Intranasal Corticosteroids Are Associated with Better Outcomes in Coronavirus Disease 2019
et al., The Journal of Allergy and Clinical Immunology: In Practice, doi:10.1016/j.jaip.2021.08.007, Aug 2021
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
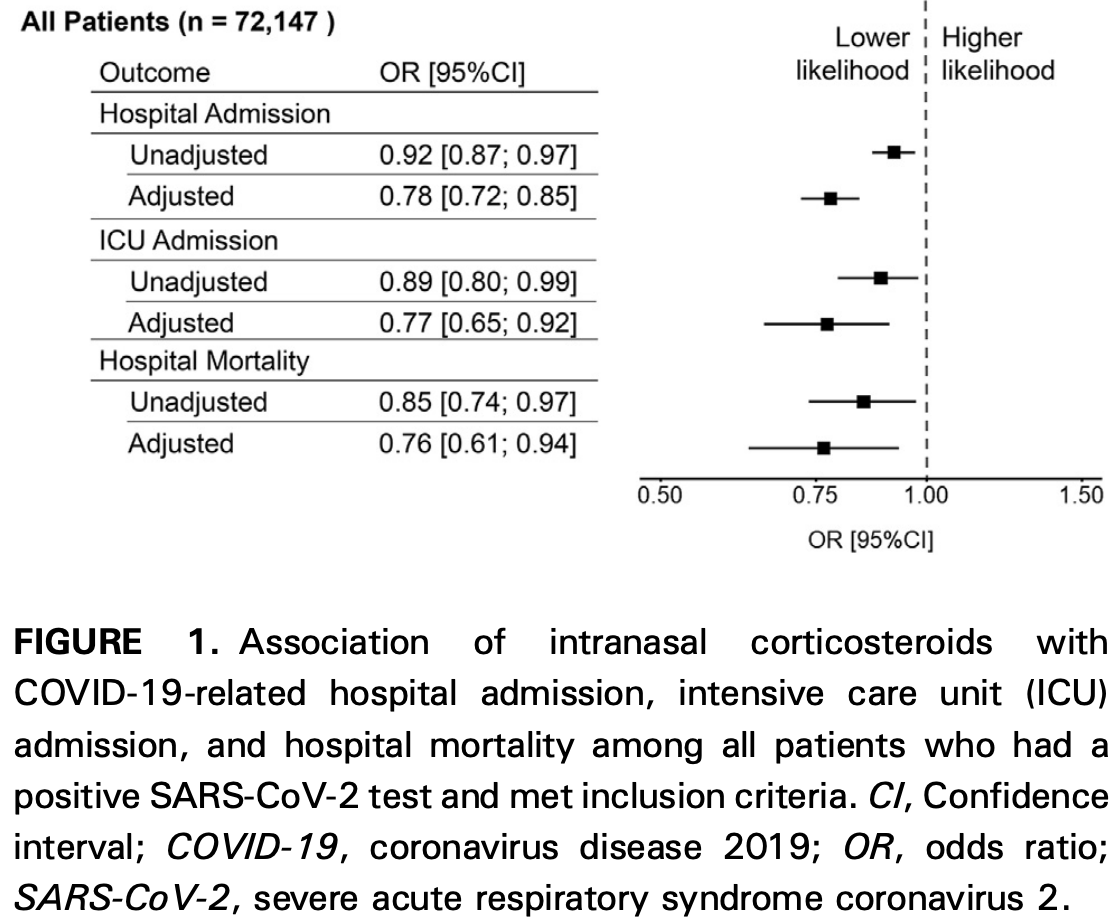
Retrospective 72,147 COVID-19+ patients in the USA, showing lower mortality, ICU admission, and hospitalization with intranasal corticosteroid use.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 23.5% lower, RR 0.76, p = 0.01, treatment 231 of 10,187 (2.3%), control 1,649 of 61,960 (2.7%), NNT 254, adjusted per study, odds ratio converted to relative risk, PSM.
|
|
risk of ICU admission, 22.3% lower, RR 0.78, p = 0.003, treatment 376 of 10,187 (3.7%), control 2,559 of 61,960 (4.1%), adjusted per study, odds ratio converted to relative risk, PSM.
|
|
risk of hospitalization, 18.9% lower, RR 0.81, p < 0.001, treatment 1,676 of 10,187 (16.5%), control 10,932 of 61,960 (17.6%), adjusted per study, odds ratio converted to relative risk, PSM.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Strauss et al., 23 Aug 2021, retrospective, USA, peer-reviewed, 8 authors, study period 1 April, 2020 - 31 March, 2021.
Intranasal Corticosteroids Are Associated with Better Outcomes in Coronavirus Disease 2019
The Journal of Allergy and Clinical Immunology: In Practice, doi:10.1016/j.jaip.2021.08.007
What is already known about this topic? Severe acute respiratory syndrome coronavirus 2 sites of entry are highly expressed in nasal epithelial cells. What does this article add to our knowledge? Intranasal corticosteroid (INCS) therapy is associated with a lower risk for coronavirus disease 2019 (COVID-19)-related hospitalization, admission to the intensive care unit, and in-hospital mortality. How does this study impact current management guidelines? Although our findings suggest a potential beneficial role for INCS use, randomized control trials are needed to determine if INCS reduces the risk for severe outcomes related to COVID-19. BACKGROUND: Sites of entry for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are highly expressed in nasal epithelial cells; however, little is known about the impact of intranasal corticosteroids (INCS) on coronavirus disease 2019 (COVID-19)-related outcomes. OBJECTIVE: To determine the association between baseline INCS use and COVID-19-related outcomes. METHODS: Using the Cleveland Clinic COVID-19 Research Registry, we performed a propensity score matching for treatment with INCS before SARS-CoV-2 infection (April 1, 2020, to March 31, 2021). Of the 82,096 individuals who tested positive, 72,147 met inclusion criteria. Our endpoints included the need for hospitalization, admission to the intensive care unit (ICU), or in-hospital mortality. RESULTS: Of the 12,608 (17.5%) who were hospitalized, 2935 (4.1%) required ICU admission and 1880 (2.6%) died during hospitalization. A significant proportion (n [ 10,187; 14.1%) were using INCS before SARS-CoV-2 infection. Compared with nonusers, INCS users demonstrated lower risk for hospitalization (adjusted odds ratio [OR] [95% confidence interval (CI)]: 0.78 [0.72; 0.85]), ICU admission (adjusted OR [95% CI]: 0.77 [0.65; 0.92]), and in-hospital mortality (adjusted OR [95% CI]: 0.76 [0.61; 0.94]). These findings were replicated in sensitivity analyses where patients on inhaled corticosteroids and those with allergic rhinitis were excluded. The beneficial effect of INCS was significant after adjustment for baseline blood eosinophil count (measured before SARS-CoV-2 testing) in a subset of 30,289 individuals. CONCLUSION: INCS therapy is associated with a lower risk for COVID-19-related hospitalization, ICU admission, or death. Future randomized control trials are needed to determine if INCS reduces the risk for severe outcomes related to COVID-19.
ONLINE REPOSITORY APPENDIX E1: DESCRIPTION OF THE REGISTRY The Cleveland Clinic COVID-19 Research Registry (CCCRR) This study uses the CCCRR, which includes all patients tested for SARS-CoV-2 at the Cleveland Clinic Healthcare System (CCHS). Data on patients' demographics, medications, comorbidities, history of SARS-CoV-2 exposure, national and international travel, disease manifestation on presentation, socioeconomic status, COVID-19-related therapy, disposition, and outcomes were extracted from electronic health records (EHR). E1 In addition, data related to hospitalization, critical care needs, and outcome were extracted for patients requiring hospitalization. Registry characterization and data collection reflect the clinical characteristics previously published on COVID-19. E2-E6 Uniform clinical templates were implemented across the CCHS using EHR to standardize the care of patients tested for SARS-CoV-2 and to facilitate data extraction. Data extraction from EHR (Epic; Epic Systems Corporation, Wisc) at the CCHS was performed manually by a trained research team and electronically using predefined processes that have been previously published. E7 This study and the CCCRR were both approved by the Cleveland Clinic Institutional Review Board (IRB #20-283 and 20-391).
COVID-19 testing protocols
References
Arentz, Yim, Klaff, Lokhandwala, Riedo et al., Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State, JAMA
Arentz, Yim, Klaff, Lokhandwala, Riedo et al., Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State, JAMA
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-final report, N Engl J Med
Bhatraju, Ghassemieh, Nichols, Kim, Jerome et al., Covid-19 in critically ill patients in the Seattle Region-case series, N Engl J Med
Bhatraju, Ghassemieh, Nichols, Kim, Jerome et al., Covid-19 in critically ill patients in the Seattle Region-case series, N Engl J Med
Bunyavanich, Do, Vicencio, Nasal gene expression of angiotensinconverting enzyme 2 in children and adults, JAMA
Camiolo, Gauthier, Kaminski, Ray, Wenzel, Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype, J Allergy Clinical Immunol
Chhiba, Patel, Vu, Chen, Guo et al., Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19, J Allergy Clin Immunol
Chowdhury, Jomo, Responding to the COVID-19 pandemic in developing countries: lessons from selected countries of the Global South, Development
Dennis, Mcgovern, Vollmer, Mateen, Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020, Crit Care Med
Fajnzylber, Regan, Coxen, Corry, Wong et al., SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun
Ferastraoaru, Hudes, Jerschow, Jariwala, Karagic et al., Eosinophilia in asthma patients is protective against severe COVID-19 illness, J Allergy Clinical Immunol Pract
Finney, Glanville, Farne, Aniscenko, Fenwick et al., Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon, J Allergy Clin Immunol
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Horwitz, Jones, Cerfolio, Francois, Greco et al., Trends in COVID-19 risk-adjusted mortality rates, J Hosp Med
Hou, Okuda, Edwards, Martinez, Asakura et al., SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell
Inselman, Rank, Zawada, Jeffery, Which people with asthma are most likely to be hospitalized with COVID-19 in the United States?, J Allergy Clinical Immunol Pract
Izquierdo, Almonacid, Gonzalez, Rio-Bermudez, Ancochea et al., The impact of COVID-19 on patients with asthma, Eur Respir J
Kasela, Ortega, Martorella, Garudadri, Nguyen et al., Genetic and non-genetic factors affecting the expression of COVID-19-relevant genes in the large airway epithelium, Genome Med
Keswani, Dhana, Rosenthal, Moore, Mahdavinia, Atopy is predictive of a decreased need for hospitalization for coronavirus disease 2019, Ann Allergy Asthma Immunol
Lauer, Grantz, Bi, Jones, Zheng et al., The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann Intern Med
Lipworth, Chan, Kuo, COVID-19: start with the nose, J Allergy Clin Immunol
Matsuyama, Kawase, Nao, Shirato, Ujike et al., The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells, J Virol
Mehta, Kalra, Nowacki, Anjewierden, Han et al., Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19), JAMA Cardiol
Milinovich, Kattan, Extracting and utilizing electronic health data from Epic for research, Ann Transl Med
Milinovich, Kattan, Extracting and utilizing electronic health data from Epic for research, Ann Transl Med
Ng, Tipih, Makoah, Vermeulen, Goedhals et al., Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis, mBio
Peters, Sajuthi, Deford, Christenson, Rios et al., COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids, Am J Resp Crit Care Med
Ramakrishnan, Nicolau, Jr, Langford, Mahdi et al., Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respir Med
References, Mehta, Kalra, Nowacki, Anjewierden et al., Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19), JAMA Cardiol
Rosenbaum, Db, The central role of propensity score in observational studies for causal effects, Biometrika
Rosenbaum, Observational Studies
Rubin, Using propensity score to help design observational studies: application to the tobacco litigation, Health Serv Outcomes Res Methodol
Shekerdemian, Mahmood, Wolfe, Riggs, Ross et al., Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units, JAMA Pediatr
Sungnak, Huang, Becavin, Berg, Queen et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med
Van Buuren, Groothuis-Oudshoorn, mice: Multivariate Imputation by Chained Equations in R, J Stat Softw
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Zein, Udeh, Teague, Koroukian, Schlitz et al., Impact of age and sex on outcomes and hospital cost of acute asthma in the United States, 2011-2012, PloS One
Zein, Whelan, Erzurum, Safety of influenza vaccine during COVID-19, J Clin Trans Sci
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.1016/j.jaip.2021.08.007",
"ISSN": [
"2213-2198"
],
"URL": "http://dx.doi.org/10.1016/j.jaip.2021.08.007",
"alternative-id": [
"S2213219821009065"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Intranasal Corticosteroids Are Associated with Better Outcomes in Coronavirus Disease 2019"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Journal of Allergy and Clinical Immunology: In Practice"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jaip.2021.08.007"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 American Academy of Allergy, Asthma & Immunology"
}
],
"author": [
{
"affiliation": [],
"family": "Strauss",
"given": "Ronald",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jawhari",
"given": "Nesreen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Attaway",
"given": "Amy H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Bo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jehi",
"given": "Lara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milinovich",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ortega",
"given": "Victor E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zein",
"given": "Joe G.",
"sequence": "additional"
}
],
"container-title": "The Journal of Allergy and Clinical Immunology: In Practice",
"container-title-short": "The Journal of Allergy and Clinical Immunology: In Practice",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"jaci-inpractice.org",
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
23
]
],
"date-time": "2021-08-23T15:56:52Z",
"timestamp": 1629734212000
},
"deposited": {
"date-parts": [
[
2022,
11,
19
]
],
"date-time": "2022-11-19T04:31:23Z",
"timestamp": 1668832283000
},
"funder": [
{
"DOI": "10.13039/100000002",
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
},
{
"DOI": "10.13039/100000050",
"award": [
"K08 HL133381"
],
"doi-asserted-by": "publisher",
"name": "National Heart, Lung, and Blood Institute"
},
{
"DOI": "10.13039/100000065",
"award": [
"R01 NS097719"
],
"doi-asserted-by": "publisher",
"name": "National Institute of Neurological Disorders and Stroke"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
8
]
],
"date-time": "2024-05-08T17:21:36Z",
"timestamp": 1715188896266
},
"is-referenced-by-count": 19,
"issue": "11",
"issued": {
"date-parts": [
[
2021,
11
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2021,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
1
]
],
"date-time": "2021-11-01T00:00:00Z",
"timestamp": 1635724800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213219821009065?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213219821009065?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "3934-3940.e9",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
11
]
]
},
"published-print": {
"date-parts": [
[
2021,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1128/mBio.03647-20",
"article-title": "Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis",
"author": "Ng",
"doi-asserted-by": "crossref",
"first-page": "e03647-20",
"journal-title": "mBio",
"key": "10.1016/j.jaip.2021.08.007_bib1",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2001737",
"article-title": "SARS-CoV-2 viral load in upper respiratory specimens of infected patients",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "1177",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jaip.2021.08.007_bib2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"article-title": "SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes",
"author": "Sungnak",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "Nat Med",
"key": "10.1016/j.jaip.2021.08.007_bib3",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.06.038",
"article-title": "COVID-19: start with the nose",
"author": "Lipworth",
"doi-asserted-by": "crossref",
"first-page": "1214",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/j.jaip.2021.08.007_bib4",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "10.1016/j.jaip.2021.08.007_bib5",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"article-title": "SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract",
"author": "Hou",
"doi-asserted-by": "crossref",
"first-page": "429",
"journal-title": "Cell",
"key": "10.1016/j.jaip.2021.08.007_bib6",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.8707",
"article-title": "Nasal gene expression of angiotensin-converting enzyme 2 in children and adults",
"author": "Bunyavanich",
"doi-asserted-by": "crossref",
"first-page": "2427",
"journal-title": "JAMA",
"key": "10.1016/j.jaip.2021.08.007_bib7",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.jaip.2020.12.045",
"article-title": "Eosinophilia in asthma patients is protective against severe COVID-19 illness",
"author": "Ferastraoaru",
"doi-asserted-by": "crossref",
"first-page": "1152",
"journal-title": "J Allergy Clinical Immunol Pract",
"key": "10.1016/j.jaip.2021.08.007_bib8",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202003-0821OC",
"article-title": "COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids",
"author": "Peters",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Am J Resp Crit Care Med",
"key": "10.1016/j.jaip.2021.08.007_bib9",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1186/s13073-021-00866-2",
"article-title": "Genetic and non-genetic factors affecting the expression of COVID-19-relevant genes in the large airway epithelium",
"author": "Kasela",
"doi-asserted-by": "crossref",
"first-page": "66",
"journal-title": "Genome Med",
"key": "10.1016/j.jaip.2021.08.007_bib10",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2020.09.034",
"article-title": "Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon",
"author": "Finney",
"doi-asserted-by": "crossref",
"first-page": "510",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/j.jaip.2021.08.007_bib11",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"article-title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial",
"author": "Ramakrishnan",
"doi-asserted-by": "crossref",
"first-page": "763",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.jaip.2021.08.007_bib12",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jamacardio.2020.1855",
"article-title": "Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19)",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1020",
"journal-title": "JAMA Cardiol",
"key": "10.1016/j.jaip.2021.08.007_bib13",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.21037/atm.2018.01.13",
"article-title": "Extracting and utilizing electronic health data from Epic for research",
"author": "Milinovich",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "Ann Transl Med",
"key": "10.1016/j.jaip.2021.08.007_bib14",
"volume": "6",
"year": "2018"
},
{
"DOI": "10.1001/jamapediatrics.2020.1948",
"article-title": "Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units",
"author": "Shekerdemian",
"doi-asserted-by": "crossref",
"first-page": "868",
"journal-title": "JAMA Pediatr",
"key": "10.1016/j.jaip.2021.08.007_bib15",
"volume": "174",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0157301",
"article-title": "Impact of age and sex on outcomes and hospital cost of acute asthma in the United States, 2011-2012",
"author": "Zein",
"doi-asserted-by": "crossref",
"first-page": "e0157301",
"journal-title": "PloS One",
"key": "10.1016/j.jaip.2021.08.007_bib16",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.1017/cts.2020.543",
"article-title": "Safety of influenza vaccine during COVID-19",
"author": "Zein",
"doi-asserted-by": "crossref",
"first-page": "E49",
"journal-title": "J Clin Trans Sci",
"key": "10.1016/j.jaip.2021.08.007_bib17",
"volume": "5",
"year": "2021"
},
{
"author": "Rosenbaum",
"key": "10.1016/j.jaip.2021.08.007_bib18",
"series-title": "Observational Studies",
"year": "2002"
},
{
"DOI": "10.1093/biomet/70.1.41",
"article-title": "The central role of propensity score in observational studies for causal effects",
"author": "Rosenbaum",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Biometrika",
"key": "10.1016/j.jaip.2021.08.007_bib19",
"volume": "70",
"year": "1983"
},
{
"DOI": "10.1056/NEJMoa2004500",
"article-title": "Covid-19 in critically ill patients in the Seattle Region—case series",
"author": "Bhatraju",
"doi-asserted-by": "crossref",
"first-page": "2012",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jaip.2021.08.007_bib20",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4326",
"article-title": "Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State",
"author": "Arentz",
"doi-asserted-by": "crossref",
"first-page": "1612",
"journal-title": "JAMA",
"key": "10.1016/j.jaip.2021.08.007_bib21",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jaip.2021.08.007_bib22",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA",
"key": "10.1016/j.jaip.2021.08.007_bib23",
"volume": "323",
"year": "2020"
},
{
"key": "10.1016/j.jaip.2021.08.007_bib24",
"unstructured": "Agency for Healthcare Research and Quality. Appendix I: Immunocompromised state diagnosis and procedure codes 2016. Accessed May 2, 2021. https://www.qualityindicators.ahrq.gov/Downloads/Modules/PSI/V60-ICD10/TechSpecs/PSI_Appendix_I.pdf"
},
{
"DOI": "10.12788/jhm.3552",
"article-title": "Trends in COVID-19 risk-adjusted mortality rates",
"author": "Horwitz",
"doi-asserted-by": "crossref",
"first-page": "90",
"journal-title": "J Hosp Med",
"key": "10.1016/j.jaip.2021.08.007_bib25",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0000000000004747",
"article-title": "Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020",
"author": "Dennis",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Crit Care Med",
"key": "10.1016/j.jaip.2021.08.007_bib26",
"volume": "49",
"year": "2021"
},
{
"article-title": "mice: Multivariate Imputation by Chained Equations in R",
"author": "Van Buuren",
"first-page": "1",
"journal-title": "J Stat Softw",
"key": "10.1016/j.jaip.2021.08.007_bib27",
"volume": "45",
"year": "2011"
},
{
"DOI": "10.1023/A:1020363010465",
"article-title": "Using propensity score to help design observational studies: application to the tobacco litigation",
"author": "Rubin",
"doi-asserted-by": "crossref",
"first-page": "169",
"journal-title": "Health Serv Outcomes Res Methodol",
"key": "10.1016/j.jaip.2021.08.007_bib28",
"volume": "2",
"year": "2001"
},
{
"DOI": "10.1183/13993003.03142-2020",
"article-title": "The impact of COVID-19 on patients with asthma",
"author": "Izquierdo",
"doi-asserted-by": "crossref",
"first-page": "2003142",
"journal-title": "Eur Respir J",
"key": "10.1016/j.jaip.2021.08.007_bib29",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.1016/j.jaip.2021.02.050",
"article-title": "Which people with asthma are most likely to be hospitalized with COVID-19 in the United States?",
"author": "Inselman",
"doi-asserted-by": "crossref",
"first-page": "2080",
"journal-title": "J Allergy Clinical Immunol Pract",
"key": "10.1016/j.jaip.2021.08.007_bib30",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2020.06.010",
"article-title": "Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19",
"author": "Chhiba",
"doi-asserted-by": "crossref",
"first-page": "307",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/j.jaip.2021.08.007_bib31",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1016/j.anai.2020.07.012",
"article-title": "Atopy is predictive of a decreased need for hospitalization for coronavirus disease 2019",
"author": "Keswani",
"doi-asserted-by": "crossref",
"first-page": "479",
"journal-title": "Ann Allergy Asthma Immunol",
"key": "10.1016/j.jaip.2021.08.007_bib32",
"volume": "125",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.05.051",
"article-title": "Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype",
"author": "Camiolo",
"doi-asserted-by": "crossref",
"first-page": "315",
"journal-title": "J Allergy Clinical Immunol",
"key": "10.1016/j.jaip.2021.08.007_bib33",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jaip.2021.08.007_bib34",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jaip.2021.08.007_bib35",
"volume": "384",
"year": "2021"
},
{
"article-title": "Responding to the COVID-19 pandemic in developing countries: lessons from selected countries of the Global South",
"author": "Chowdhury",
"first-page": "162",
"journal-title": "Development (Rome)",
"key": "10.1016/j.jaip.2021.08.007_bib36",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1128/JVI.01648-20",
"article-title": "The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells",
"author": "Matsuyama",
"doi-asserted-by": "crossref",
"first-page": "e01648-20",
"journal-title": "J Virol",
"key": "10.1016/j.jaip.2021.08.007_bib37",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 viral load is associated with increased disease severity and mortality",
"author": "Fajnzylber",
"doi-asserted-by": "crossref",
"first-page": "5493",
"journal-title": "Nat Commun",
"key": "10.1016/j.jaip.2021.08.007_bib38",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1001/jamacardio.2020.1855",
"article-title": "Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19)",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1020",
"journal-title": "JAMA Cardiol",
"key": "10.1016/j.jaip.2021.08.007_bib39",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4326",
"article-title": "Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State",
"author": "Arentz",
"doi-asserted-by": "crossref",
"first-page": "1612",
"journal-title": "JAMA",
"key": "10.1016/j.jaip.2021.08.007_bib40",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2004500",
"article-title": "Covid-19 in critically ill patients in the Seattle Region—case series",
"author": "Bhatraju",
"doi-asserted-by": "crossref",
"first-page": "2012",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jaip.2021.08.007_bib41",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jaip.2021.08.007_bib42",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.7326/M20-0504",
"article-title": "The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application",
"author": "Lauer",
"doi-asserted-by": "crossref",
"first-page": "577",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.jaip.2021.08.007_bib43",
"volume": "172",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA",
"key": "10.1016/j.jaip.2021.08.007_bib44",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.21037/atm.2018.01.13",
"article-title": "Extracting and utilizing electronic health data from Epic for research",
"author": "Milinovich",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "Ann Transl Med",
"key": "10.1016/j.jaip.2021.08.007_bib45",
"volume": "6",
"year": "2018"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213219821009065"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Intranasal Corticosteroids Are Associated with Better Outcomes in Coronavirus Disease 2019",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "9"
}
