27
]
]
},
"published-online": {
"date-parts": [
[
2023,
2,
27
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1080/10408363.2020.1783198",
"author": "M Ciotti",
"doi-asserted-by": "publisher",
"first-page": "365",
"journal-title": "Crit Rev Clin Lab Sci",
"key": "463_CR1",
"unstructured": "Ciotti M, Ciccozzi M, Terrinoni A, Jiang W-C, Wang C-B, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57:365–88. https://doi.org/10.1080/10408363.2020.1783198.",
"volume": "57",
"year": "2020"
},
{
"DOI": "10.3906/sag-2004-127",
"author": "M Hasöksüz",
"doi-asserted-by": "publisher",
"first-page": "549",
"journal-title": "Turkish Journal of Medical Sciences",
"key": "463_CR2",
"unstructured": "Hasöksüz M, Kilic S, Saraç F. Coronaviruses and sars-cov-2. Turkish Journal of Medical Sciences. 2020;50:549–56.",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.1016/B978-0-7020-4089-4.00072-X",
"doi-asserted-by": "publisher",
"key": "463_CR3",
"unstructured": "Peiris JSM. Coronaviruses. Medical Microbiology 2012:587–93. https://doi.org/10.1016/B978-0-7020-4089-4.00072-X."
},
{
"DOI": "10.1007/978-1-4939-2438-7_1",
"author": "AR Fehr",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Methods Mol Biol",
"key": "463_CR4",
"unstructured": "Fehr AR, Perlman S, Maier HJ, Bickerton E, Britton P. An overview of their replication and pathogenesis; genomic organization. Methods Mol Biol. 2015;1282:1–23.",
"volume": "1282",
"year": "2015"
},
{
"DOI": "10.1016/j.cell.2020.07.012",
"author": "Q Li",
"doi-asserted-by": "publisher",
"first-page": "1284",
"journal-title": "Cell",
"key": "463_CR5",
"unstructured": "Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020;182:1284–94.",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1007/978-981-15-5936-5_1",
"author": "SJ Fong",
"doi-asserted-by": "publisher",
"journal-title": "Artif Intell Cor Outbreak",
"key": "463_CR6",
"unstructured": "Fong SJ, Dey N, Chaki J. An Introduction to COVID-19. Artif Intell Cor Outbreak. 2020. https://doi.org/10.1007/978-981-15-5936-5_1.",
"year": "2020"
},
{
"DOI": "10.1007/s13577-021-00512-4",
"author": "S Kashte",
"doi-asserted-by": "publisher",
"first-page": "711",
"journal-title": "Hum Cell",
"key": "463_CR7",
"unstructured": "Kashte S, Gulbake A, El-Amin Iii SF, Gupta A. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum Cell. 2021;34:711–33. https://doi.org/10.1007/s13577-021-00512-4.",
"volume": "34",
"year": "2021"
},
{
"DOI": "10.1007/s10729-021-09563-3",
"author": "T Dai",
"doi-asserted-by": "publisher",
"first-page": "455",
"journal-title": "Health Care Manag Sci",
"key": "463_CR8",
"unstructured": "Dai T, Song J-S. Transforming COVID-19 vaccines into vaccination : challenges and opportunities for management scientists. Health Care Manag Sci. 2021;24:455–9. https://doi.org/10.1007/s10729-021-09563-3.",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.18683/germs.2021.1280",
"doi-asserted-by": "crossref",
"key": "463_CR9",
"unstructured": "Ayenigbara IO, Adegboro JS, Ayenigbara GO, Adeleke OR, Olofintuyi OO. The challenges to a successful COVID-19 vaccination programme in Africa. Germs 2021:427–40."
},
{
"DOI": "10.1097/INF.0000000000003042",
"author": "M Yigit",
"doi-asserted-by": "publisher",
"first-page": "e134",
"journal-title": "Pediatr Infect Dis J",
"key": "463_CR10",
"unstructured": "Yigit M, Ozkaya-Parlakay A, Senel E. Evaluation of COVID-19 vaccine refusal in parents. Pediatr Infect Dis J. 2021;40:e134–6. https://doi.org/10.1097/INF.0000000000003042.",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.3389/fpubh.2021.709127",
"author": "ET Tagoe",
"doi-asserted-by": "publisher",
"journal-title": "Front Public Health",
"key": "463_CR11",
"unstructured": "Tagoe ET, Sheikh N, Morton A, Nonvignon J, Sarker AR, Williams L, et al. COVID-19 vaccination in lower-middle income countries: national stakeholder views on challenges, barriers, and potential solutions. Front Public Health. 2021. https://doi.org/10.3389/fpubh.2021.709127.",
"year": "2021"
},
{
"DOI": "10.1016/j.ijpe.2021.108193",
"author": "ST Alam",
"doi-asserted-by": "publisher",
"journal-title": "Int J Prod Econ",
"key": "463_CR12",
"unstructured": "Alam ST, Ahmed S, Ali SM, Sarker S, Kabir G, Ul-Islam A. Challenges to COVID-19 vaccine supply chain: implications for sustainable development goals. Int J Prod Econ. 2021. https://doi.org/10.1016/j.ijpe.2021.108193.",
"year": "2021"
},
{
"DOI": "10.1016/j.jhin.2021.10.019",
"author": "TL Meister",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "J Hosp Infect",
"key": "463_CR13",
"unstructured": "Meister TL, Todt D, Brüggemann Y, Steinmann J, Banava S, Brill FHH, et al. Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2. J Hosp Infect. 2022;120:9–13. https://doi.org/10.1016/j.jhin.2021.10.019.",
"volume": "120",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03673-2",
"author": "Z Ku",
"doi-asserted-by": "publisher",
"first-page": "718",
"journal-title": "Nature",
"key": "463_CR14",
"unstructured": "Ku Z, Xie X, Hinton PR, Liu X, Ye X, Muruato AE, et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature. 2021;595:718–23.",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30354-4",
"author": "E Pujadas",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "463_CR15",
"unstructured": "Pujadas E, Chaudhry F, McBride R, Richter F, Zhao S, Wajnberg A, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8: e70.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/pharmaceutics13101612",
"author": "B Pilicheva",
"doi-asserted-by": "publisher",
"first-page": "1612",
"journal-title": "Pharmaceutics",
"key": "463_CR16",
"unstructured": "Pilicheva B, Boyuklieva R. Can the nasal cavity help tackle COVID-19? Pharmaceutics. 2021;13:1612.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.4049/jimmunol.168.4.1796",
"author": "AW Zuercher",
"doi-asserted-by": "publisher",
"first-page": "1796",
"journal-title": "J Immunol",
"key": "463_CR17",
"unstructured": "Zuercher AW, Coffin SE, Thurnheer MC, Fundova P, Cebra JJ. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J Immunol. 2002;168:1796–803.",
"volume": "168",
"year": "2002"
},
{
"DOI": "10.1038/nri1439",
"author": "H Kiyono",
"doi-asserted-by": "publisher",
"first-page": "699",
"journal-title": "Nat Rev Immunol",
"key": "463_CR18",
"unstructured": "Kiyono H, Fukuyama S. NALT-versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710.",
"volume": "4",
"year": "2004"
},
{
"DOI": "10.1111/j.1574-695X.2007.00359.x",
"author": "SC Corr",
"doi-asserted-by": "publisher",
"first-page": "2",
"journal-title": "FEMS Immunol Med Microbiol",
"key": "463_CR19",
"unstructured": "Corr SC, Gahan CCGM, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008;52:2–12.",
"volume": "52",
"year": "2008"
},
{
"DOI": "10.1007/s004289900177",
"author": "Y Fujimura",
"doi-asserted-by": "publisher",
"first-page": "560",
"journal-title": "Virchows Arch",
"key": "463_CR20",
"unstructured": "Fujimura Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. 2000;436:560–6.",
"volume": "436",
"year": "2000"
},
{
"DOI": "10.1517/14712598.3.3.519",
"author": "JD Suman",
"doi-asserted-by": "publisher",
"first-page": "519",
"journal-title": "Expert Opin Biol Ther",
"key": "463_CR21",
"unstructured": "Suman JD. Nasal drug delivery. Expert Opin Biol Ther. 2003;3:519–23.",
"volume": "3",
"year": "2003"
},
{
"DOI": "10.1146/annurev-immunol-032414-112315",
"author": "A Iwasaki",
"doi-asserted-by": "publisher",
"first-page": "575",
"journal-title": "Annu Rev Immunol",
"key": "463_CR22",
"unstructured": "Iwasaki A. Exploiting mucosal immunity for antiviral vaccines. Annu Rev Immunol. 2016;34:575–608.",
"volume": "34",
"year": "2016"
},
{
"DOI": "10.1038/s41587-020-0732-8",
"author": "L DeFrancesco",
"doi-asserted-by": "publisher",
"first-page": "1242",
"journal-title": "Nat Biotechnol",
"key": "463_CR23",
"unstructured": "DeFrancesco L. COVID-19 antibodies on trial. Nat Biotechnol. 2020;38:1242–52.",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "463_CR24",
"unstructured": "Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–51.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"author": "YJ Hou",
"doi-asserted-by": "publisher",
"first-page": "429",
"journal-title": "Cell",
"key": "463_CR25",
"unstructured": "Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH III, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–46.",
"volume": "182",
"year": "2020"
},
{
"author": "M Birkhoff",
"first-page": "729",
"journal-title": "Indian J Pharm Sci",
"key": "463_CR26",
"unstructured": "Birkhoff M, Leitz M, Marx D. Advantages of intranasal vaccination and considerations on device selection. Indian J Pharm Sci. 2009;71:729.",
"volume": "71",
"year": "2009"
},
{
"DOI": "10.1021/acschemneuro.0c00288",
"author": "R Siddiqui",
"doi-asserted-by": "publisher",
"first-page": "1523",
"journal-title": "ACS Chem Neurosci",
"key": "463_CR27",
"unstructured": "Siddiqui R, Khan NA. Proposed intranasal route for drug administration in the management of central nervous system manifestations of COVID-19. ACS Chem Neurosci. 2020;11:1523–4.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1186/2049-6958-8-46",
"author": "L Bellussi",
"doi-asserted-by": "publisher",
"first-page": "46",
"journal-title": "Multidiscip Respir Med",
"key": "463_CR28",
"unstructured": "Bellussi L, Cambi J, Passali D. Functional maturation of nasal mucosa: role of secretory immunoglobulin A (SIgA). Multidiscip Respir Med. 2013;8:46. https://doi.org/10.1186/2049-6958-8-46.",
"volume": "8",
"year": "2013"
},
{
"author": "M Kilian",
"first-page": "99",
"journal-title": "Bull Eur Physiopathol Respir",
"key": "463_CR29",
"unstructured": "Kilian M, Reinholdt J, Mortensen SB, Sørensen CH. Perturbation of mucosal immune defence mechanisms by bacterial IgA proteases. Bull Eur Physiopathol Respir. 1983;19:99–104.",
"volume": "19",
"year": "1983"
},
{
"DOI": "10.1177/000348948909800407",
"author": "Y Kurono",
"doi-asserted-by": "publisher",
"first-page": "273",
"journal-title": "Ann Otol Rhinol Laryngol",
"key": "463_CR30",
"unstructured": "Kurono Y, Fujiyoshi T, Mogi G. Secretory IgA and bacterial adherence to nasal mucosal cells. Ann Otol Rhinol Laryngol. 1989;98:273–7. https://doi.org/10.1177/000348948909800407.",
"volume": "98",
"year": "1989"
},
{
"DOI": "10.1126/scitranslmed.abf1555",
"author": "Z Wang",
"doi-asserted-by": "publisher",
"first-page": "1555",
"journal-title": "Sci Translat Med",
"key": "463_CR31",
"unstructured": "Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Viant C, Gaebler C, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Translat Med. 2021;13:1555.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell",
"key": "463_CR32",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"author": "R Lu",
"doi-asserted-by": "publisher",
"first-page": "565",
"journal-title": "The Lancet",
"key": "463_CR33",
"unstructured": "Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395:565–74.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1126/science.abb2507",
"author": "D Wrapp",
"doi-asserted-by": "publisher",
"first-page": "1260",
"journal-title": "Science",
"key": "463_CR34",
"unstructured": "Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3.",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2180-5",
"author": "J Lan",
"doi-asserted-by": "publisher",
"first-page": "215",
"journal-title": "Nature",
"key": "463_CR35",
"unstructured": "Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20.",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.08.026",
"author": "AO Hassan",
"doi-asserted-by": "publisher",
"first-page": "169",
"journal-title": "Cell",
"key": "463_CR36",
"unstructured": "Hassan AO, Kafai NM, Dmitriev IP, Fox JM, Smith BK, Harvey IB, et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169–84.",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1164/arrd.1982.125.5.607",
"author": "JK Czop",
"doi-asserted-by": "publisher",
"first-page": "607",
"journal-title": "Am Rev Respir Dis",
"key": "463_CR37",
"unstructured": "Czop JK, McGowan SE, Center DM. Opsonin-independent phagocytosis by human alveolar macrophages: augmentation by human plasma fibronectin. Am Rev Respir Dis. 1982;125:607–9.",
"volume": "125",
"year": "1982"
},
{
"DOI": "10.1164/arrd.1986.133.1.136",
"author": "S Jonsson",
"doi-asserted-by": "publisher",
"first-page": "136",
"journal-title": "Am Rev Respir Dis",
"key": "463_CR38",
"unstructured": "Jonsson S, Musher DM, Goree A, Clinton LE. Human alveolar lining material and antibacterial defenses. Am Rev Respir Dis. 1986;133:136–40.",
"volume": "133",
"year": "1986"
},
{
"author": "JD Coonrod",
"first-page": "118",
"journal-title": "Semin Respir Infect",
"key": "463_CR39",
"unstructured": "Coonrod JD. The role of extracellular bactericidal factors in pulmonary host defense. Semin Respir Infect. 1986;1:118–29.",
"volume": "1",
"year": "1986"
},
{
"DOI": "10.1172/JCI108102",
"author": "HY Reynolds",
"doi-asserted-by": "publisher",
"first-page": "376",
"journal-title": "J Clin Investig",
"key": "463_CR40",
"unstructured": "Reynolds HY, Kazmierowski JA, Newball HH. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Investig. 1975;56:376–85.",
"volume": "56",
"year": "1975"
},
{
"DOI": "10.1046/j.1365-2273.2000.00432.x",
"author": "J Hanif",
"doi-asserted-by": "publisher",
"first-page": "461",
"journal-title": "Clin Otolaryngol Allied Sci",
"key": "463_CR41",
"unstructured": "Hanif J, Jawad SSM, Eccles R. The nasal cycle in health and disease. Clin Otolaryngol Allied Sci. 2000;25:461–7.",
"volume": "25",
"year": "2000"
},
{
"DOI": "10.1016/S0169-409X(97)00058-6",
"author": "N Mygind",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Adv Drug Deliv Rev",
"key": "463_CR42",
"unstructured": "Mygind N, Dahl R. Anatomy, physiology and function of the nasal cavities in health and disease. Adv Drug Deliv Rev. 1998;29:3–12.",
"volume": "29",
"year": "1998"
},
{
"DOI": "10.4193/RHINOL/18.021",
"author": "AL Pendolino",
"doi-asserted-by": "publisher",
"first-page": "67",
"journal-title": "Rhinol Onl",
"key": "463_CR43",
"unstructured": "Pendolino AL, Lund VJ, Nardello E, Ottaviano G. The nasal cycle: a comprehensive review. Rhinol Onl. 2018;1:67–76.",
"volume": "1",
"year": "2018"
},
{
"author": "NG Papadopoulos",
"first-page": "215",
"journal-title": "Immunol Aller Clinics",
"key": "463_CR44",
"unstructured": "Papadopoulos NG, Guibas GV. Rhinitis subtypes, endotypes, and definitions. Immunol Aller Clinics. 2016;36:215–33.",
"volume": "36",
"year": "2016"
},
{
"DOI": "10.1016/j.drudis.2021.07.021",
"author": "VP Chavda",
"doi-asserted-by": "publisher",
"first-page": "2619",
"journal-title": "Drug Discovery Today",
"key": "463_CR45",
"unstructured": "Chavda VP, Vora LK, Pandya AK, Patravale VB. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discovery Today. 2021;26:2619–36. https://doi.org/10.1016/j.drudis.2021.07.021.",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1002/mco2.101",
"author": "J Xi",
"doi-asserted-by": "publisher",
"first-page": "569",
"journal-title": "MedComm",
"key": "463_CR46",
"unstructured": "Xi J, Lei LR, Zouzas W, April SX. Nasally inhaled therapeutics and vaccination for COVID-19: developments and challenges. MedComm. 2021;2:569–86. https://doi.org/10.1002/mco2.101.",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1152/ajplegacy.1975.229.3.721",
"author": "W Silen",
"doi-asserted-by": "publisher",
"first-page": "721",
"journal-title": "Am J Physiol",
"key": "463_CR47",
"unstructured": "Silen W, Machen TE, Forte JG. Acid-base balance in amphibian gastric mucosa. Am J Physiol. 1975;229:721–30. https://doi.org/10.1152/ajplegacy.1975.229.3.721.",
"volume": "229",
"year": "1975"
},
{
"DOI": "10.1152/ajplegacy.1975.229.3.754",
"author": "IC Ehrhart",
"doi-asserted-by": "publisher",
"first-page": "754",
"journal-title": "Am J Physiol",
"key": "463_CR48",
"unstructured": "Ehrhart IC, Parker PE, Weidner WJ, Dabney JM, Scott JB, Haddy FJ. Coronary vascular and myocardial responses to carotid body stimulation in the dog. Am J Physiol. 1975;229:754–60. https://doi.org/10.1152/ajplegacy.1975.229.3.754.",
"volume": "229",
"year": "1975"
},
{
"DOI": "10.4161/hv.27449",
"author": "A Smith",
"doi-asserted-by": "publisher",
"first-page": "797",
"journal-title": "Hum Vaccin Immunother",
"key": "463_CR49",
"unstructured": "Smith A, Perelman M, Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum Vaccin Immunother. 2014;10:797–807. https://doi.org/10.4161/hv.27449.",
"volume": "10",
"year": "2014"
},
{
"DOI": "10.1155/2016/1459394",
"author": "JS de Apostólico",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol Res",
"key": "463_CR50",
"unstructured": "de Apostólico JS, Lunardelli VAS, Coirada FC, Boscardin SB, Rosa DS. Adjuvants: classification, modus operandi, and licensing. J Immunol Res. 2016. https://doi.org/10.1155/2016/1459394.",
"year": "2016"
},
{
"DOI": "10.1002/fsn3.1236",
"author": "S Xiang",
"doi-asserted-by": "publisher",
"first-page": "3883",
"journal-title": "Food Sci Nutr",
"key": "463_CR51",
"unstructured": "Xiang S, Fu J, Ye K, Zheng Y, Zhu X, Chen J, et al. Effect of Lactobacillus gasseri PA3 on gut microbiota in an in vitro colonic simulation. Food Sci Nutr. 2019;7:3883–91. https://doi.org/10.1002/fsn3.1236.",
"volume": "7",
"year": "2019"
},
{
"key": "463_CR52",
"unstructured": "Covid-19 Vaccine Tracker: Latest Updates - The New York Times n.d. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed October 29, 2022)."
},
{
"DOI": "10.1038/s41598-022-14601-3",
"author": "A Castellarnau",
"doi-asserted-by": "publisher",
"first-page": "10210",
"journal-title": "Sci Rep",
"key": "463_CR53",
"unstructured": "Castellarnau A, Heery GP, Seta A, Luscombe CA, Kinghorn GR, Button P, et al. Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial. Sci Rep. 2022;12:10210. https://doi.org/10.1038/s41598-022-14601-3.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1152/ajplegacy.1975.229.6.1726",
"author": "RP Durbin",
"doi-asserted-by": "publisher",
"first-page": "1726",
"journal-title": "Am J Physiol",
"key": "463_CR54",
"unstructured": "Durbin RP. Letter: acid secretion by gastric mucous membrane. Am J Physiol. 1975;229:1726. https://doi.org/10.1152/ajplegacy.1975.229.6.1726.",
"volume": "229",
"year": "1975"
},
{
"DOI": "10.1088/1752-7163/abc302",
"author": "FLM Ricciardolo",
"doi-asserted-by": "publisher",
"journal-title": "J Breath Res",
"key": "463_CR55",
"unstructured": "Ricciardolo FLM, Bertolini F, Carriero V, Högman M. Nitric oxide’s physiologic effects and potential as a therapeutic agent against COVID-19. J Breath Res. 2020. https://doi.org/10.1088/1752-7163/abc302.",
"year": "2020"
},
{
"DOI": "10.3390/v13081656",
"author": "JRA Paull",
"doi-asserted-by": "publisher",
"first-page": "1656",
"journal-title": "Viruses",
"key": "463_CR56",
"unstructured": "Paull JRA, Luscombe CA, Castellarnau A, Heery GP, Bobardt MD, Gallay PA. Protective effects of astodrimer sodium 1% nasal spray formulation against SARS-CoV-2 nasal challenge in K18-hACE2 mice. Viruses. 2021;13:1656. https://doi.org/10.3390/v13081656.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1089/jamp.2020.1622",
"author": "JP Mitchell",
"doi-asserted-by": "publisher",
"first-page": "235",
"journal-title": "J Aerosol Med Pulm Drug Deliv",
"key": "463_CR57",
"unstructured": "Mitchell JP, Berlinski A, Canisius S, Cipolla D, Dolovich MB, Gonda I, et al. Urgent appeal from international society for aerosols in medicine (ISAM) during COVID-19: clinical decision makers and governmental agencies should consider the inhaled route of administration: a statement from the ISAM regulatory and standardization issues networking group. J Aerosol Med Pulm Drug Deliv. 2020;33:235–8. https://doi.org/10.1089/jamp.2020.1622.",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1016/j.virol.2009.09.007",
"author": "S Åkerström",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Virology",
"key": "463_CR58",
"unstructured": "Åkerström S, Gunalan V, Keng CT, Tan Y-J, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1–9. https://doi.org/10.1016/j.virol.2009.09.007.",
"volume": "395",
"year": "2009"
},
{
"author": "LB Mekler",
"first-page": "501",
"journal-title": "Acta Virol",
"key": "463_CR59",
"unstructured": "Mekler LB. On the problem of oncogene of tumour viruses. Acta Virol. 1975;19:501–8.",
"volume": "19",
"year": "1975"
},
{
"key": "463_CR60",
"unstructured": "UK Clinical Trial Confirms SaNOtize’s Breakthrough Treatment for COVID-19 2021. https://www.businesswire.com/news/home/20210315005197/en/UK-Clinical-Trial-Confirms-SaNOtize%E2%80%99s-Breakthrough-Treatment-for-COVID-19 (accessed October 29, 2022)."
},
{
"DOI": "10.1016/j.niox.2013.03.007",
"author": "G Regev-Shoshani",
"doi-asserted-by": "publisher",
"first-page": "48",
"journal-title": "Nitric Oxide",
"key": "463_CR61",
"unstructured": "Regev-Shoshani G, Vimalanathan S, McMullin B, Road J, Av-Gay Y, Miller C. Gaseous nitric oxide reduces influenza infectivity in vitro. Nitric Oxide. 2013;31:48–53. https://doi.org/10.1016/j.niox.2013.03.007.",
"volume": "31",
"year": "2013"
},
{
"DOI": "10.1080/14787210.2021.1908127",
"author": "K Shmuel",
"doi-asserted-by": "publisher",
"first-page": "1325",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "463_CR62",
"unstructured": "Shmuel K, Dalia M, Tair L, Yaakov N. Low pH Hypromellose (Taffix) nasal powder spray could reduce SARS-CoV-2 infection rate post mass-gathering event at a highly endemic community: an observational prospective open label user survey. Expert Rev Anti Infect Ther. 2021;19:1325–30. https://doi.org/10.1080/14787210.2021.1908127.",
"volume": "19",
"year": "2021"
},
{
"key": "463_CR63",
"unstructured": "Chulalongkorn University. A Phase I Double-blind, Randomized, Placebo-controlled Study to Evaluate Safety of Hypromellose-based Nasal Spray Solution Containing Human IgG1 Anti-SARS-CoV-2 Antibody Cocktail in Healthy Volunteers. clinicaltrials.gov; 2022."
},
{
"DOI": "10.1007/s10811-020-02143-y",
"author": "L Pereira",
"doi-asserted-by": "publisher",
"first-page": "1875",
"journal-title": "J Appl Phycol",
"key": "463_CR64",
"unstructured": "Pereira L, Critchley AT. The COVID 19 novel coronavirus pandemic 2020: seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J Appl Phycol. 2020;32:1875–7. https://doi.org/10.1007/s10811-020-02143-y.",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0259943",
"author": "S Bansal",
"doi-asserted-by": "publisher",
"journal-title": "PLoS ONE",
"key": "463_CR65",
"unstructured": "Bansal S, Jonsson CB, Taylor SL, Figueroa JM, Dugour AV, Palacios C, et al. Iota-carrageenan and xylitol inhibit SARS-CoV-2 in Vero cell culture. PLoS ONE. 2021. https://doi.org/10.1371/journal.pone.0259943.",
"year": "2021"
},
{
"DOI": "10.1002/adma.202008304",
"author": "RJA Moakes",
"doi-asserted-by": "publisher",
"first-page": "2008304",
"journal-title": "Adv Mater",
"key": "463_CR66",
"unstructured": "Moakes RJA, Davies SP, Stamataki Z, Grover LM. Formulation of a composite nasal spray enabling enhanced surface coverage and prophylaxis of SARS-COV-2. Adv Mater. 2021;33:2008304. https://doi.org/10.1002/adma.202008304.",
"volume": "33",
"year": "2021"
},
{
"key": "463_CR67",
"unstructured": "Eva Prieschl-Grassauer. Nasal spray works against COVID-19 variants. 21–04–2021 2021. https://www.thepharmaletter.com/article/nasal-spray-works-against-covid-19-variants? (Accessed April 25, 2021)."
},
{
"DOI": "10.1101/2020.12.08.416677",
"author": "H Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Bioengineering",
"key": "463_CR68",
"unstructured": "Zhang H, Yang Z, Xiang J, Cui Z, Liu J, Liu C. Intranasal administration of SARS-CoV-2 neutralizing human antibody prevents infection in mice. Bioengineering. 2020. https://doi.org/10.1101/2020.12.08.416677.",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.815812",
"author": "TG Moreira",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "463_CR69",
"unstructured": "Moreira TG, Matos KTF, De Paula GS, Santana TMM, Da Mata RG, Pansera FC, et al. Corrigendum: nasal administration of anti-CD3 monoclonal antibody (foralumab) reduces lung inflammation and blood inflammatory biomarkers in mild to moderate COVID-19 patients: a pilot study. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2021.815812.",
"year": "2022"
},
{
"key": "463_CR70",
"unstructured": "Dr. Fabian Buller. Neurimmune and Ethris Sign Collaboration Agreement to Rapidly Develop Inhaled mRNA-based Antibody Therapy for the Treatment of Covid-19. Neurimmune AG 2020. https://www.neurimmune.com/news/neurimmune-and-ethris-sign-collaboration-agreement-to-rapidly-develop-inhaled-mrna-based-antibody-therapy-for-the-treatment-of-covid-19 (accessed April 25, 2021)."
},
{
"key": "463_CR71",
"unstructured": "Chakraverty A. Swiss and German Team to Develop Inhaled mRNA Coronavirus Treatment. LabiotechEu 2020. https://www.labiotech.eu/trends-news/ethris-neurimmune-mrna-coronavirus/ (accessed October 29, 2022)."
},
{
"DOI": "10.1128/JVI.01648-20",
"author": "S Matsuyama",
"doi-asserted-by": "publisher",
"first-page": "e01648",
"journal-title": "J Virol",
"key": "463_CR72",
"unstructured": "Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95:e01648-e1720. https://doi.org/10.1128/JVI.01648-20.",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1016/j.jiac.2020.04.007",
"author": "K Iwabuchi",
"doi-asserted-by": "publisher",
"first-page": "625",
"journal-title": "J Infect Chemother",
"key": "463_CR73",
"unstructured": "Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases. J Infect Chemother. 2020;26:625–32. https://doi.org/10.1016/j.jiac.2020.04.007.",
"volume": "26",
"year": "2020"
},
{
"key": "463_CR74",
"unstructured": "Covis Pharma. Covis Pharma B.V. Initiates Phase 3 Clinical Trial of Alvesco (Ciclesonide) Inhaler for the Treatment of COVID-19. Prnewswire 2020. https://www.prnewswire.com/news-releases/covis-pharma-bv-initiates-phase-3-clinical-trial-of-alvesco-ciclesonide-inhaler-for-the-treatment-of-covid-19-301061105.html (accessed April 25, 2021)."
},
{
"key": "463_CR75",
"unstructured": "Covis Pharma S.à.r.l. A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Efficacy of Ciclesonide Metered-Dose Inhaler in Non-hospitalized Patients 12 Years of Age and Older With Symptomatic COVID-19 Infection. clinicaltrials.gov; 2022."
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"author": "L Caly",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "463_CR76",
"unstructured": "Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020. https://doi.org/10.1016/j.antiviral.2020.104787.",
"year": "2020"
},
{
"DOI": "10.21873/invivo.12134",
"doi-asserted-by": "publisher",
"key": "463_CR77",
"unstructured": "Lehrer S, Rheinstein PH. Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2. In Vivo 2020 https://doi.org/10.21873/invivo.12134."
},
{
"DOI": "10.1016/j.xphs.2021.01.017",
"author": "J Errecalde",
"doi-asserted-by": "publisher",
"first-page": "2501",
"journal-title": "J Pharm Sci",
"key": "463_CR78",
"unstructured": "Errecalde J, Lifschitz A, Vecchioli G, Ceballos L, Errecalde F, Ballent M, et al. Safety and pharmacokinetic assessments of a novel ivermectin nasal spray formulation in a pig model. J Pharm Sci. 2021;110:2501–7. https://doi.org/10.1016/j.xphs.2021.01.017.",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1371/journal.pbio.3001060",
"author": "L Kashkooli",
"doi-asserted-by": "publisher",
"journal-title": "PLoS Biol",
"key": "463_CR79",
"unstructured": "Kashkooli L, Rozema D, Espejo-Ramirez L, Lasko P, Fagotto F. Ectoderm to mesoderm transition by down-regulation of actomyosin contractility. PLoS Biol. 2021. https://doi.org/10.1371/journal.pbio.3001060.",
"year": "2021"
},
{
"DOI": "10.4193/Rhin20.251",
"author": "C Huart",
"doi-asserted-by": "publisher",
"first-page": "623",
"journal-title": "Rhin",
"key": "463_CR80",
"unstructured": "Huart C, Philpott C, Konstantinidis I, Altundag A, Whitcroft KL, Trecca EMC, et al. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhin. 2020;58:623–5. https://doi.org/10.4193/Rhin20.251.",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2021.107871",
"author": "H Kasiri",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "463_CR81",
"unstructured": "Kasiri H, Rouhani N, Salehifar E, Ghazaeian M, Fallah S. Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: a randomized, double blind clinical trial. Int Immunopharmacol. 2021. https://doi.org/10.1016/j.intimp.2021.107871.",
"year": "2021"
},
{
"DOI": "10.1002/lary.30353",
"author": "M Hosseinpoor",
"doi-asserted-by": "publisher",
"first-page": "2209",
"journal-title": "Laryngoscope",
"key": "463_CR82",
"unstructured": "Hosseinpoor M, Kabiri M, Rajati Haghi M, Ghadam Soltani T, Rezaei A, Faghfouri A, et al. Intranasal corticosteroid treatment on recovery of long-term olfactory dysfunction due to COVID-19. Laryngoscope. 2022;132:2209–16. https://doi.org/10.1002/lary.30353.",
"volume": "132",
"year": "2022"
},
{
"DOI": "10.3389/fmicb.2018.02643",
"author": "W Xu",
"doi-asserted-by": "publisher",
"first-page": "2643",
"journal-title": "Front Microbiol",
"key": "463_CR83",
"unstructured": "Xu W, Xia S, Pu J, Wang Q, Li P, Lu L, et al. The antihistamine drugs carbinoxamine maleate and chlorpheniramine maleate exhibit potent antiviral activity against a broad spectrum of influenza viruses. Front Microbiol. 2018;9:2643. https://doi.org/10.3389/fmicb.2018.02643.",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.21203/rs.3.rs-138252/v1",
"doi-asserted-by": "publisher",
"key": "463_CR84",
"unstructured": "Torres J, Go CC, Chohan FA, L. GC, Sanchez-Gonzalez MA, Ferrer G. Chlorpheniramine maleate nasal spray in COVID-19 patients: Case Series. In Review 2021 https://doi.org/10.21203/rs.3.rs-138252/v1."
},
{
"DOI": "10.1176/appi.ajp.2014.12101342",
"doi-asserted-by": "crossref",
"key": "463_CR85",
"unstructured": "Michael R. Liebowitz,Ester Salman,Humberto Nicolini NR. Effect of an Acute Intranasal Aerosol Dose of PH94B on Social and Performance Anxiety in Women With Social Anxiety Disorder. Am J Psychiatry 2014;171."
},
{
"key": "463_CR86",
"unstructured": "VistaGen Therapeutics Inc. PH94B in the treatment of adjustment disorder with anxiety. May 27, 2020 2020. https://clinicaltrials.gov/ct2/show/NCT04404192 (accessed April 25, 2021)."
},
{
"DOI": "10.1176/appi.ajp.2014.12101342",
"author": "MR Liebowitz",
"doi-asserted-by": "publisher",
"first-page": "675",
"journal-title": "AJP",
"key": "463_CR87",
"unstructured": "Liebowitz MR, Salman E, Nicolini H, Rosenthal N, Hanover R, Monti L. Effect of an acute intranasal aerosol dose of PH94B on social and performance anxiety in women with social anxiety disorder. AJP. 2014;171:675–82. https://doi.org/10.1176/appi.ajp.2014.12101342.",
"volume": "171",
"year": "2014"
},
{
"DOI": "10.1016/j.jinf.2021.05.009",
"author": "S Winchester",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "J Infect",
"key": "463_CR88",
"unstructured": "Winchester S, John S, Jabbar K, John I. Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection. J Infect. 2021;83:237–79. https://doi.org/10.1016/j.jinf.2021.05.009.",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.32412/pjohns.v35i2.1503",
"author": "JB Lo",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "Philipp J Otolaryngol Head Neck Surg",
"key": "463_CR89",
"unstructured": "Lo JB, Cruz ET. Efficacy of Carragelose® nasal spray impregnated versus mupirocin ointment impregnated nasal packs on mucosal healing after endoscopic sinus surgery: a double-blind, non-randomized, right-left side comparison. Philipp J Otolaryngol Head Neck Surg. 2020;35:11.",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-20789-7",
"author": "Z Ku",
"doi-asserted-by": "publisher",
"first-page": "469",
"journal-title": "Nat Commun",
"key": "463_CR90",
"unstructured": "Ku Z, Xie X, Davidson E, Ye X, Su H, Menachery VD, et al. Molecular determinants and mechanism for antibody cocktail preventing SARS-CoV-2 escape. Nat Commun. 2021;12:469. https://doi.org/10.1038/s41467-020-20789-7.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41590-020-0778-2",
"author": "ES Winkler",
"doi-asserted-by": "publisher",
"first-page": "1327",
"journal-title": "Nat Immunol",
"key": "463_CR91",
"unstructured": "Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21:1327–35. https://doi.org/10.1038/s41590-020-0778-2.",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1017/S0022215120002455",
"author": "LA Vaira",
"doi-asserted-by": "publisher",
"first-page": "1123",
"journal-title": "J Laryngol Otol",
"key": "463_CR92",
"unstructured": "Vaira LA, Hopkins C, Sandison A, Manca A, Machouchas N, Turilli D, et al. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J Laryngol Otol. 2020;134:1123–7. https://doi.org/10.1017/S0022215120002455.",
"volume": "134",
"year": "2020"
},
{
"DOI": "10.2147/IJN.S313093",
"author": "ZF Aref",
"doi-asserted-by": "publisher",
"first-page": "4063",
"journal-title": "IJN",
"key": "463_CR93",
"unstructured": "Aref ZF, Bazeed SEES, Hassan MH, Hassan AS, Rashad A, Hassan RG, et al. Clinical, biochemical and molecular evaluations of ivermectin mucoadhesive nanosuspension nasal spray in reducing upper respiratory symptoms of mild COVID-19. IJN. 2021;16:4063–72. https://doi.org/10.2147/IJN.S313093.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.5694/mja2.51305",
"author": "D Fraile Navarro",
"doi-asserted-by": "publisher",
"first-page": "255",
"journal-title": "Med J Aust",
"key": "463_CR94",
"unstructured": "Fraile Navarro D, Tendal B, Tingay D, Vasilunas N, Anderson L, Best J, et al. Clinical care of children and adolescents with COVID-19: recommendations from the national COVID-19 clinical evidence taskforce. Med J Aust. 2022;216:255–63. https://doi.org/10.5694/mja2.51305.",
"volume": "216",
"year": "2022"
},
{
"DOI": "10.1139/y75-101",
"author": "AH Jansen",
"doi-asserted-by": "publisher",
"first-page": "726",
"journal-title": "Can J Physiol Pharmacol",
"key": "463_CR95",
"unstructured": "Jansen AH, Russell BJ, Chernick V. Respiratory effects of H+ and dinitrophenol injections into the brain stem subarachnoid space of fetal lambs. Can J Physiol Pharmacol. 1975;53:726–33. https://doi.org/10.1139/y75-101.",
"volume": "53",
"year": "1975"
},
{
"DOI": "10.1002/ppul.24748",
"author": "LM Yonker",
"doi-asserted-by": "publisher",
"first-page": "1085",
"journal-title": "Pediatr Pulmonol",
"key": "463_CR96",
"unstructured": "Yonker LM, Shen K, Kinane TB. Lessons unfolding from pediatric cases of COVID-19 disease caused by SARS-CoV-2 infection. Pediatr Pulmonol. 2020;55:1085–6. https://doi.org/10.1002/ppul.24748.",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1111/j.1365-2044.1994.tb04360.x",
"author": "RWM Walker",
"doi-asserted-by": "publisher",
"journal-title": "Anaesthesia",
"key": "463_CR97",
"unstructured": "Walker RWM, Darowski M, Morris P, Wraith JE. Anaesthesia and mucopolysaccharidoses: a review of airway problems in children. Anaesthesia. 1994. https://doi.org/10.1111/j.1365-2044.1994.tb04360.x.",
"year": "1994"
},
{
"DOI": "10.1542/peds.2010-0616",
"author": "TR Wolfe",
"doi-asserted-by": "publisher",
"first-page": "532",
"journal-title": "Pediatrics",
"key": "463_CR98",
"unstructured": "Wolfe TR, Braude DA. Intranasal medication delivery for children: a brief review and update. Pediatrics. 2010;126:532–7. https://doi.org/10.1542/peds.2010-0616.",
"volume": "126",
"year": "2010"
},
{
"DOI": "10.1016/0005-2744(75)90218-1",
"author": "F Yoshimura",
"doi-asserted-by": "publisher",
"first-page": "167",
"journal-title": "Biochim Biophys Acta",
"key": "463_CR99",
"unstructured": "Yoshimura F, Suzuki T. Calcium-stimulated adenosine triphosphatase in the microsomal fraction of tooth germ from porcine fetus. Biochim Biophys Acta. 1975;410:167–77. https://doi.org/10.1016/0005-2744(75)90218-1.",
"volume": "410",
"year": "1975"
},
{
"DOI": "10.1007/978-1-4614-7978-9",
"doi-asserted-by": "crossref",
"key": "463_CR100",
"unstructured": "Kolhe P, Shah M, Rathore N, (2013). American association of pharmaceutical scientists, editors. Sterile product development: formulation, process, quality and regulatory considerations. New York: Springer : AAPS Press."
},
{
"DOI": "10.2105/ajph.66.1.64",
"author": "JK Glenn",
"doi-asserted-by": "publisher",
"first-page": "64",
"journal-title": "Am J Public Health",
"key": "463_CR101",
"unstructured": "Glenn JK, Goldman J. Task delegation to physician extenders–some comparisons. Am J Public Health. 1976;66:64–6. https://doi.org/10.2105/ajph.66.1.64.",
"volume": "66",
"year": "1976"
},
{
"DOI": "10.1002/14651858.CD013627.pub2",
"author": "MJ Burton",
"doi-asserted-by": "publisher",
"journal-title": "Coch Database Syst Rev",
"key": "463_CR102",
"unstructured": "Burton MJ, Clarkson JE, Goulao B, Glenny A-M, McBain AJ, Schilder AG, et al. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Coch Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD013627.pub2.",
"year": "2020"
},
{
"DOI": "10.1007/s43441-020-00214-3",
"author": "JD Momper",
"doi-asserted-by": "publisher",
"first-page": "282",
"journal-title": "Ther Innov Regul Sci",
"key": "463_CR103",
"unstructured": "Momper JD, Green DJ, Park K, Burckart GJ, Snyder DL. Ethical considerations for pediatric placebo-controlled trials: FDA outcomes and perspectives. Ther Innov Regul Sci. 2021;55:282–303. https://doi.org/10.1007/s43441-020-00214-3.",
"volume": "55",
"year": "2021"
},
{
"DOI": "10.1177/0145561320932318",
"author": "S Frank",
"doi-asserted-by": "publisher",
"first-page": "586",
"journal-title": "Ear Nose Throat J",
"key": "463_CR104",
"unstructured": "Frank S, Capriotti J, Brown SM, Tessema B. Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era. Ear Nose Throat J. 2020;99:586–93. https://doi.org/10.1177/0145561320932318.",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.3390/vaccines9080881",
"author": "RG King",
"doi-asserted-by": "publisher",
"first-page": "881",
"journal-title": "Vaccines",
"key": "463_CR105",
"unstructured": "King RG, Silva-Sanchez A, Peel JN, Botta D, Dickson AM, Pinto AK, et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects mice from lethal challenge. Vaccines. 2021;9:881. https://doi.org/10.3390/vaccines9080881.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3390/vaccines8040771",
"author": "W Sun",
"doi-asserted-by": "publisher",
"first-page": "771",
"journal-title": "Vaccines",
"key": "463_CR106",
"unstructured": "Sun W, McCroskery S, Liu W-C, Leist SR, Liu Y, Albrecht RA, et al. A newcastle disease virus (NDV) expressing a membrane-anchored spike as a cost-effective inactivated SARS-CoV-2 vaccine. Vaccines. 2020;8:771. https://doi.org/10.3390/vaccines8040771.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciac448",
"author": "Y Lin",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "463_CR107",
"unstructured": "Lin Y, Yue S, Yang Y, Yang S, Pan Z, Yang X, et al. Nasal spray of neutralizing monoclonal antibody 35B5 confers potential prophylaxis against severe acute respiratory syndrome coronavirus 2 variants of concern: a small-scale clinical trial. Clin Infect Dis. 2022. https://doi.org/10.1093/cid/ciac448.",
"year": "2022"
},
{
"DOI": "10.1080/21645515.2022.2045853",
"author": "K Dhama",
"doi-asserted-by": "publisher",
"first-page": "2045853",
"journal-title": "Hum Vaccin Immunother",
"key": "463_CR108",
"unstructured": "Dhama K, Dhawan M, Tiwari R, Emran TB, Mitra S, Rabaan AA, et al. COVID-19 intranasal vaccines: current progress, advantages, prospects, and challenges. Hum Vaccin Immunother. 2022;18:2045853. https://doi.org/10.1080/21645515.2022.2045853.",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics10030082",
"author": "E Touitou",
"doi-asserted-by": "publisher",
"first-page": "82",
"journal-title": "Pharmaceutics",
"key": "463_CR109",
"unstructured": "Touitou E, Natsheh H, Duchi S. Buspirone nanovesicular nasal system for non-hormonal hot flushes treatment. Pharmaceutics. 2018;10:82. https://doi.org/10.3390/pharmaceutics10030082.",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.3109/10611869308996066",
"author": "A Harris",
"doi-asserted-by": "publisher",
"first-page": "101",
"journal-title": "J Drug Target",
"key": "463_CR110",
"unstructured": "Harris A. Review: clinical opportunities provided by the nasal administration of peptides. J Drug Target. 1993;1:101–16. https://doi.org/10.3109/10611869308996066.",
"volume": "1",
"year": "1993"
},
{
"DOI": "10.1016/S0168-3659(02)00363-2",
"author": "L Illum",
"doi-asserted-by": "publisher",
"first-page": "187",
"journal-title": "J Control Release",
"key": "463_CR111",
"unstructured": "Illum L. Nasal drug delivery—possibilities, problems and solutions. J Control Release. 2003;87:187–98. https://doi.org/10.1016/S0168-3659(02)00363-2.",
"volume": "87",
"year": "2003"
},
{
"DOI": "10.1080/17425247.2020.1714589",
"author": "B Forbes",
"doi-asserted-by": "publisher",
"first-page": "127",
"journal-title": "Expert Opin Drug Deliv",
"key": "463_CR112",
"unstructured": "Forbes B, Bommer R, Goole J, Hellfritzsch M, De Kruijf W, Lambert P, et al. A consensus research agenda for optimising nasal drug delivery. Expert Opin Drug Deliv. 2020;17:127–32. https://doi.org/10.1080/17425247.2020.1714589.",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1007/s13346-020-00891-5",
"author": "L-A Keller",
"doi-asserted-by": "publisher",
"first-page": "735",
"journal-title": "Drug Deliv and Transl Res",
"key": "463_CR113",
"unstructured": "Keller L-A, Merkel O, Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv and Transl Res. 2022;12:735–57. https://doi.org/10.1007/s13346-020-00891-5.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.ijpharm.2019.118813",
"author": "MY Lobaina",
"doi-asserted-by": "publisher",
"journal-title": "Int J Pharmaceut",
"key": "463_CR114",
"unstructured": "Lobaina MY. Nasal route for vaccine and drug delivery: features and current opportunities. Int J Pharmaceut. 2019. https://doi.org/10.1016/j.ijpharm.2019.118813.",
"year": "2019"
},
{
"DOI": "10.1007/s11095-011-0417-6",
"author": "V Kundoor",
"doi-asserted-by": "publisher",
"first-page": "1895",
"journal-title": "Pharm Res",
"key": "463_CR115",
"unstructured": "Kundoor V, Dalby RN. Effect of formulation- and administration-related variables on deposition pattern of nasal spray pumps evaluated using a nasal cast. Pharm Res. 2011;28:1895–904. https://doi.org/10.1007/s11095-011-0417-6.",
"volume": "28",
"year": "2011"
},
{
"DOI": "10.1089/jam.2004.17.249",
"author": "PG Djupesland",
"doi-asserted-by": "publisher",
"first-page": "249",
"journal-title": "J Aerosol Med",
"key": "463_CR116",
"unstructured": "Djupesland PG, Skretting A, Winderen M, Holand T. Bi-directional nasal delivery of aerosols can prevent lung deposition. J Aerosol Med. 2004;17:249–59. https://doi.org/10.1089/jam.2004.17.249.",
"volume": "17",
"year": "2004"
},
{
"DOI": "10.1208/s12249-015-0317-x",
"author": "K Berkenfeld",
"doi-asserted-by": "publisher",
"first-page": "479",
"journal-title": "AAPS PharmSciTech",
"key": "463_CR117",
"unstructured": "Berkenfeld K, Lamprecht A, McConville JT. Devices for dry powder drug delivery to the lung. AAPS PharmSciTech. 2015;16:479–90. https://doi.org/10.1208/s12249-015-0317-x.",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1016/j.otohns.2008.03.009",
"author": "SJ Kanowitz",
"doi-asserted-by": "publisher",
"first-page": "131",
"journal-title": "Otolaryngol Head Neck Surg",
"key": "463_CR118",
"unstructured": "Kanowitz SJ, Batra PS, Citardi MJ. Topical budesonide via mucosal atomization device in refractory postoperative chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2008;139:131–6. https://doi.org/10.1016/j.otohns.2008.03.009.",
"volume": "139",
"year": "2008"
},
{
"key": "463_CR119",
"unstructured": "Marino D. Aptar Pharma’s Nasal Unidose Device Approved by US FDA. Drug Development and Delivery 2020. https://drug-dev.com/aptar-pharmas-nasal-unidose-device-approved-by-us-fda/ (accessed October 29, 2022)."
},
{
"key": "463_CR120",
"unstructured": "SinusTM Pulsating Aerosol System - PARI n.d. https://www.pari.com/us/products/nebulizer-systems-for-the-sinuses-and-upper-airway-us/sinus-pulsating-aerosol-system-us/ (accessed October 29, 2022)."
},
{
"DOI": "10.1016/j.jconrel.2012.01.024",
"author": "L Illum",
"doi-asserted-by": "publisher",
"first-page": "254",
"journal-title": "J Control Release",
"key": "463_CR121",
"unstructured": "Illum L. Nasal drug delivery: recent developments and future prospects. J Control Release. 2012;161:254–63. https://doi.org/10.1016/j.jconrel.2012.01.024.",
"volume": "161",
"year": "2012"
},
{
"DOI": "10.7759/cureus.10501",
"doi-asserted-by": "publisher",
"key": "463_CR122",
"unstructured": "Westover JB, Ferrer G, Vazquez H, Bethencourt-Mirabal A, Go CC. In Vitro Virucidal Effect of Intranasally Delivered Chlorpheniramine Maleate Compound Against Severe Acute Respiratory Syndrome Coronavirus 2. Cureus 2020. https://doi.org/10.7759/cureus.10501"
}
],
"reference-count": 122,
"references-count": 122,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s43440-023-00463-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"General Medicine"
],
"subtitle": [],
"title": "Nasal sprays for treating COVID-19: a scientific note",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}
