A Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients Reduces Viral Load and SARS-CoV-2 Detection Time in the Nasal Cavity
et al., Journal of Personalized Medicine, doi:10.3390/jpm13071093, NCT05729204, Jul 2023
32nd treatment shown to reduce risk in
November 2021, now with p = 0.0000000039 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
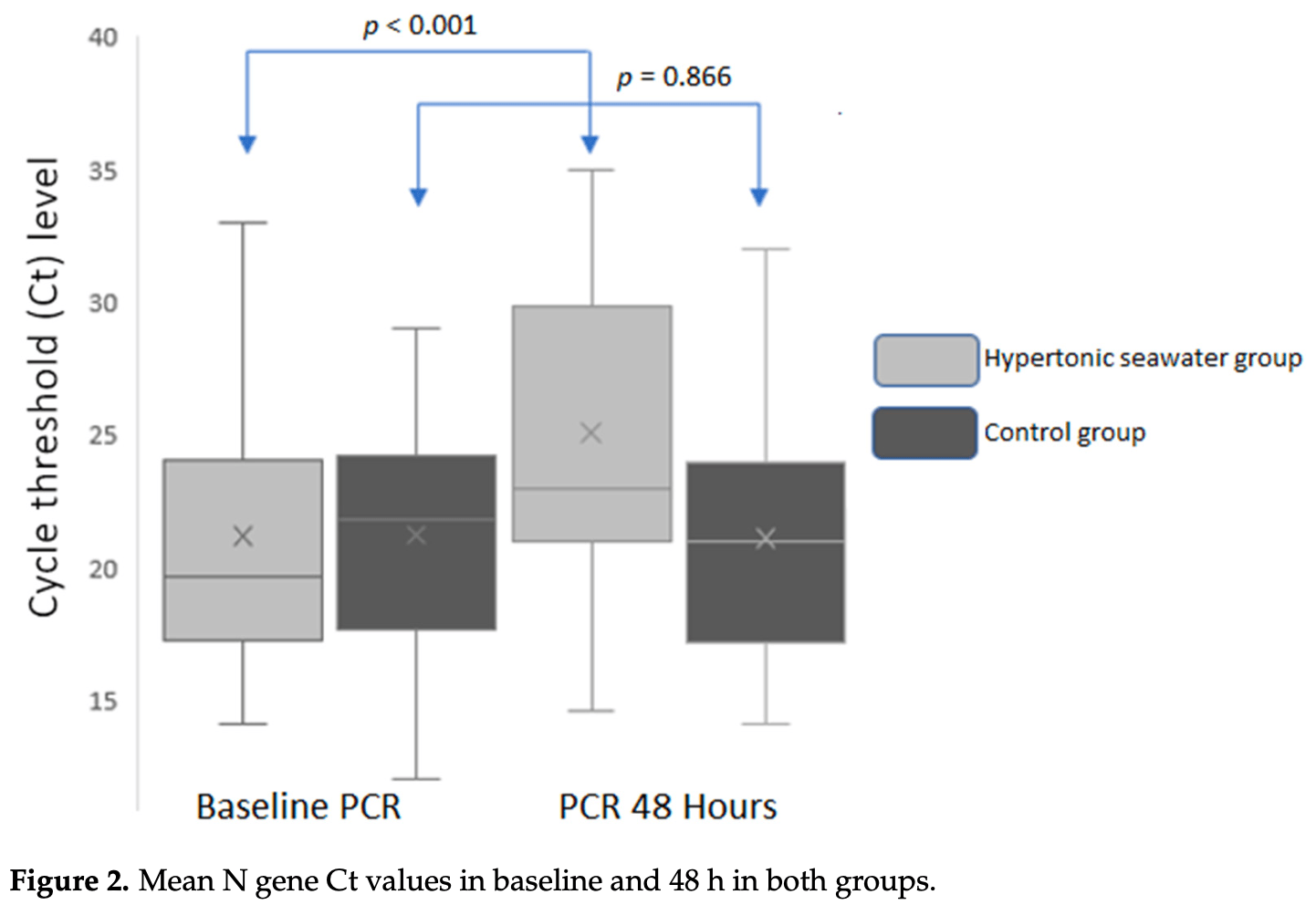
RCT 56 severe COVID-19 patients, showing significantly decreased viral load with Sinomarin Plus Algae nasal irrigation. Sinomarin Plus Algae is a hypertonic seawater solution with algal and herbal natural ingredients with a pH of 7.5-81.
The treatment group received nasal irrigation every 4 hours, 16 hours per day, for 2 days. Nasopharyngeal swabs were taken at baseline and 48 hours later to measure viral load. The treatment group showed a significant increase in cycle threshold values, indicating decreased viral load, while no difference was seen in the control group. The treatment was well tolerated with only mild adverse effects.
Alkalinization is one possible mechanism of action - SARS-CoV-2 requires acidic pH for infection2 and the solution has pH 7.5-8. Other possible mechanisms include antiviral activity of ingredients (e.g., fucoidan from Undaria pinnatifida) and physical removal of viral particles.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action and reduced systemic side effects (early treatment may be more beneficial).
|
risk of death, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 28 (0.0%), control 1 of 28 (3.6%), NNT 28, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 28 (0.0%), control 1 of 28 (3.6%), NNT 28, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of oxygen therapy, 80.0% lower, RR 0.20, p = 0.49, treatment 0 of 28 (0.0%), control 2 of 28 (7.1%), NNT 14, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), high flow nasal cannula or non-invasive ventilation.
|
|
risk of no viral clearance, 42.1% lower, RR 0.58, p = 0.04, treatment 11 of 28 (39.3%), control 19 of 28 (67.9%), NNT 3.5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pantazopoulos et al., 3 Jul 2023, Single Blind Randomized Controlled Trial, Greece, peer-reviewed, mean age 63.6, 7 authors, study period June 2022 - December 2022, average treatment delay 9.5 days, trial NCT05729204 (history).
Contact: pantazopoulosioannis@yahoo.com (corresponding author), michaelspan1988@gmail.com, kellymiz95@yahoo.com, gerovasileiou@yahoo.com, kgourg@uth.gr, thanoschalkias@yahoo.gr, errouka@uth.gr.
A Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients Reduces Viral Load and SARS-CoV-2 Detection Time in the Nasal Cavity
Journal of Personalized Medicine, doi:10.3390/jpm13071093
Nasal irrigation is thought to decrease the viral load present in the nasal cavity. Our aim was to assess the effect of a hypertonic seawater solution [with algal and herbal natural ingredients (Sinomarin ® )] on the viral load of nasopharynx in patients hospitalized with severe COVID-19 pneumonia. We conducted a prospective, randomized, controlled trial from June 2022 to December 2022. We allocated 56 patients with COVID-19 pneumonia into two groups (28 in each group)-the hypertonic seawater group [nasal irrigations with a hypertonic seawater solution (Sinomarin ® ) every 4 h for 16 h per day, for two consecutive days] and the control group (no nasal irrigations). A second nasopharyngeal swab was collected 48 h after the baseline nasopharyngeal swab (8 h after the last wash in the hypertonic seawater group) to estimate the SARS-CoV-2 viral load as determined by cycle threshold (Ct) values. In the hypertonic seawater group, the mean Ct values significantly increased two days after the initial measurement [∆Ct 48-0 h = 3.86 ± 3.03 cycles, p < 0.001 (95%CI: 2.69 to 5.04)]. No significant differences in the Ct values were observed in the control group [∆Ct 48-0 h = -0.14 ± 4.29, p = 0.866 (95%CI: -1.80 to -1.52)]. At follow-up, 17 patients from the hypertonic seawater group had negative test results compared to only 9 patients from the control group (p = 0.03). Nasal irrigations with a hypertonic seawater solution containing algal and herbal natural ingredients significantly decreased nasopharyngeal viral load and the detection time of SARS-CoV-2 in the nasal cavity.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflict of interest.
References
Ao, Lan, He, Liu, Chen et al., SARS-CoV-2 Omicron Variant: Immune Escape and Vaccine Development, MedComm, doi:10.1002/mco2.126
Barrera Saldaña, Rivera Santiago, Rodríguez Palacios, SARS-CoV-2: Challenges in Reconverting Diagnostic Laboratories to Combat the Pandemic, Microbiol. Spectr, doi:10.1128/spectrum.01477-22
Baxter, Schwartz, Johnson, Kuchinski, Swartout et al., Rapid Initiation of Nasal Saline Irrigation to Reduce Severity in High-Risk COVID+ Outpatients, Ear. Nose. Throat J, doi:10.1177/01455613221123737
Cao, Shi, Wen, Peng, Miao et al., Can Nasal Irrigation with Chlorine Dioxide Be Considered as a Potential Alternative Therapy for Respiratory Infectious Diseases? The Example of COVID-19, Biosci. Trends, doi:10.5582/bst.2022.01495
Casale, Rinaldi, Sabatino, Moffa, Ciccozzi, Could Nasal Irrigation and Oral Rinse Reduce the Risk for COVID-19 Infection?, Int. J. Immunopathol. Pharmacol, doi:10.1177/2058738420941757
Cegolon, Mastrangelo, Emanuelli, Camerotto, Spinato et al., Early Negativization of SARS-CoV-2 Infection by Nasal Spray of Seawater plus Additives: The RENAISSANCE Open-Label Controlled Clinical Trial, Pharmaceutics, doi:10.3390/pharmaceutics14112502
Gangadi, Georgiou, Moschotzopoulou, Antronikou, Kainis et al., Efficacy and Safety of a Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients in Patients with COVID-19, Eur. Rev. Med. Pharmacol. Sci, doi:10.26355/eurrev_202212_30495
Guy, Jackson, Acharya, Sturrock, Hooper et al., Converting Enzyme-2 (ACE2): Comparative Modeling of the Active Site, Specificity Requirements, and Chloride Dependence, doi:10.1021/bi035268s
Hasan, Paray, Hussain, Qadir, Attar et al., A Review on the Cleavage Priming of the Spike Protein on Coronavirus by Angiotensin-Converting Enzyme-2 and Furin, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1754293
Huijghebaert, Hoste, Vanham, Essentials in Saline Pharmacology for Nasal or Respiratory Hygiene in Times of COVID-19, Eur. J. Clin. Pharmacol, doi:10.1007/s00228-021-03102-3
Izidoro, Gouvea, Santos, Assis, Oliveira et al., A Study of Human Furin Specificity Using Synthetic Peptides Derived from Natural Substrates, and Effects of Potassium Ions, Arch. Biochem. Biophys, doi:10.1016/j.abb.2009.05.013
Kamal Arefin, Banu, Nasir Uddin, Nurul Fattah Rumi, Khan et al., Virucidal Effect of Povidone Iodine on SARS-CoV-2 in Nasopharynx: An Open-Label Randomized Clinical Trial, Indian J. Otolaryngol. Head Neck Surg, doi:10.1007/s12070-022-03106-0
Kanjanawasee, Seresirikachorn, Chitsuthipakorn, Snidvongs, Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-Analysis, Am. J. Rhinol. Allergy, doi:10.1177/1945892418773566
Kong, Wang, Fang, Yang, Tong, False-Positive Results of SARS-CoV-2 RT-PCR in Oropharyngeal Swabs From Vaccinators, Front. Med, doi:10.3389/fmed.2022.847407
Kumar, Ahmad, Kushwaha, Shokeen, Negi et al., Selection of Ideal Reference Genes for Gene Expression Analysis in COVID-19 and Mucormycosis, Microbiol. Spectr, doi:10.1128/spectrum.01656-22
Kwon, Oh, Kwon, Jin, Zhang et al., Sulfated Polysaccharides Effectively Inhibit SARS-CoV-2 in Vitro, doi:10.1038/s41421-020-00192-8
Liu, Xie, Li, Su, Zhu, Effect of Nasal Irrigation in Adults Infected with Omicron Variant of COVID-19: A Quasi-Experimental Study, Front. Public Health, doi:10.3389/fpubh.2022.1046112
Machado, Glaser, Araujo, Petiz, Oliveira et al., Inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 Replication by Hypertonic Saline Solution in Lung and Kidney Epithelial Cells, ACS Pharmacol. Transl. Sci, doi:10.1021/acsptsci.1c00080
Moher, Hopewell, Schulz, Montori, Gøtzsche et al., Explanation and Elaboration: Updated Guidelines for Reporting Parallel Group Randomised Trials, BMJ, doi:10.1136/bmj.c869
Pantazopoulos, Chalkias, Mavrovounis, Dimeas, Sinis et al., Nasopharyngeal Wash with Normal Saline Decreases SARS-CoV-2 Viral Load: A Randomized Pilot Controlled Trial, Can. Respir. J, doi:10.1155/2022/8794127
Pradhan, Nayak, Patra, Bhuyan, Behera et al., A State-of-the-Art Review on Fucoidan as an Antiviral Agent to Combat Viral Infections, Carbohydr. Polym, doi:10.1016/j.carbpol.2022.119551
Puhach, Meyer, Eckerle, SARS-CoV-2 Viral Load and Shedding Kinetics, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00822-w
Ramalingam, Cai, Wong, Twomey, Chen et al., Antiviral Innate Immune Response in Non-Myeloid Cells Is Augmented by Chloride Ions via an Increase in Intracellular Hypochlorous Acid Levels, Sci. Rep, doi:10.1038/s41598-018-31936-y
Ramalingam, Graham, Dove, Morrice, Sheikh et al., Open Labelled, Randomised Controlled Trial of Hypertonic Saline Nasal Irrigation and Gargling for the Common Cold, Sci. Rep, doi:10.1038/s41598-018-37703-3
Ramalingam, Graham, Dove, Morrice, Sheikh, Hypertonic Saline Nasal Irrigation and Gargling Should Be Considered as a Treatment Option for COVID-19, J. Glob. Health, doi:10.7189/jogh.10.010332
Salamanna, Maglio, Landini, Fini, Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2, doi:10.3389/fmed.2020.594495
Slapak, Skoupá, Strnad, Horník, Efficacy of Isotonic Nasal Wash (Seawater) in the Treatment and Prevention of Rhinitis in Children, Arch. Otolaryngol. Head Neck Surg, doi:10.1001/archoto.2007.19
Sungnak, Huang, Bécavin, Berg, Queen et al., SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes, Nat. Med, doi:10.1038/s41591-020-0868-6
Tian, Sun, Xu, Ye, The Emergence and Epidemic Characteristics of the Highly Mutated SARS-CoV-2 Omicron Variant, J. Med. Virol, doi:10.1002/jmv.27643
To, Li, Lung, Ip, Chan et al., False Coronavirus Disease 2019 Cases Due to Contamination by Inactivated Virus Vaccine, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am, doi:10.1093/cid/ciab684
Tugizov, Virus-Associated Disruption of Mucosal Epithelial Tight Junctions and Its Role in Viral Transmission and Spread, Tissue Barriers, doi:10.1080/21688370.2021.1943274
Wang, Zhang, Zhang, Mao, Yan et al., Efficacy of Nasal Irrigation and Oral Rinse with Sodium Bicarbonate Solution on Virus Clearance for COVID-19 Patients, Front. Public Health, doi:10.3389/fpubh.2023.1145669
Yildiz, Koca Yildiz, Kuzu, Günebakan, Bucak et al., Comparison of the Healing Effect of Nasal Saline Irrigation with Triamcinolone Acetonide Versus Nasal Saline Irrigation Alone in COVID-19 Related Olfactory Dysfunction: A Randomized Controlled Study, Indian J. Otolaryngol. Head Neck Surg, doi:10.1007/s12070-021-02749-9
Yilmaz, Yilmaz, Ozdemir, Kocazeybek, Karaali et al., Effects of Hypertonic Alkaline Nasal Irrigation on COVID-19, Laryngoscope Investig. Otolaryngol, doi:10.1002/lio2.686
Zhang, Peng, Lai, Jiang, Wang et al., Differential Susceptibility to SARS-CoV-2 in the Normal Nasal Mucosa and in Chronic Sinusitis, Eur. J. Immunol, doi:10.1002/eji.202249805
Zhang, Zhang, Li, Pang, Liu et al., Epithelial Barrier in the Nasal Mucosa, Related Risk Factors and Diseases, Int. Arch. Allergy Immunol, doi:10.1159/000528969
DOI record:
{
"DOI": "10.3390/jpm13071093",
"ISSN": [
"2075-4426"
],
"URL": "http://dx.doi.org/10.3390/jpm13071093",
"abstract": "<jats:p>Nasal irrigation is thought to decrease the viral load present in the nasal cavity. Our aim was to assess the effect of a hypertonic seawater solution [with algal and herbal natural ingredients (Sinomarin®)] on the viral load of nasopharynx in patients hospitalized with severe COVID-19 pneumonia. We conducted a prospective, randomized, controlled trial from June 2022 to December 2022. We allocated 56 patients with COVID-19 pneumonia into two groups (28 in each group)—the hypertonic seawater group [nasal irrigations with a hypertonic seawater solution (Sinomarin®) every 4 h for 16 h per day, for two consecutive days] and the control group (no nasal irrigations). A second nasopharyngeal swab was collected 48 h after the baseline nasopharyngeal swab (8 h after the last wash in the hypertonic seawater group) to estimate the SARS-CoV-2 viral load as determined by cycle threshold (Ct) values. In the hypertonic seawater group, the mean Ct values significantly increased two days after the initial measurement [ΔCt 48−0 h = 3.86 ± 3.03 cycles, p < 0.001 (95%CI: 2.69 to 5.04)]. No significant differences in the Ct values were observed in the control group [ΔCt 48−0 h = −0.14 ± 4.29, p = 0.866 (95%CI: −1.80 to −1.52)]. At follow-up, 17 patients from the hypertonic seawater group had negative test results compared to only 9 patients from the control group (p = 0.03). Nasal irrigations with a hypertonic seawater solution containing algal and herbal natural ingredients significantly decreased nasopharyngeal viral load and the detection time of SARS-CoV-2 in the nasal cavity.</jats:p>",
"alternative-id": [
"jpm13071093"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8846-519X",
"affiliation": [
{
"name": "Department of Emergency Medicine, Faculty of Medicine, University of Thessaly, 41500 Larissa, Greece"
},
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, 41500 Larissa, Greece"
}
],
"authenticated-orcid": false,
"family": "Pantazopoulos",
"given": "Ioannis",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7634-4665",
"affiliation": [
{
"name": "Department of Anaesthesiology, Faculty of Medicine, University of Thessaly, 41500 Larissa, Greece"
},
{
"name": "Institute for Translational Medicine and Therapeutics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA"
},
{
"name": "Outcomes Research Consortium, Cleveland, OH 44195, USA"
}
],
"authenticated-orcid": false,
"family": "Chalkias",
"given": "Athanasios",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, 41500 Larissa, Greece"
}
],
"family": "Miziou",
"given": "Angeliki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Faculty of Medicine, University of Thessaly, 41500 Larissa, Greece"
}
],
"family": "Spanos",
"given": "Michalis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, 41500 Larissa, Greece"
}
],
"family": "Gerovasileiou",
"given": "Efrosyni",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2885-4510",
"affiliation": [
{
"name": "Faculty of Nursing, University of Thessaly, 45550 Larissa, Greece"
}
],
"authenticated-orcid": false,
"family": "Rouka",
"given": "Erasmia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9541-1010",
"affiliation": [
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, 41500 Larissa, Greece"
}
],
"authenticated-orcid": false,
"family": "Gourgoulianis",
"given": "Konstantinos",
"sequence": "additional"
}
],
"container-title": "Journal of Personalized Medicine",
"container-title-short": "JPM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
4
]
],
"date-time": "2023-07-04T05:32:18Z",
"timestamp": 1688448738000
},
"deposited": {
"date-parts": [
[
2023,
7,
4
]
],
"date-time": "2023-07-04T07:26:28Z",
"timestamp": 1688455588000
},
"indexed": {
"date-parts": [
[
2023,
7,
5
]
],
"date-time": "2023-07-05T04:24:25Z",
"timestamp": 1688531065331
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2023,
7,
3
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2023,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
7,
3
]
],
"date-time": "2023-07-03T00:00:00Z",
"timestamp": 1688342400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2075-4426/13/7/1093/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1093",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
7,
3
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
3
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1002/jmv.27643",
"article-title": "The Emergence and Epidemic Characteristics of the Highly Mutated SARS-CoV-2 Omicron Variant",
"author": "Tian",
"doi-asserted-by": "crossref",
"first-page": "2376",
"journal-title": "J. Med. Virol.",
"key": "ref_1",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1002/mco2.126",
"article-title": "SARS-CoV-2 Omicron Variant: Immune Escape and Vaccine Development",
"author": "Ao",
"doi-asserted-by": "crossref",
"first-page": "e126",
"journal-title": "MedComm",
"key": "ref_2",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"article-title": "SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes",
"author": "Sungnak",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "Nat. Med.",
"key": "ref_3",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1002/eji.202249805",
"article-title": "Differential Susceptibility to SARS-CoV-2 in the Normal Nasal Mucosa and in Chronic Sinusitis",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1308",
"journal-title": "Eur. J. Immunol.",
"key": "ref_4",
"volume": "52",
"year": "2022"
},
{
"article-title": "SARS-CoV-2 Viral Load and Shedding Kinetics",
"author": "Puhach",
"first-page": "147",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_5",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1155/2022/8794127",
"article-title": "Nasopharyngeal Wash with Normal Saline Decreases SARS-CoV-2 Viral Load: A Randomized Pilot Controlled Trial",
"author": "Pantazopoulos",
"doi-asserted-by": "crossref",
"first-page": "8794127",
"journal-title": "Can. Respir. J.",
"key": "ref_6",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.1177/1945892418773566",
"article-title": "Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-Analysis",
"author": "Kanjanawasee",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Am. J. Rhinol. Allergy",
"key": "ref_7",
"volume": "32",
"year": "2018"
},
{
"DOI": "10.1038/s41421-020-00192-8",
"article-title": "Sulfated Polysaccharides Effectively Inhibit SARS-CoV-2 in Vitro",
"author": "Kwon",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Cell Discov.",
"key": "ref_8",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.carbpol.2022.119551",
"article-title": "A State-of-the-Art Review on Fucoidan as an Antiviral Agent to Combat Viral Infections",
"author": "Pradhan",
"doi-asserted-by": "crossref",
"first-page": "119551",
"journal-title": "Carbohydr. Polym.",
"key": "ref_9",
"volume": "291",
"year": "2022"
},
{
"DOI": "10.1001/jama.2013.281053",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "(2013). World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA, 310, 2191–2194."
},
{
"key": "ref_11",
"unstructured": "(2023, June 22). Clinical Spectrum, Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/."
},
{
"key": "ref_12",
"unstructured": "(2023, June 22). Hospitalized Adults: Therapeutic Management, Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/hospitalized-adults--therapeutic-management/."
},
{
"DOI": "10.1136/bmj.c869",
"article-title": "CONSORT 2010 Explanation and Elaboration: Updated Guidelines for Reporting Parallel Group Randomised Trials",
"author": "Moher",
"doi-asserted-by": "crossref",
"first-page": "c869",
"journal-title": "BMJ",
"key": "ref_13",
"volume": "340",
"year": "2010"
},
{
"DOI": "10.1080/21688370.2021.1943274",
"article-title": "Virus-Associated Disruption of Mucosal Epithelial Tight Junctions and Its Role in Viral Transmission and Spread",
"author": "Tugizov",
"doi-asserted-by": "crossref",
"first-page": "1943274",
"journal-title": "Tissue Barriers",
"key": "ref_14",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1159/000528969",
"article-title": "Epithelial Barrier in the Nasal Mucosa, Related Risk Factors and Diseases",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Int. Arch. Allergy Immunol.",
"key": "ref_15",
"volume": "184",
"year": "2023"
},
{
"DOI": "10.3389/fmed.2020.594495",
"article-title": "Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2",
"author": "Salamanna",
"doi-asserted-by": "crossref",
"first-page": "594495",
"journal-title": "Front. Med.",
"key": "ref_16",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1021/bi035268s",
"article-title": "Angiotensin-Converting Enzyme-2 (ACE2): Comparative Modeling of the Active Site, Specificity Requirements, and Chloride Dependence",
"author": "Guy",
"doi-asserted-by": "crossref",
"first-page": "13185",
"journal-title": "Biochemistry",
"key": "ref_17",
"volume": "42",
"year": "2003"
},
{
"DOI": "10.1021/acsptsci.1c00080",
"article-title": "Inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 Replication by Hypertonic Saline Solution in Lung and Kidney Epithelial Cells",
"author": "Machado",
"doi-asserted-by": "crossref",
"first-page": "1514",
"journal-title": "ACS Pharmacol. Transl. Sci.",
"key": "ref_18",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1007/s00228-021-03102-3",
"article-title": "Essentials in Saline Pharmacology for Nasal or Respiratory Hygiene in Times of COVID-19",
"author": "Huijghebaert",
"doi-asserted-by": "crossref",
"first-page": "1275",
"journal-title": "Eur. J. Clin. Pharmacol.",
"key": "ref_19",
"volume": "77",
"year": "2021"
},
{
"DOI": "10.1080/07391102.2020.1754293",
"article-title": "A Review on the Cleavage Priming of the Spike Protein on Coronavirus by Angiotensin-Converting Enzyme-2 and Furin",
"author": "Hasan",
"doi-asserted-by": "crossref",
"first-page": "3025",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_20",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1016/j.abb.2009.05.013",
"article-title": "A Study of Human Furin Specificity Using Synthetic Peptides Derived from Natural Substrates, and Effects of Potassium Ions",
"author": "Izidoro",
"doi-asserted-by": "crossref",
"first-page": "105",
"journal-title": "Arch. Biochem. Biophys.",
"key": "ref_21",
"volume": "487",
"year": "2009"
},
{
"DOI": "10.1038/s41598-018-31936-y",
"article-title": "Antiviral Innate Immune Response in Non-Myeloid Cells Is Augmented by Chloride Ions via an Increase in Intracellular Hypochlorous Acid Levels",
"author": "Ramalingam",
"doi-asserted-by": "crossref",
"first-page": "13630",
"journal-title": "Sci. Rep.",
"key": "ref_22",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1177/2058738420941757",
"article-title": "Could Nasal Irrigation and Oral Rinse Reduce the Risk for COVID-19 Infection?",
"author": "Casale",
"doi-asserted-by": "crossref",
"first-page": "2058738420941757",
"journal-title": "Int. J. Immunopathol. Pharmacol.",
"key": "ref_23",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1038/s41598-018-37703-3",
"article-title": "A Pilot, Open Labelled, Randomised Controlled Trial of Hypertonic Saline Nasal Irrigation and Gargling for the Common Cold",
"author": "Ramalingam",
"doi-asserted-by": "crossref",
"first-page": "1015",
"journal-title": "Sci. Rep.",
"key": "ref_24",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.7189/jogh.10.010332",
"article-title": "Hypertonic Saline Nasal Irrigation and Gargling Should Be Considered as a Treatment Option for COVID-19",
"author": "Ramalingam",
"doi-asserted-by": "crossref",
"first-page": "010332",
"journal-title": "J. Glob. Health",
"key": "ref_25",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1001/archoto.2007.19",
"article-title": "Efficacy of Isotonic Nasal Wash (Seawater) in the Treatment and Prevention of Rhinitis in Children",
"author": "Slapak",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "Arch. Otolaryngol. Head Neck Surg.",
"key": "ref_26",
"volume": "134",
"year": "2008"
},
{
"DOI": "10.1007/s12070-021-02749-9",
"article-title": "Comparison of the Healing Effect of Nasal Saline Irrigation with Triamcinolone Acetonide Versus Nasal Saline Irrigation Alone in COVID-19 Related Olfactory Dysfunction: A Randomized Controlled Study",
"author": "Yildiz",
"doi-asserted-by": "crossref",
"first-page": "3022",
"journal-title": "Indian J. Otolaryngol. Head Neck Surg.",
"key": "ref_27",
"volume": "74",
"year": "2022"
},
{
"article-title": "Efficacy and Safety of a Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients in Patients with COVID-19",
"author": "Gangadi",
"first-page": "112",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref_28",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics14112502",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Cegolon, L., Mastrangelo, G., Emanuelli, E., Camerotto, R., Spinato, G., and Frezza, D. (2022). Early Negativization of SARS-CoV-2 Infection by Nasal Spray of Seawater plus Additives: The RENAISSANCE Open-Label Controlled Clinical Trial. Pharmaceutics, 14."
},
{
"DOI": "10.3389/fpubh.2022.1046112",
"article-title": "Effect of Nasal Irrigation in Adults Infected with Omicron Variant of COVID-19: A Quasi-Experimental Study",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1046112",
"journal-title": "Front. Public Health",
"key": "ref_30",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.5582/bst.2022.01495",
"article-title": "Can Nasal Irrigation with Chlorine Dioxide Be Considered as a Potential Alternative Therapy for Respiratory Infectious Diseases? The Example of COVID-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Biosci. Trends",
"key": "ref_31",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1002/lio2.686",
"article-title": "Effects of Hypertonic Alkaline Nasal Irrigation on COVID-19",
"author": "Yilmaz",
"doi-asserted-by": "crossref",
"first-page": "1240",
"journal-title": "Laryngoscope Investig. Otolaryngol.",
"key": "ref_32",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1177/01455613221123737",
"doi-asserted-by": "crossref",
"key": "ref_33",
"unstructured": "Baxter, A.L., Schwartz, K.R., Johnson, R.W., Kuchinski, A.-M., Swartout, K.M., Srinivasa Rao, A.S.R., Gibson, R.W., Cherian, E., Giller, T., and Boomer, H. (2022). Rapid Initiation of Nasal Saline Irrigation to Reduce Severity in High-Risk COVID+ Outpatients. Ear. Nose. Throat J."
},
{
"DOI": "10.3389/fpubh.2023.1145669",
"article-title": "Efficacy of Nasal Irrigation and Oral Rinse with Sodium Bicarbonate Solution on Virus Clearance for COVID-19 Patients",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1145669",
"journal-title": "Front. Public Health",
"key": "ref_34",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1007/s12070-022-03106-0",
"article-title": "Virucidal Effect of Povidone Iodine on SARS-CoV-2 in Nasopharynx: An Open-Label Randomized Clinical Trial",
"author": "Banu",
"doi-asserted-by": "crossref",
"first-page": "3283",
"journal-title": "Indian J. Otolaryngol. Head Neck Surg.",
"key": "ref_35",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1128/spectrum.01656-22",
"article-title": "Selection of Ideal Reference Genes for Gene Expression Analysis in COVID-19 and Mucormycosis",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "e0165622",
"journal-title": "Microbiol. Spectr.",
"key": "ref_36",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2022.847407",
"article-title": "False-Positive Results of SARS-CoV-2 RT-PCR in Oropharyngeal Swabs From Vaccinators",
"author": "Kong",
"doi-asserted-by": "crossref",
"first-page": "847407",
"journal-title": "Front. Med.",
"key": "ref_37",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciab684",
"article-title": "False Coronavirus Disease 2019 Cases Due to Contamination by Inactivated Virus Vaccine",
"author": "To",
"doi-asserted-by": "crossref",
"first-page": "1485",
"journal-title": "Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am.",
"key": "ref_38",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1128/spectrum.01477-22",
"article-title": "SARS-CoV-2: Challenges in Reconverting Diagnostic Laboratories to Combat the Pandemic",
"doi-asserted-by": "crossref",
"first-page": "e0147722",
"journal-title": "Microbiol. Spectr.",
"key": "ref_39",
"volume": "10",
"year": "2022"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2075-4426/13/7/1093"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": "A Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients Reduces Viral Load and SARS-CoV-2 Detection Time in the Nasal Cavity",
"type": "journal-article",
"volume": "13"
}
