RETRACTED: Evaluating the efficacy and safety of a novel prophylactic nasal spray in the prevention of SARS-CoV-2 infection: A multi-centre, double blind, placebo-controlled, randomised trial.
et al., Journal of Clinical Virology, doi:10.1016/j.jcv.2022.105248, Jul 2022
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000019 from 42 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
This study was retracted.
Study covers zinc and pHOXWELL.
Balmforth et al., 25 Jul 2022, peer-reviewed, 15 authors.
Evaluating the efficacy and safety of a novel prophylactic nasal spray in the prevention of SARS-CoV-2 infection: A multi-centre, double blind, placebo-controlled, randomised trial.
Journal of Clinical Virology, doi:10.1016/j.jcv.2022.105248
Evaluating the efficacy and safety of a novel prophylactic nasal spray in the prevention of SARS-CoV-2 infection: A multi-centre, double blind, placebo-controlled, randomised trial.,
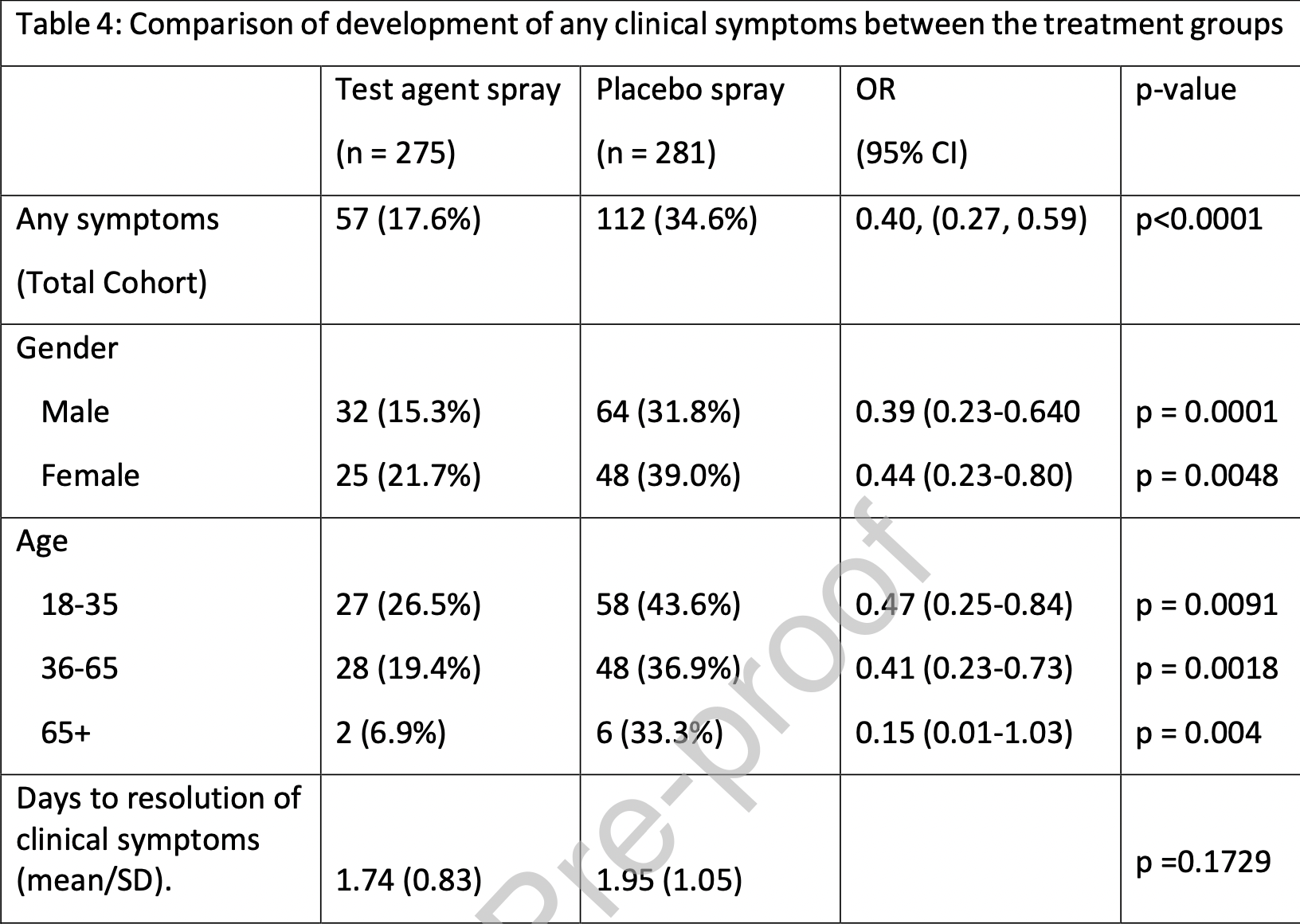
A statistically significant reduction in experiencing any clinical symptom was seen in those treated with the test agent compared to placebo [57 cases (17.6%) vs 112 cases (34.6%); OR 0.40, (95% CI; 0.27-0.59), p<0.0001]. As per the primary endpoint, these results were reflected across sex (male 15.3% vs 31.8% p= 0.0001; female 21.7% vs 39% p= 0.0048) and all age groups (18-35; 26.5% vs 43.6% p= 0.009: 36-65; 19.4% vs 36.9% p= 0.0018: 65+; 6.9% vs 33/3% p= 0.0407). A breakdown of all symptoms experienced in either treatment arm is given in Appendix 2; Supplementary information. Mean time to resolution of symptoms was 1.74 days in the test agent group compared to 1.95 days for the placebo (p=0.1729) (table 4). A comparison of SARS-CoV-2 IgGS antibody status and symptom status for each treatment group is shown in table 5 . This analysis shows that for participants who did not contract SARS-CoV-2 infection during the study period as evidenced by a negative IgGS antibody result, reported symptoms were significantly lower in the test agent group [n=35 (14.5%)] than in the placebo group [n= 67 (36.4%)] (p<0.001). No significant difference was seen in the proportion of patients who reported symptoms amongst patients who tested positive for IgGS. Acceptability testing showed that subjects had a positive experience of using both the test agent and placebo nasal sprays (figure 2 ). Both sprays were comfortable to use, easy to carry, easy to use and had an acceptable odour..
References
Abdukahil, Abe, Abel, COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study, Infection
Bentley, Stanton, Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread, Viruses
Bestle, Heindl, Limburg, TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells, Life Sci Alliance
Bovard, Van Der Toorn, Schlage, Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia, Biochem Biophys Rep
Burton, Clarkson, Goulao, Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection, Cochrane Database of Systematic Reviews
Figueroa, Lombardo, Dogliotti, Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease, Int J Gen Med
Gomes, Fernandes, Casimiro, Cathepsin L in COVID-19: From Pharmacological Evidences to Genetics, Front Cell Infect Microbiol
Hiscott, Alexandridi, Muscolini, The global impact of the coronavirus pandemic, Cytokine & Growth Factor Reviews
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat Rev Mol Cell Biol
Johnson, Xie, Bailey, Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis, Nature
Khan, Chen, Geiger, Role of Endolysosomes in Severe Acute Respiratory Syndrome Coronavirus-2 Infection and Coronavirus Disease 2019 Pathogenesis: Implications for Potential Treatments, Front Pharmacol
Ku, Xie, Hinton, Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants, Nature
Luo, Liu, Li, Dynamic changes and prevalence of SARS-CoV-2 IgG/IgM antibodies: Analysis of multiple factors, Int J Infect Dis
Manisty, Treibel, Jensen, Characterising heterogeneity and sero-reversion in antibody responses to mild SARS⍰CoV-2 infection: a cohort study using time series analysis and mechanistic modelling, medRxiv
Paull, Luscombe, Castellarnau, Heery, Bobardt et al., Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice, Viruses
Prather, Marr, Schooley, Mcdiarmid, Wilson et al., Airborne transmission of SARS-CoV-2, Science
Wu, Zheng, Yang, A Potential Therapeutic Target for COVID-19, iScience
Xia, Lan, Su, The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin, Signal Transduct Target Ther
Xie, Guo, Lopez-Hernadez, Teng, Li, The pH Effects on SARS-CoV and SARS-CoV-2 Spike Proteins in the Process of Binding to hACE2, Res Sq
Zhao, Yang, Yang, Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development, Signal Transduct Target Ther
Zhou, Ayeh, Chidambaram, Karakousis, Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions, BMC Infect Dis
DOI record:
{
"DOI": "10.1016/j.jcv.2022.105248",
"ISSN": [
"1386-6532"
],
"URL": "http://dx.doi.org/10.1016/j.jcv.2022.105248",
"alternative-id": [
"S1386653222001809"
],
"article-number": "105248",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Evaluating the efficacy and safety of a novel prophylactic nasal spray in the prevention of SARS-CoV-2 infection: A multi-centre, double blind, placebo-controlled, randomised trial."
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Clinical Virology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jcv.2022.105248"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Authors. Published by Elsevier B.V."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8897-7972",
"affiliation": [],
"authenticated-orcid": false,
"family": "Balmforth",
"given": "Damian",
"sequence": "first"
},
{
"affiliation": [],
"family": "Swales",
"given": "James A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silpa",
"given": "Laurence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dunton",
"given": "Alan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Kay E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Stephen G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamath",
"given": "Archana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Jayanti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Sandeep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Masood",
"given": "M. Abid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McKnight",
"given": "Áine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rees",
"given": "Doug",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russell",
"given": "Angela J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaggi",
"given": "Manu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uppal",
"given": "Rakesh",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Virology",
"container-title-short": "Journal of Clinical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
26
]
],
"date-time": "2022-07-26T00:17:12Z",
"timestamp": 1658794632000
},
"deposited": {
"date-parts": [
[
2022,
7,
26
]
],
"date-time": "2022-07-26T05:53:42Z",
"timestamp": 1658814822000
},
"indexed": {
"date-parts": [
[
2022,
7,
27
]
],
"date-time": "2022-07-27T04:54:24Z",
"timestamp": 1658897664527
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
1
]
],
"date-time": "2022-07-01T00:00:00Z",
"timestamp": 1656633600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 24,
"start": {
"date-parts": [
[
2022,
7,
25
]
],
"date-time": "2022-07-25T00:00:00Z",
"timestamp": 1658707200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1386653222001809?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1386653222001809?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105248",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
7
]
]
},
"published-print": {
"date-parts": [
[
2022,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.cytogfr.2020.05.010",
"article-title": "The global impact of the coronavirus pandemic",
"author": "Hiscott",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Cytokine & Growth Factor Reviews",
"key": "10.1016/j.jcv.2022.105248_bib0001",
"volume": "53",
"year": "2020"
},
{
"article-title": "Airborne transmission of SARS-CoV-2",
"author": "Prather",
"first-page": "303",
"issue": "6514",
"journal-title": "Science",
"key": "10.1016/j.jcv.2022.105248_bib0002",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06222-4",
"article-title": "Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "496",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jcv.2022.105248_bib0003",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 entry into cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"issue": "1",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "10.1016/j.jcv.2022.105248_bib0004",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "10.1016/j.jcv.2022.105248_bib0005",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2020.595888",
"article-title": "Role of Endolysosomes in Severe Acute Respiratory Syndrome Coronavirus-2 Infection and Coronavirus Disease 2019 Pathogenesis: Implications for Potential Treatments",
"author": "Khan",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.jcv.2022.105248_bib0006",
"volume": "11",
"year": "2020"
},
{
"article-title": "The pH Effects on SARS-CoV and SARS-CoV-2 Spike Proteins in the Process of Binding to hACE2",
"author": "Xie",
"journal-title": "Res Sq",
"key": "10.1016/j.jcv.2022.105248_bib0007",
"year": "2021"
},
{
"DOI": "10.1038/s41392-020-0184-0",
"article-title": "The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin",
"author": "Xia",
"doi-asserted-by": "crossref",
"first-page": "92",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.jcv.2022.105248_bib0008",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.isci.2020.101642",
"article-title": "Furin: A Potential Therapeutic Target for COVID-19",
"author": "Wu",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "iScience",
"key": "10.1016/j.jcv.2022.105248_bib0009",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03237-4",
"article-title": "Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "293",
"issue": "7849",
"journal-title": "Nature",
"key": "10.1016/j.jcv.2022.105248_bib0010",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.26508/lsa.202000786",
"article-title": "TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells",
"author": "Bestle",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "Life Sci Alliance",
"key": "10.1016/j.jcv.2022.105248_bib0011",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00558-8",
"article-title": "Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "134",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.jcv.2022.105248_bib0012",
"volume": "6",
"year": "2021"
},
{
"article-title": "Cathepsin L in COVID-19: From Pharmacological Evidences to",
"author": "Gomes",
"journal-title": "Genetics. Front Cell Infect Microbiol",
"key": "10.1016/j.jcv.2022.105248_bib0013",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1101/2020.11.04.20225920",
"doi-asserted-by": "crossref",
"key": "10.1016/j.jcv.2022.105248_bib0014",
"unstructured": "Manisty C, Treibel T, Jensen M, et al. Characterising heterogeneity and sero-reversion in antibody responses to mild SARS⍰CoV-2 infection: a cohort study using time series analysis and mechanistic modelling. medRxiv 2020: 2020.11.04.20225920."
},
{
"DOI": "10.1016/j.ijid.2021.04.078",
"article-title": "Dynamic changes and prevalence of SARS-CoV-2 IgG/IgM antibodies: Analysis of multiple factors",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jcv.2022.105248_bib0015",
"volume": "108",
"year": "2021"
},
{
"DOI": "10.1007/s15010-021-01599-5",
"article-title": "COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study",
"author": "Abdukahil",
"doi-asserted-by": "crossref",
"first-page": "889",
"issue": "5",
"journal-title": "Infection",
"key": "10.1016/j.jcv.2022.105248_bib0016",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.3390/v13122345",
"article-title": "Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread",
"author": "Bentley",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "Viruses",
"key": "10.1016/j.jcv.2022.105248_bib0017",
"volume": "13",
"year": "2021"
},
{
"article-title": "Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia",
"author": "Bovard",
"journal-title": "Biochem Biophys Rep",
"key": "10.1016/j.jcv.2022.105248_bib0018",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.3390/v13081656",
"article-title": "Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice",
"author": "Paull",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "Viruses",
"key": "10.1016/j.jcv.2022.105248_bib0019",
"volume": "13",
"year": "2021"
},
{
"article-title": "Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection",
"author": "Burton",
"issue": "9",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "10.1016/j.jcv.2022.105248_bib0020",
"year": "2020"
},
{
"DOI": "10.2147/IJGM.S328486",
"article-title": "Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease",
"author": "Figueroa",
"doi-asserted-by": "crossref",
"first-page": "6277",
"journal-title": "Int J Gen Med",
"key": "10.1016/j.jcv.2022.105248_bib0021",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03673-2",
"article-title": "Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants",
"author": "Ku",
"doi-asserted-by": "crossref",
"first-page": "718",
"issue": "7869",
"journal-title": "Nature",
"key": "10.1016/j.jcv.2022.105248_bib0022",
"volume": "595",
"year": "2021"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1386653222001809"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Evaluating the efficacy and safety of a novel prophylactic nasal spray in the prevention of SARS-CoV-2 infection: A multi-centre, double blind, placebo-controlled, randomised trial.",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
