Povidone-Iodine Use in Sinonasal and Oral Cavities: A Review of Safety in the COVID-19 Era
et al., Ear, Nose & Throat Journal, doi:10.1177/0145561320932318, Jun 2020
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
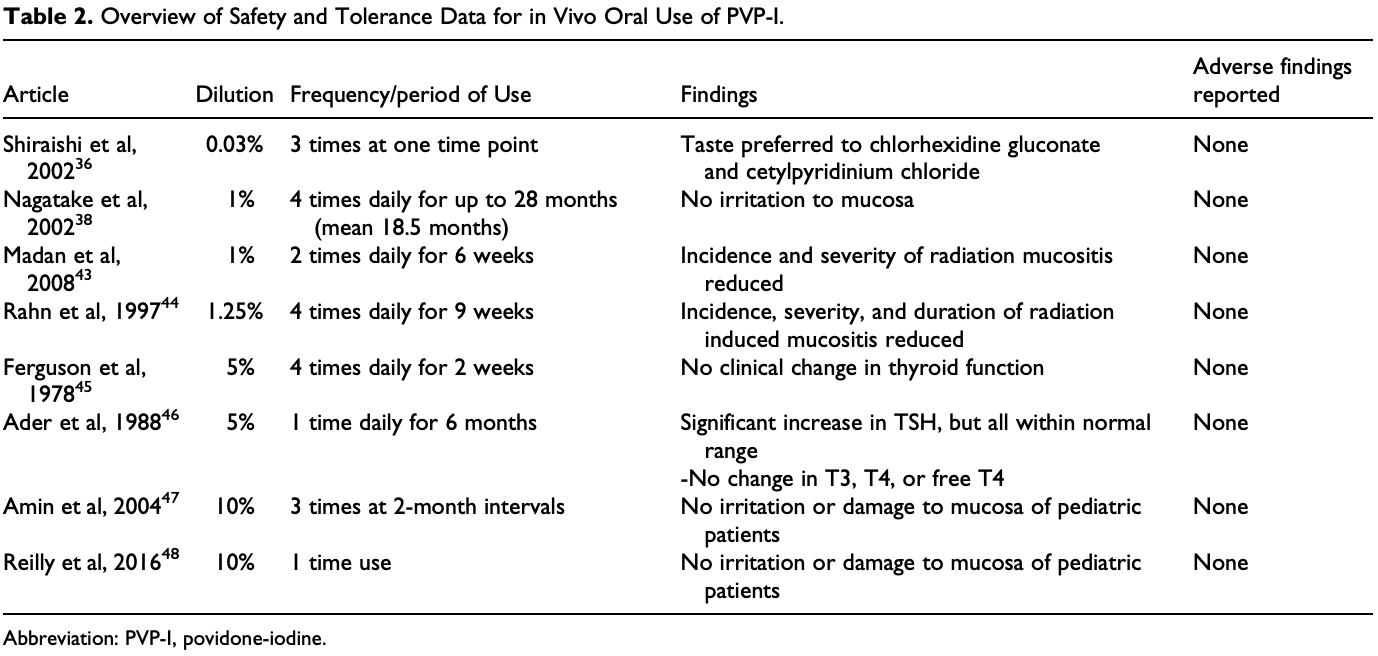
Review of povidone-iodine finding that it can safely be used in the nose at concentrations up to 1.25% and in the mouth at concentrations up to 2.5% for up to 5 months.
Frank et al., 10 Jun 2020, peer-reviewed, 4 authors.
Povidone-Iodine Use in Sinonasal and Oral Cavities: A Review of Safety in the COVID-19 Era
Ear, Nose & Throat Journal, doi:10.1177/0145561320932318
Objectives: Approaches to nasal and oral decontamination with povidone-iodine (PVP-I) have been published to reduce nosocomial spread of Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). The safety of PVP-I topically applied to the nasal and oral cavity is addressed by a literature review. The specific efficacy of PVP-I against coronaviruses and its potential efficacy against SARS-CoV-2 is discussed. Methods: A review was performed utilizing PubMed and Cochrane Databases. All citations in protocols for nasal and oral PVP-I use regarding COVID-19 were independently reviewed. Results: Povidone-iodine has been safely administered for up to 5 months in the nasal cavity and 6 months in the oral cavity. Concentrations less than 2.5% in vitro do not reduce ciliary beat frequency or cause pathological changes in ciliated nasal epithelium, upper respiratory, or mucosal cells. Adverse events with oral use have not been reported in conscious adults or children. Allergy and contact sensitivity is rare. Chronic mucosal use up to 5% has not been shown to result in clinical thyroid disease. PVP-I is rapidly virucidal and inactivates coronaviruses, including SARS-CoV and Middle East Respiratory Syndrome (MERS). Conclusions: Povidone-iodine can safely be used in the nose at concentrations up to 1.25% and in the mouth at concentrations up to 2.5% for up to 5 months. Povidone-iodine rapidly inactivates coronaviruses, including SARS and MERS, when applied for as little as 15 seconds. There is optimism that PVP-I can inactivate SARS-CoV-2, but in vitro efficacy has not yet been demonstrated.
References
Ader, Paul, Reinhardt, Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function, J Clin Endocrinol Metab
Amin, Harrison, Benton, Roberts, Weinstein, Effect of povidone-iodine on Streptococcus mutans in children with extensive dental caries, Pediatr Dent
Balakrishnan, Schechtman, Hogikyan, Teoh, Mcgrath et al., COVID-19 pandemic: what every otolaryngologist-head and neck surgeon needs to know for safe airway management, Otolaryngol Head Neck Surg
Bebko, Green, Awad, Effect of a preoperative decontamination protocol on surgical site infections in patients undergoing elective orthopedic surgery with hardware implantation, JAMA Surg
Beeching, Fletcher, Beadsworth, Covid-19: testing times, BMJ
Below, Behrens-Baumann, Bernhardt, Vo ¨lzke, Kramer et al., Systemic iodine absorption after preoperative antisepsis using povidone-iodine in cataract surgery-an open controlled study, Dermatology
Berkelman, Holland, Anderson, Increased bactericidal activity of dilute preparations of povidone-iodine solutions, J Clin Microbiol
Bhagwat, Iny, Pedi, Stabilizing packaged iodophor and minimizing leaching of iodine through packaging
Butowt, Bilinska, SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection, ACS Chem Neurosci
Capriotti, Capriotti, Topical iodophor preparations: chemistry, microbiology, and clinical utility, Dermatol Online J
Cheong, Yang, Choi, Lung injury induced by the pulmonary instillation of povidone-iodine in rats, J Anesth
Chepla, Gosain, Insterstitial pneumonitis after betadine aspiration, J Craniofac Surg
Choi, Park, Cheon, Son, Aspiration pneumonitis due to povidone-iodine aspiration during a facial bone fracture reduction operation, J Craniofac Surg
Dexter, Parra, Brown, Loftus, Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management, Anesth Analg
Eggers, Eickmann, Zorn, Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA), Infect Dis Ther
Eggers, Koburger-Janssen, Eickmann, Zorn, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/ mouthwash against respiratory and oral tract pathogens, Infect Dis Ther
Ferguson, Geddes, Wray, The effect of a povidoneiodine mouthwash upon thyroid function and plaque accumulation, Br Dent J
Foley, The relationship between autoimmune thyroid disease and iodine intake: a review, Endokrynol Pol
Furudate, Nishimaki, Muto, 125I uptake competing with iodine absorption by the thyroid gland following povidone-iodine skin application, Exp Anim
Gluck, Martin, Bosse, Reimer, Mueller, A clinical study on the tolerability of a liposomal povidone-iodine nasal spray: implications for further development, ORL J Otorhinolaryngol Relat Spec
Gray, Katelaris, Lipson, Recurrent anaphylaxis caused by topical povidoneiodine (Betadine), J Paediatr Child Health
Hitosugi, Tsukamoto, Yokoyama, Pneumonia due to aspiration of povidone iodine after preoperative disinfection of the oral cavity, Oral Maxillofac Surg
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidoneiodine, physical conditions and chemical reagents, Dermatology
Kawana, Kitamura, Nakagomi, Inactivation of human viruses by povidoneiodine in comparison with other antiseptics, Dermatology
Kim, Rimmer, Mrad, Ahmadzada, Harvey, Betadine has a ciliotoxic effect on ciliated human respiratory cells, J Laryngol Otol
Kirk-Bayley, Combes, Sunkaraneni, The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers
Kitamura, Satomura, Kawamura, Great Cold Investigators-I. Can we prevent influenza-like illnesses by gargling?, Intern Med
Kovesi, The use of betadine antiseptic in the treatment of oral surgical, paradontological and oral mucosal diseases
Lachapelle, A comparison of the irritant and allergenic properties of antiseptics, Eur J Dermatol
Lachapelle, Allergic contact dermatitis from povidone-iodine: a re-evaluation study, Contact Dermatitis
Leung, Chu, Shiu, Respiratory virus shedding in exhaled breath and efficacy of face masks, Nat Med
Madan, Sequeira, Shenoy, Shetty, The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: a randomized control trial, J Cancer Res Ther
Mady, Kubik, Baddour, Snyderman, Rowan, Consideration of povidone-iodine as a public healthy intervention for COVID-19: utilization as ''personal protective equipment'' for frontline providers exposed in high-risk head and neck and skull base oncology care, Oral Oncol
Managutti, Managutti, Patel, Puthanakar, Evaluation of post-surgical bacteremia with use of povidone-iodine and chlorhexidine during mandibular third molar surgery, J Maxillofac Oral Surg
Mullings, Panchmatia, Samoy, Topical povidoneiodine as an adjunctive treatment for recalcitrant chronic rhinosinusitis, Eur J Rhinol Allergy
Nagata, Takasu, Akamine, Urinary iodine and thyroid antibodies in Okinawa, Yamagata, Hyogo, and Nagano, Japan: the differences in iodine intake do not affect thyroid antibody positivity, Endocrine J
Nagatake, Ahmed, Oishi, Prevention of respiratory infections by povidone-iodine gargle, Dermatology
Nelson, Palmes, The absorption, excretion, and physiological effect of iodine in normal human subjects, J Clin Invest
Nesvadbova, Crosera, Maina, Filon, Povidone iodine skin absorption: an ex-vivo study, Toxicol Lett
Ngai, Van Arsdale, Govindappagari, Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial, Obstet Gynecol
Panchmatia, Payandeh, Al-Salman, The efficacy of diluted topical povidoneiodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study, Eur Arch Otorhinolaryngol
Parhar, Tasche, Brody, Topical preparations to reduce SARS-CoV-2 aerosolization in head and neck mucosal surgery
Peng, Wang, Zhai, Weng, Feng, Effectiveness of preoperative decolonization with nasal povidone iodine in Chinese patients undergoing elective orthopedic surgery: a prospective cross-sectional study, Braz J Med Biol Res
Perl, Cullen, Wenzel, Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections, N Engl J Med
Plamondon, Ophthalmic solution comprising iodinepolyvinylpyrrolidone complex
Rackur, New aspects of mechanism of action of povidoneiodine, J Hosp Infect
Rahn, Adamietz, Boettcher, Schaefer, Reimer et al., Povidone-iodine to prevent mucositis in patients during antineoplastic radiochemotherapy, Dermatology
Ramaswamykanive, Nanavati, Mackie, Linderman, Lavee, Cardiovascular collapse following povidone-iodine wash, Anaesth Intensive Care
Ramezanpour, Smith, Psaltis, Wormald, Vreugde, In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells, Int Forum Allergy Rhinol
Reilly, Goettl, Steinmetz, Nikrad, Jones, Short-term effects of povidone iodine and sodium fluoride therapy on plaque levels and microbiome diversity, Oral Dis
Reimer, Wichelhaus, Schafer, Antimicrobial effectiveness of povidone-iodine and consequences for new application areas, Dermatology
Reyazulla, Gopinath, Vaibhav, Raut, An unusual complication of late onset allergic contact dermatitis to povidone iodine in oral & maxillofacial surgery-a report of 2 cases, Eur Ann Allergy Clin Immunol
Rezapoor, Nicholson, Tabatabaee, Chen, Maltenfort et al., Povidone-iodine-based solutions for decolonization of nasal staphylococcus aureus: a randomized, prospective, placebo-controlled study, J Arthroplasty
Sato, Miyake, Hazama, Omori, Povidone-iodineinduced cell death in cultured human epithelial HeLa cells and rat oral mucosal tissue, Drug Chem Toxicol
Satomura, Kitamura, Kawamura, Prevention of upper respiratory tract infections by gargling: a randomized trial, Am J Prev Med
Shiraishi, Nakagawa, Evaluation of the bactericidal activity of povidone-iodine and commercially available gargle preparations, Dermatology
Sizun, Yu, Talbot, Survival of human coronaviruses 229E and OC43 in suspension and after drying on surfaces: a possible source of hospital-acquired infections, J Hosp Infect
Sriwilaijaroen, Wilairat, Hiramatsu, Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities, Virol J
Sungnak, Huang, Bécavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med
Tahirovic, Toromanovic, Grbic, Bogdanovic, Fatusic et al., Maternal and neonatal urinary iodine excretion and neonatal TSH in relation to use of antiseptic during caesarean section in an iodine sufficient area, J Pediatr Endocrinol Metab
To, Tsang, Chik-Yan Yip, Consistent detection of 2019 novel coronavirus in saliva, Clin Infect Dis
Tomada, Kitano, Uruno, Transcutaneous iodine absorption in adult patients with thyroid cancer disinfected with povidone-iodine at operation, Thyroid
Valizadeh, Nazeri, Fazli, Application of povidoneiodine at delivery significantly increase maternal urinary iodine but not neonatal thyrotropin in an area with iodine sufficiency, J Pediatr Endocrinol Metab
Van Doremalen, Bushmaker, Morris, Aerosol and surface stability of SARS-CoV-2 compared with SARS-CoV-1, N Engl J Med
Verger, Aurengo, Geoffroy, Guen, Iodine kinetics and effectiveness of stable iodine prophylaxis after intake of radioactive iodine: a review, Thyroid
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan China, JAMA
Workman, Welling, Carter, Endonasal instrumentation and aerosolization risk in the era of COVID-19: simulation, literature review, and proposed mitigation strategies, Int Forum Allergy Rhinol
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Wu, Zheng, Single-cell RNA expression profiling shows that ACE2, the putative receptor of Wuhan 2019-nCoV, has significant expression in the nasal, mouth, lung and colon tissues, and tends to be co-expressed with HLA-DRB1 in the four tissues, Preprints
Xu, Zhong, Deng, High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, Int J Oral Sci
Yu, Du, Ojcius, Pan, Jiang, Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China, Microbes Infect
Zhang, Du, Li, Molecular and serological investigation of 2019-nCOV infected patients: implication of multiple shedding routes, Emerg Microbes Infect
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.1177/0145561320932318",
"ISSN": [
"0145-5613",
"1942-7522"
],
"URL": "http://dx.doi.org/10.1177/0145561320932318",
"abstract": "<jats:sec><jats:title>Objectives:</jats:title><jats:p>Approaches to nasal and oral decontamination with povidone-iodine (PVP-I) have been published to reduce nosocomial spread of Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). The safety of PVP-I topically applied to the nasal and oral cavity is addressed by a literature review. The specific efficacy of PVP-I against coronaviruses and its potential efficacy against SARS-CoV-2 is discussed.</jats:p></jats:sec><jats:sec><jats:title>Methods:</jats:title><jats:p>A review was performed utilizing PubMed and Cochrane Databases. All citations in protocols for nasal and oral PVP-I use regarding COVID-19 were independently reviewed.</jats:p></jats:sec><jats:sec><jats:title>Results:</jats:title><jats:p>Povidone-iodine has been safely administered for up to 5 months in the nasal cavity and 6 months in the oral cavity. Concentrations less than 2.5% in vitro do not reduce ciliary beat frequency or cause pathological changes in ciliated nasal epithelium, upper respiratory, or mucosal cells. Adverse events with oral use have not been reported in conscious adults or children. Allergy and contact sensitivity is rare. Chronic mucosal use up to 5% has not been shown to result in clinical thyroid disease. PVP-I is rapidly virucidal and inactivates coronaviruses, including SARS-CoV and Middle East Respiratory Syndrome (MERS).</jats:p></jats:sec><jats:sec><jats:title>Conclusions:</jats:title><jats:p>Povidone-iodine can safely be used in the nose at concentrations up to 1.25% and in the mouth at concentrations up to 2.5% for up to 5 months. Povidone-iodine rapidly inactivates coronaviruses, including SARS and MERS, when applied for as little as 15 seconds. There is optimism that PVP-I can inactivate SARS-CoV-2, but in vitro efficacy has not yet been demonstrated.</jats:p></jats:sec>",
"alternative-id": [
"10.1177/0145561320932318"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5068-1065",
"affiliation": [
{
"name": "University of Connecticut School of Medicine, Farmington, USA"
}
],
"authenticated-orcid": false,
"family": "Frank",
"given": "Samantha",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Veloce BioPharma, Fort Lauderdale, FL, USA"
}
],
"family": "Capriotti",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Connecticut School of Medicine, Farmington, USA"
},
{
"name": "ProHealth Ear Nose and Throat, Farmington, CT, USA"
}
],
"family": "Brown",
"given": "Seth M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7418-6034",
"affiliation": [
{
"name": "University of Connecticut School of Medicine, Farmington, USA"
},
{
"name": "ProHealth Ear Nose and Throat, Farmington, CT, USA"
}
],
"authenticated-orcid": false,
"family": "Tessema",
"given": "Belachew",
"sequence": "additional"
}
],
"container-title": "Ear, Nose & Throat Journal",
"container-title-short": "Ear Nose Throat J",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2020,
6,
10
]
],
"date-time": "2020-06-10T17:31:55Z",
"timestamp": 1591810315000
},
"deposited": {
"date-parts": [
[
2022,
10,
27
]
],
"date-time": "2022-10-27T19:18:32Z",
"timestamp": 1666898312000
},
"indexed": {
"date-parts": [
[
2024,
5,
7
]
],
"date-time": "2024-05-07T13:11:25Z",
"timestamp": 1715087485664
},
"is-referenced-by-count": 64,
"issue": "9",
"issued": {
"date-parts": [
[
2020,
6,
10
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2020,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
6,
10
]
],
"date-time": "2020-06-10T00:00:00Z",
"timestamp": 1591747200000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/0145561320932318",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/0145561320932318",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/0145561320932318",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "586-593",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2020,
6,
10
]
]
},
"published-online": {
"date-parts": [
[
2020,
6,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
11
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "bibr1-0145561320932318"
},
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "publisher",
"key": "bibr2-0145561320932318"
},
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "publisher",
"key": "bibr3-0145561320932318"
},
{
"author": "To KK",
"first-page": "1319",
"journal-title": "Clin Infect Dis",
"key": "bibr4-0145561320932318",
"volume": "361",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0843-2",
"doi-asserted-by": "publisher",
"key": "bibr5-0145561320932318"
},
{
"DOI": "10.1021/acschemneuro.0c00172",
"doi-asserted-by": "publisher",
"key": "bibr6-0145561320932318"
},
{
"author": "Wu C",
"first-page": "2020020247",
"journal-title": "Preprints",
"key": "bibr7-0145561320932318",
"year": "2020"
},
{
"DOI": "10.1038/s41368-020-0074-x",
"doi-asserted-by": "publisher",
"key": "bibr8-0145561320932318"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "publisher",
"key": "bibr9-0145561320932318"
},
{
"DOI": "10.1136/bmj.m1403",
"doi-asserted-by": "publisher",
"key": "bibr10-0145561320932318"
},
{
"DOI": "10.1080/22221751.2020.1729071",
"doi-asserted-by": "publisher",
"key": "bibr11-0145561320932318"
},
{
"DOI": "10.1056/NEJMc2004973",
"doi-asserted-by": "publisher",
"key": "bibr12-0145561320932318"
},
{
"author": "Balakrishnan K",
"first-page": "194599820919751",
"journal-title": "Otolaryngol Head Neck Surg",
"key": "bibr13-0145561320932318",
"year": "2020"
},
{
"DOI": "10.1002/alr.22577",
"doi-asserted-by": "publisher",
"key": "bibr14-0145561320932318"
},
{
"DOI": "10.1016/S0195-6701(85)80041-4",
"doi-asserted-by": "publisher",
"key": "bibr15-0145561320932318"
},
{
"DOI": "10.1186/1743-422X-6-124",
"doi-asserted-by": "publisher",
"key": "bibr16-0145561320932318"
},
{
"DOI": "10.1016/j.oraloncology.2020.104724",
"doi-asserted-by": "publisher",
"key": "bibr18-0145561320932318"
},
{
"DOI": "10.1002/hed.26200",
"doi-asserted-by": "publisher",
"key": "bibr19-0145561320932318"
},
{
"DOI": "10.1213/ANE.0000000000004829",
"doi-asserted-by": "publisher",
"key": "bibr20-0145561320932318"
},
{
"DOI": "10.5070/D39RP912J2",
"author": "Capriotti K",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "11",
"journal-title": "Dermatol Online J",
"key": "bibr24-0145561320932318",
"volume": "18",
"year": "2012"
},
{
"DOI": "10.1128/JCM.15.4.635-639.1982",
"doi-asserted-by": "publisher",
"key": "bibr25-0145561320932318"
},
{
"DOI": "10.1056/NEJMoa003069",
"doi-asserted-by": "publisher",
"key": "bibr26-0145561320932318"
},
{
"DOI": "10.1590/1414-431x20176736",
"doi-asserted-by": "publisher",
"key": "bibr27-0145561320932318"
},
{
"DOI": "10.1001/jamasurg.2014.3480",
"doi-asserted-by": "publisher",
"key": "bibr28-0145561320932318"
},
{
"DOI": "10.1016/j.arth.2017.04.039",
"doi-asserted-by": "publisher",
"key": "bibr29-0145561320932318"
},
{
"DOI": "10.1007/s00405-019-05628-w",
"doi-asserted-by": "publisher",
"key": "bibr30-0145561320932318"
},
{
"DOI": "10.5152/ejra.2019.166",
"doi-asserted-by": "publisher",
"key": "bibr31-0145561320932318"
},
{
"DOI": "10.1159/000097758",
"doi-asserted-by": "publisher",
"key": "bibr32-0145561320932318"
},
{
"DOI": "10.1002/alr.22575",
"doi-asserted-by": "publisher",
"key": "bibr33-0145561320932318"
},
{
"DOI": "10.1017/S0022215114002746",
"doi-asserted-by": "publisher",
"key": "bibr34-0145561320932318"
},
{
"DOI": "10.1159/000057738",
"doi-asserted-by": "publisher",
"key": "bibr35-0145561320932318"
},
{
"DOI": "10.1159/000057723",
"doi-asserted-by": "publisher",
"key": "bibr36-0145561320932318"
},
{
"DOI": "10.1007/s12663-016-0976-5",
"doi-asserted-by": "publisher",
"key": "bibr37-0145561320932318"
},
{
"DOI": "10.1159/000057722",
"doi-asserted-by": "publisher",
"key": "bibr38-0145561320932318"
},
{
"DOI": "10.1016/j.amepre.2005.06.013",
"doi-asserted-by": "publisher",
"key": "bibr39-0145561320932318"
},
{
"DOI": "10.2169/internalmedicine.46.0104",
"doi-asserted-by": "publisher",
"key": "bibr40-0145561320932318"
},
{
"author": "Committee for the Japanese Respiratory Society Guidelines in Management of Respiratory",
"first-page": "48",
"issue": "1",
"journal-title": "Respirology",
"key": "bibr41-0145561320932318",
"volume": "9",
"year": "2004"
},
{
"DOI": "10.3109/01480545.2013.846364",
"doi-asserted-by": "publisher",
"key": "bibr42-0145561320932318"
},
{
"DOI": "10.4103/0973-1482.39597",
"doi-asserted-by": "publisher",
"key": "bibr43-0145561320932318"
},
{
"DOI": "10.1159/000246032",
"doi-asserted-by": "publisher",
"key": "bibr44-0145561320932318"
},
{
"DOI": "10.1038/sj.bdj.4804017",
"doi-asserted-by": "publisher",
"key": "bibr45-0145561320932318"
},
{
"DOI": "10.1210/jcem-66-3-632",
"doi-asserted-by": "publisher",
"key": "bibr46-0145561320932318"
},
{
"author": "Amin MS",
"first-page": "5",
"issue": "1",
"journal-title": "Pediatr Dent",
"key": "bibr47-0145561320932318",
"volume": "26",
"year": "2004"
},
{
"DOI": "10.1111/odi.12407",
"doi-asserted-by": "publisher",
"key": "bibr48-0145561320932318"
},
{
"author": "Kovesi G",
"first-page": "243",
"issue": "8",
"journal-title": "Fogorv Sz",
"key": "bibr49-0145561320932318",
"volume": "92",
"year": "1999"
},
{
"DOI": "10.1007/s10006-019-00800-2",
"doi-asserted-by": "publisher",
"key": "bibr50-0145561320932318"
},
{
"DOI": "10.1097/SCS.0000000000000526",
"doi-asserted-by": "publisher",
"key": "bibr51-0145561320932318"
},
{
"DOI": "10.1097/SCS.0b013e31826cf57b",
"doi-asserted-by": "publisher",
"key": "bibr52-0145561320932318"
},
{
"DOI": "10.1007/s00540-011-1242-0",
"doi-asserted-by": "publisher",
"key": "bibr53-0145561320932318"
},
{
"DOI": "10.1089/thy.2005.15.600",
"doi-asserted-by": "publisher",
"key": "bibr54-0145561320932318"
},
{
"DOI": "10.1159/000089198",
"doi-asserted-by": "publisher",
"key": "bibr55-0145561320932318"
},
{
"DOI": "10.1172/JCI101809",
"doi-asserted-by": "publisher",
"key": "bibr57-0145561320932318"
},
{
"DOI": "10.1089/10507250152039082",
"doi-asserted-by": "publisher",
"key": "bibr58-0145561320932318"
},
{
"DOI": "10.1016/j.toxlet.2015.04.004",
"doi-asserted-by": "publisher",
"key": "bibr59-0145561320932318"
},
{
"author": "Foley TP",
"first-page": "53",
"issue": "1",
"journal-title": "Endokrynol Pol",
"key": "bibr60-0145561320932318",
"volume": "43",
"year": "1992"
},
{
"DOI": "10.1507/endocrj.45.797",
"doi-asserted-by": "publisher",
"key": "bibr61-0145561320932318"
},
{
"DOI": "10.1177/0310057X1103900121",
"doi-asserted-by": "publisher",
"key": "bibr62-0145561320932318"
},
{
"DOI": "10.1538/expanim.46.197",
"doi-asserted-by": "publisher",
"key": "bibr63-0145561320932318"
},
{
"DOI": "10.1684/ejd.2013.2198",
"doi-asserted-by": "publisher",
"key": "bibr64-0145561320932318"
},
{
"DOI": "10.1111/jpc.12232",
"doi-asserted-by": "publisher",
"key": "bibr65-0145561320932318"
},
{
"DOI": "10.1111/j.0105-1873.2005.00479.x",
"doi-asserted-by": "publisher",
"key": "bibr66-0145561320932318"
},
{
"author": "Reyazulla MA",
"first-page": "157",
"issue": "4",
"journal-title": "Eur Ann Allergy Clin Immunol",
"key": "bibr67-0145561320932318",
"volume": "46",
"year": "2014"
},
{
"DOI": "10.1097/AOG.0000000000001118",
"doi-asserted-by": "publisher",
"key": "bibr68-0145561320932318"
},
{
"DOI": "10.1515/jpem-2017-0087",
"doi-asserted-by": "publisher",
"key": "bibr69-0145561320932318"
},
{
"DOI": "10.1515/JPEM.2009.22.12.1145",
"doi-asserted-by": "publisher",
"key": "bibr70-0145561320932318"
},
{
"DOI": "10.1159/000246027",
"doi-asserted-by": "publisher",
"key": "bibr71-0145561320932318"
},
{
"DOI": "10.1053/jhin.2000.0795",
"doi-asserted-by": "publisher",
"key": "bibr72-0145561320932318"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"doi-asserted-by": "publisher",
"key": "bibr73-0145561320932318"
},
{
"DOI": "10.1007/s40121-015-0091-9",
"doi-asserted-by": "publisher",
"key": "bibr74-0145561320932318"
},
{
"DOI": "10.1159/000089211",
"doi-asserted-by": "publisher",
"key": "bibr75-0145561320932318"
},
{
"DOI": "10.1016/j.micinf.2020.01.003",
"doi-asserted-by": "publisher",
"key": "bibr76-0145561320932318"
}
],
"reference-count": 71,
"references-count": 71,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/0145561320932318"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Povidone-Iodine Use in Sinonasal and Oral Cavities: A Review of Safety in the COVID-19 Era",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "99"
}
