Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients
et al., Ear, Nose & Throat Journal, doi:10.1177/01455613221123737, NCT04559035, Aug 2022
32nd treatment shown to reduce risk in
November 2021, now with p = 0.0000000039 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
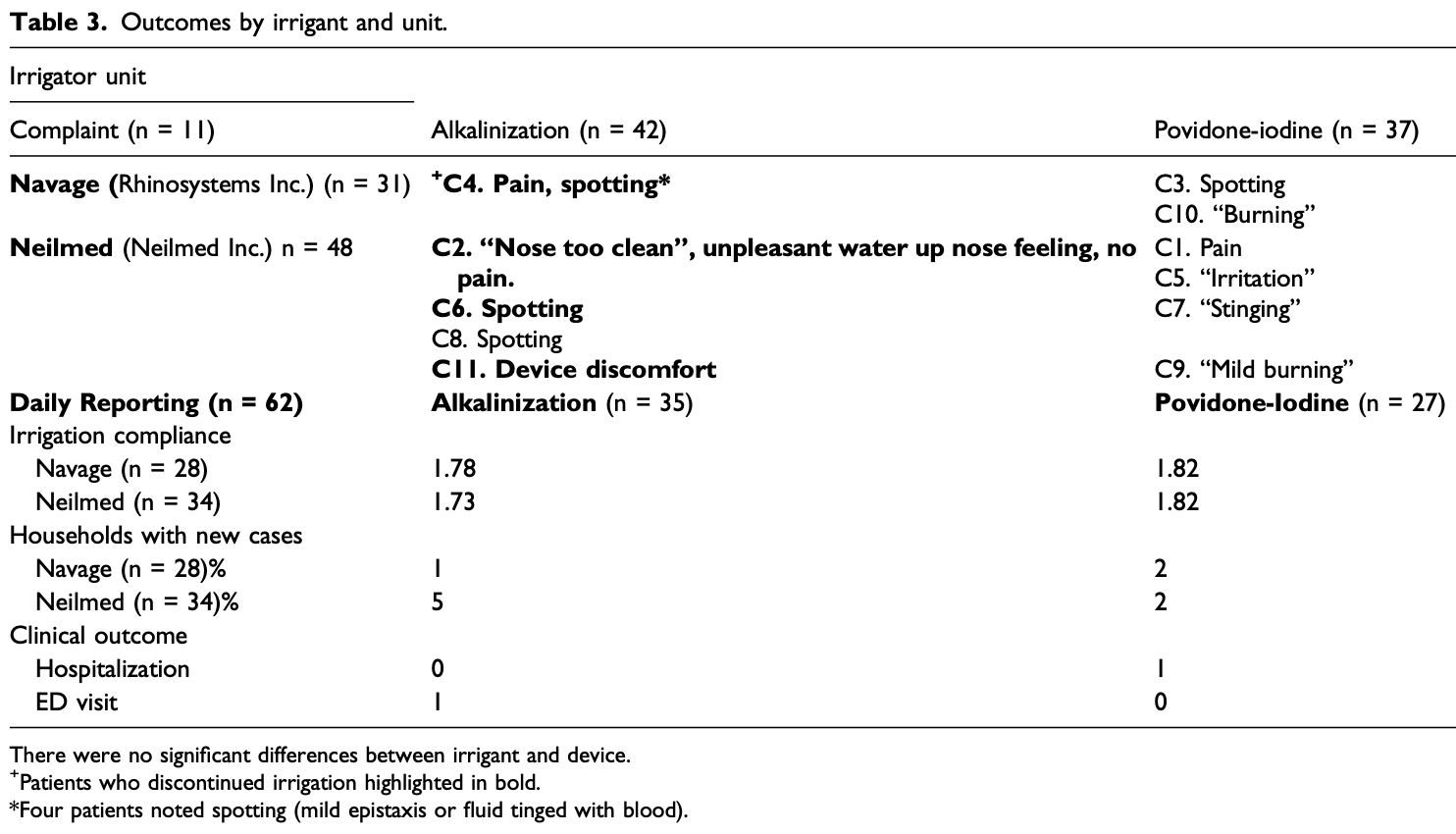
Small RCT 79 PCR+ patients 55+ comparing pressure-based nasal irrigation with povidone-iodine and sodium bicarbonate, showing significantly lower hospitalization when compared with CDC data.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers alkalinization and povidone-iodine.
|
risk of hospitalization, 65.3% lower, RR 0.35, p = 1.00, treatment 0 of 37 (0.0%), control 1 of 42 (2.4%), NNT 42, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), vs. PVP-I.
|
|
risk of hospitalization, 94.1% lower, RR 0.06, p = 0.004, nasal irrigation vs. CDC data.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Baxter et al., 25 Aug 2022, Randomized Controlled Trial, USA, peer-reviewed, 12 authors, study period 24 September, 2020 - 21 December, 2020, this trial compares with another treatment - results may be better when compared to placebo, trial NCT04559035 (history).
Contact: abaxter@augusta.edu, @AmyBaxterMD.
Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients
Ear, Nose & Throat Journal, doi:10.1177/01455613221123737
Objective: To determine whether initiating saline nasal irrigation after COVID-19 diagnosis reduces hospitalization and death in highrisk outpatients compared with observational controls, and if irrigant composition impacts severity. Methods: Participants 55 and older were enrolled within 24 hours of a + PCR COVID-19 test between September 24 and December 21, 2020. Among 826 screened, 79 participants were enrolled and randomly assigned to add 2.5 mL povidone-iodine 10% or 2.5 mL sodium bicarbonate to 240 mL of isotonic nasal irrigation twice daily for 14 days. The primary outcome was hospitalization or death from COVID-19 within 28 days of enrollment by daily self-report confirmed with phone calls and hospital records, compared to the CDC Surveillance Dataset covering the same time. Secondary outcomes compared symptom resolution by irrigant additive. Results: Seventy-nine high-risk participants were enrolled (mean [SD] age, 64 [8] years; 36 [46%] women; 71% Non-Hispanic White), with mean BMI 30.3. Analyzed by intention-to-treat, by day 28, COVID-19 symptoms resulted in one ED visit and no hospitalizations in 42 irrigating with alkalinization, one hospitalization of 37 in the povidone-iodine group, (1.27%) and no deaths. Of nearly three million CDC cases, 9.47% were known to be hospitalized, with an additional 1.5% mortality in those without hospitalization data. Age, sex, and percentage with pre-existing conditions did not significantly differ by exact binomial test from the CDC dataset, while reported race and hospitalization rate did. The total risk of hospitalization or death (11%) was 8.57 times that of enrolled nasal irrigation participants (SE = 2.74; P = .006). Sixty-two participants completed daily surveys (78%), averaging 1.8 irrigations/day. Eleven reported irrigation-related complaints and four discontinued use. Symptom resolution was more likely for those reporting twice daily irrigation (X 2 = 8.728, P = .0031) regardless of additive. Conclusion: SARS-CoV-2+ participants initiating nasal irrigation were over 8 times less likely to be hospitalized than the national rate.
Ethical approval The study was approved by the institutional review board at Augusta University in Augusta, Georgia and was registered at Clinical-Trials.gov NCT04559035.
Informed consent Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs Amy L Baxter https://orcid.org/0000-0001-7123-0733 Taylor Giller https://orcid.org/0000-0002-0350-8243 Richard Schwartz https://orcid.org/0000-0003-0947-3034
Supplemental Material Supplemental material for this article is available online.
References
Brandt, Beck, Mersha, Air pollution, racial disparities, and COVID-19 mortality, J Allergy Clin Immunol
Bunyavanich, Do, Vicencio, Nasal gene expression of angiotensin-converting enzyme 2 in children and adults, JAMA
Burton, Clarkson, Goulao, Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them, Cochrane Database Syst Rev
Burton, Fort, Seoane, Hospitalization and mortality among black patients and white patients with Covid-19, N Engl J Med
Cardenas, Rifas-Shiman, Sordillo, DNA methylation architecture of the ACE2 gene in nasal cells of children, Sci Rep
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Chen, Shen, Rowan, Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication, Eur Respir J
Eggers, Eickmann, Zorn, Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus ankara (MVA), Infect Dis Therapy
Eggers, Koburger-Janssen, Eickmann, Zorn, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/ mouthwash against respiratory and oral tract pathogens, Infect Dis Therapy
Esther, Kimura, Mikami, Pharmacokinetic-based failure of a detergent virucidal for severe acute respiratory syndromecoronavirus-2 (SARS-CoV-2) nasal infections: A preclinical study and randomized controlled trial, Int Forum Allergy Rhinol
Farrell, Klatt-Cromwell, Schneider, Benefits and safety of nasal saline irrigations in a pandemic-washing COVID-19 away, JAMA Otolaryngol Head Neck Surg
Faíco-Filho, Passarelli, Bellei, Is higher viral load in SARS-CoV-2 associated with death?, Am J Trop Med Hyg
Frank, Capriotti, Brown, Tessema, Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 Era, Ear Nose Throat J
Gallagher, Escarmis, Buchmeier, Alteration of the pH dependence of coronavirus-induced cell fusion: effect of mutations in the spike glycoprotein, J Virol
Gandhi, Rutherford, Facial masking for Covid-19--potential for "variolation" as we await a vaccine, N Engl J Med
Goyal, Reeves, Cardozo-Ojeda, Schiffer, Mayer, Wrong person, place and time: viral load and contact network structure predict SARS-CoV-2 transmission and superspreading events
Guenezan, Garcia, Strasters, Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: A randomized clinical trial, JAMA Otolaryngol Head Neck Surg
Gutiérrez-García, De, Cerda-Ángeles, Cabrera-Licona, Delgado-Enciso et al., Nasopharyngeal and oropharyngeal rinses with neutral electrolyzed water prevents COVID-19 in front-line health professionals: A randomized, open-label, controlled trial in a general hospital in Mexico City, Biomed Rep
Hou, Okuda, Edwards, SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell
Huijghebaert, Hoste, Vanham, Essentials in saline pharmacology for nasal or respiratory hygiene in times of COVID-19, Eur J Clin Pharmacol
Keeler, Patki, Woodard, Do, A computational study of nasal spray deposition pattern in four ethnic groups, J Aerosol Med Pulmonary Drug Deliv
Khan, Parab, Paranjape, Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid-19 pandemic, Am J Otolaryngol
Kimura, Freeman, Wessinger, Interim analysis of an open-label randomized controlled trial evaluating nasal irrigations in non-hospitalized patients with coronavirus disease 2019, Int Forum Allergy Rhinol
Krantz, Rao, Level of underreporting including underdiagnosis before the first peak of COVID-19 in various countries: Preliminary retrospective results based on wavelets and deterministic modeling, Infect Control Hosp Epidemiol
Lei, Xu, Xiao, Wu, Shu, Household transmission of COVID-19-a systematic review and meta-analysis, J Infect
Levin, Hanage, Owusu-Boaitey, Cochran, Walsh et al., Assessing the age specificity of infection fatality rates for COVID-19: systematic review, metaanalysis, and public policy implications, Eur J Epidemiol
Li, Li, Hypertonic saline and aprotinin based blockage of SARS-CoV-2 specific furin site cleavage by inhibition of nasal protease activity
Likus, Bajor, Gruszczyńska, Baron, Markowski, Nasal region dimensions in children: a CT study and clinical implications, BioMed Res Int
Loftus, Wise, Nieto, Panella, Aiken et al., Intranasal volume increases with age: Computed tomography volumetric analysis in adults, Laryngoscope
Mackey, Ayers, Kondo, Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review, Ann Intern Med
Madewell, Yang, Longini, Halloran, Dean, a systematic review and meta-analysis of secondary attack rate
Meng, Deng, Dai, Meng, COVID-19 and anosmia: A review based on up-to-date knowledge, Am J Otolaryngol
Palaiodimos, Kokkinidis, Li, Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, Metabolism
Panchmatia, Payandeh, Al-Salman, The efficacy of diluted topical povidone-iodine rinses in the management of recalcitrant chronic rhinosinusitis: a prospective cohort study, Eur Arch Oto-Rhino-Laryngol
Panta, Chatti, Andhavarapu, Do saline water gargling and nasal irrigation confer protection against COVID-19? Explore
Pelletier, Tessema, Frank, Westover, Brown et al., Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndromecoronavirus 2 (SARS-CoV-2), Ear Nose Throat J
Piromchai, Puvatanond, Kirtsreesakul, Chaiyasate, Thanaviratananich, Effectiveness of nasal irrigation devices: a Thai multicentre survey, PeerJ, doi:10.7717/peerj.7000
Radulesco, Lechien, Saussez, Hopkins, Michel, Safety and impact of nasal lavages during viral infections such as SARS-CoV-2, Ear Nose Throat J
Radzikowska, Ding, Tan, Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors, Allergy
Ramalingam, Graham, Dove, Morrice, Sheikh, A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold, Sci Rep
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TO-GETHER randomised, platform clinical trial, Lancet Glob Health
Rieser, Moskal, Cost efficacy of methicillin-resistant Staphylococcus aureus decolonization with intranasal povidone-iodine, J Arthroplasty
Sajuthi, Deford, Jackson, Type 2 and interferon inflammation strongly regulate SARS-CoV-2 related gene expression in the airway epithelium
Scudellari, How the coronavirus infects cells -and why Delta is so dangerous, Nature
Sungnak, Huang, Becavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med
Tenforde, Kim, Lindsell, Symptom Duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network -United States, March, MMWR Morb Mortal Wkly Rep
Viner, Mytton, Bonell, Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis, JAMA Pediatr
Yoder, Straif-Bourgeois, Roy, Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water, Clin Infect Dis
DOI record:
{
"DOI": "10.1177/01455613221123737",
"ISSN": [
"0145-5613",
"1942-7522"
],
"URL": "http://dx.doi.org/10.1177/01455613221123737",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p> To determine whether initiating saline nasal irrigation after COVID-19 diagnosis reduces hospitalization and death in high-risk outpatients compared with observational controls, and if irrigant composition impacts severity. </jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p> Participants 55 and older were enrolled within 24 hours of a + PCR COVID-19 test between September 24 and December 21, 2020. Among 826 screened, 79 participants were enrolled and randomly assigned to add 2.5 mL povidone-iodine 10% or 2.5 mL sodium bicarbonate to 240 mL of isotonic nasal irrigation twice daily for 14 days. The primary outcome was hospitalization or death from COVID-19 within 28 days of enrollment by daily self-report confirmed with phone calls and hospital records, compared to the CDC Surveillance Dataset covering the same time. Secondary outcomes compared symptom resolution by irrigant additive. </jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p> Seventy-nine high-risk participants were enrolled (mean [SD] age, 64 [8] years; 36 [46%] women; 71% Non-Hispanic White), with mean BMI 30.3. Analyzed by intention-to-treat, by day 28, COVID-19 symptoms resulted in one ED visit and no hospitalizations in 42 irrigating with alkalinization, one hospitalization of 37 in the povidone-iodine group, (1.27%) and no deaths. Of nearly three million CDC cases, 9.47% were known to be hospitalized, with an additional 1.5% mortality in those without hospitalization data. Age, sex, and percentage with pre-existing conditions did not significantly differ by exact binomial test from the CDC dataset, while reported race and hospitalization rate did. The total risk of hospitalization or death (11%) was 8.57 times that of enrolled nasal irrigation participants (SE = 2.74; P = .006). Sixty-two participants completed daily surveys (78%), averaging 1.8 irrigations/day. Eleven reported irrigation-related complaints and four discontinued use. Symptom resolution was more likely for those reporting twice daily irrigation ( X<jats:sup>2</jats:sup> = 8.728, P = .0031) regardless of additive. </jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p> SARS-CoV-2+ participants initiating nasal irrigation were over 8 times less likely to be hospitalized than the national rate. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/01455613221123737"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7123-0733",
"affiliation": [
{
"name": "Department of Emergency Medicine, Augusta University, Augusta, GA, USA"
}
],
"authenticated-orcid": false,
"family": "Baxter",
"given": "Amy L",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Edinburgh Napier University, Edinburgh, UK"
}
],
"family": "Schwartz",
"given": "Kyle R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical College of Georgia, Augusta University, Augusta, GA, USA"
}
],
"family": "Johnson",
"given": "Ryan W",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Augusta University, Augusta, GA, USA"
}
],
"family": "Kuchinski",
"given": "Ann-Marie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Psychology, Georgia State University, Atlanta, GA, USA"
}
],
"family": "Swartout",
"given": "Kevin M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory for Theory and Mathematical Modeling, Department of Medicine-Division of Infectious Diseases, Medical College of Georgia, Augusta University, Augusta, GA, USA"
},
{
"name": "Department of Mathematics, Augusta University, Augusta, GA, USA"
}
],
"family": "Srinivasa Rao",
"given": "Arni S R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Augusta University, Augusta, GA, USA"
}
],
"family": "Gibson",
"given": "Robert W",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical College of Georgia, Augusta University, Augusta, GA, USA"
}
],
"family": "Cherian",
"given": "Erica",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0350-8243",
"affiliation": [
{
"name": "Medical College of Georgia, Augusta University, Augusta, GA, USA"
}
],
"authenticated-orcid": false,
"family": "Giller",
"given": "Taylor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Augusta University, Augusta, GA, USA"
}
],
"family": "Boomer",
"given": "Houlton",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Augusta University, Augusta, GA, USA"
}
],
"family": "Lyon",
"given": "Matthew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0947-3034",
"affiliation": [
{
"name": "Department of Emergency Medicine, Augusta University, Augusta, GA, USA"
}
],
"authenticated-orcid": false,
"family": "Schwartz",
"given": "Richard",
"sequence": "additional"
}
],
"container-title": "Ear, Nose & Throat Journal",
"container-title-short": "Ear Nose Throat J",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
25
]
],
"date-time": "2022-08-25T18:40:10Z",
"timestamp": 1661452810000
},
"deposited": {
"date-parts": [
[
2022,
8,
25
]
],
"date-time": "2022-08-25T18:40:36Z",
"timestamp": 1661452836000
},
"funder": [
{
"name": "Bernard and Anne Gray Donor Advised Fund"
},
{
"award": [
"Supplied Materials"
],
"name": "Neilmed Inc."
},
{
"DOI": "10.13039/100010716",
"doi-asserted-by": "publisher",
"name": "Community Foundation for Greater Atlanta"
},
{
"award": [
"Supplied Materials"
],
"name": "Rhinosystems Inc."
}
],
"indexed": {
"date-parts": [
[
2022,
8,
25
]
],
"date-time": "2022-08-25T19:12:04Z",
"timestamp": 1661454724062
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
25
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
25
]
],
"date-time": "2022-08-25T00:00:00Z",
"timestamp": 1661385600000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/01455613221123737",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/01455613221123737",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/01455613221123737",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "014556132211237",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2022,
8,
25
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
25
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "publisher",
"key": "bibr1-01455613221123737"
},
{
"DOI": "10.1038/d41586-021-02039-y",
"doi-asserted-by": "publisher",
"key": "bibr2-01455613221123737"
},
{
"author": "Li C",
"journal-title": "bioRxiv",
"key": "bibr3-01455613221123737",
"year": "2021"
},
{
"DOI": "10.1038/s41598-018-37703-3",
"doi-asserted-by": "publisher",
"key": "bibr4-01455613221123737"
},
{
"author": "Burton MJ",
"first-page": "Cd013627",
"journal-title": "Cochrane Database Syst Rev",
"key": "bibr5-01455613221123737",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1001/jamaoto.2020.1622",
"doi-asserted-by": "publisher",
"key": "bibr6-01455613221123737"
},
{
"DOI": "10.1177/0145561320932318",
"doi-asserted-by": "publisher",
"key": "bibr7-01455613221123737"
},
{
"DOI": "10.1007/s40121-015-0091-9",
"doi-asserted-by": "publisher",
"key": "bibr8-01455613221123737"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"doi-asserted-by": "publisher",
"key": "bibr9-01455613221123737"
},
{
"author": "Pelletier JS",
"first-page": "145561320957237",
"journal-title": "Ear Nose Throat J",
"key": "bibr10-01455613221123737",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1128/jvi.65.4.1916-1928.1991",
"doi-asserted-by": "publisher",
"key": "bibr11-01455613221123737"
},
{
"DOI": "10.1016/j.jinf.2020.08.033",
"doi-asserted-by": "publisher",
"key": "bibr12-01455613221123737"
},
{
"author": "Madewell ZJ",
"journal-title": "medRxiv",
"key": "bibr13-01455613221123737",
"year": "2020"
},
{
"DOI": "10.1007/s10654-020-00698-1",
"doi-asserted-by": "publisher",
"key": "bibr14-01455613221123737"
},
{
"author": "Control CfD",
"key": "bibr15-01455613221123737",
"volume-title": "CDC COVID-19 Case Surveillance Public Use Data"
},
{
"DOI": "10.7717/peerj.7000",
"doi-asserted-by": "publisher",
"key": "bibr16-01455613221123737"
},
{
"DOI": "10.15585/mmwr.mm6930e1",
"doi-asserted-by": "publisher",
"key": "bibr17-01455613221123737"
},
{
"DOI": "10.1002/alr.22703",
"doi-asserted-by": "publisher",
"key": "bibr18-01455613221123737"
},
{
"DOI": "10.1016/j.amjoto.2020.102581",
"doi-asserted-by": "publisher",
"key": "bibr19-01455613221123737"
},
{
"DOI": "10.1183/13993003.01948-2020",
"doi-asserted-by": "publisher",
"key": "bibr20-01455613221123737"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"doi-asserted-by": "publisher",
"key": "bibr21-01455613221123737"
},
{
"DOI": "10.1016/j.metabol.2020.154262",
"doi-asserted-by": "publisher",
"key": "bibr22-01455613221123737"
},
{
"DOI": "10.1111/all.14429",
"doi-asserted-by": "publisher",
"key": "bibr23-01455613221123737"
},
{
"DOI": "10.1002/lary.26064",
"doi-asserted-by": "publisher",
"key": "bibr24-01455613221123737"
},
{
"DOI": "10.1016/j.jaci.2020.04.035",
"doi-asserted-by": "publisher",
"key": "bibr25-01455613221123737"
},
{
"DOI": "10.1001/jama.2020.8707",
"doi-asserted-by": "publisher",
"key": "bibr26-01455613221123737"
},
{
"DOI": "10.1155/2014/125810",
"doi-asserted-by": "publisher",
"key": "bibr27-01455613221123737"
},
{
"DOI": "10.1056/NEJMp2026913",
"doi-asserted-by": "publisher",
"key": "bibr28-01455613221123737"
},
{
"author": "Viner RM",
"first-page": "25",
"journal-title": "JAMA Pediatr",
"key": "bibr29-01455613221123737",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1089/jamp.2014.1205",
"doi-asserted-by": "publisher",
"key": "bibr30-01455613221123737"
},
{
"DOI": "10.1038/s41598-021-86494-7",
"doi-asserted-by": "publisher",
"key": "bibr31-01455613221123737"
},
{
"author": "Sajuthi SP",
"journal-title": "bioRxiv",
"key": "bibr32-01455613221123737",
"year": "2020"
},
{
"DOI": "10.1007/s00405-019-05628-w",
"doi-asserted-by": "publisher",
"key": "bibr33-01455613221123737"
},
{
"DOI": "10.1016/j.arth.2018.01.033",
"doi-asserted-by": "publisher",
"key": "bibr34-01455613221123737"
},
{
"DOI": "10.4269/ajtmh.20-0954",
"doi-asserted-by": "publisher",
"key": "bibr35-01455613221123737"
},
{
"author": "Goyal A",
"journal-title": "medRxiv",
"key": "bibr36-01455613221123737",
"year": "2020"
},
{
"DOI": "10.1016/j.amjoto.2020.102618",
"doi-asserted-by": "publisher",
"key": "bibr37-01455613221123737"
},
{
"DOI": "10.1177/0145561320950491",
"doi-asserted-by": "publisher",
"key": "bibr38-01455613221123737"
},
{
"DOI": "10.1016/j.explore.2020.09.010",
"doi-asserted-by": "publisher",
"key": "bibr39-01455613221123737"
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"doi-asserted-by": "publisher",
"key": "bibr40-01455613221123737"
},
{
"DOI": "10.1002/alr.22975",
"doi-asserted-by": "publisher",
"key": "bibr41-01455613221123737"
},
{
"DOI": "10.1007/s00228-021-03102-3",
"doi-asserted-by": "publisher",
"key": "bibr42-01455613221123737"
},
{
"DOI": "10.3892/br.2021.1494",
"doi-asserted-by": "publisher",
"key": "bibr43-01455613221123737"
},
{
"DOI": "10.1017/ice.2020.116",
"doi-asserted-by": "publisher",
"key": "bibr44-01455613221123737"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"doi-asserted-by": "publisher",
"key": "bibr45-01455613221123737"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "bibr46-01455613221123737"
},
{
"DOI": "10.7326/M20-6306",
"doi-asserted-by": "publisher",
"key": "bibr47-01455613221123737"
},
{
"DOI": "10.1056/NEJMsa2011686",
"doi-asserted-by": "publisher",
"key": "bibr48-01455613221123737"
},
{
"DOI": "10.1093/cid/cis626",
"doi-asserted-by": "publisher",
"key": "bibr49-01455613221123737"
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/01455613221123737"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Otorhinolaryngology"
],
"subtitle": [],
"title": "Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy"
}
baxter
