Effect of 0.5% povidone-iodine on the nasopharyngeal and oropharyngeal viral loads in patients with COVID-19: A double-blind placebo-controlled randomized clinical trial
et al., Journal of Family Medicine and Primary Care, doi:10.4103/jfmpc.jfmpc_446_22, CTRI/2020/11/029063, Oct 2022
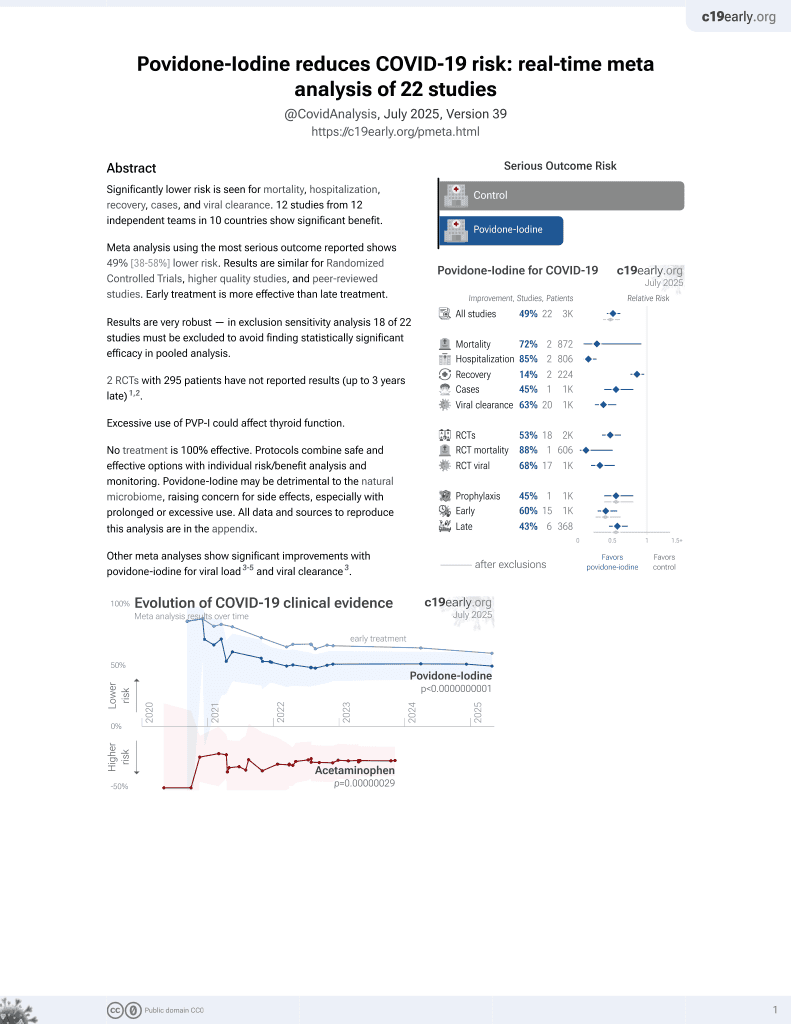
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 32 patients in India, showing greater reduction in viral load with PVP-I treatment, without statistical significance.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
This study is excluded in the after exclusion results of meta-analysis:
study only provides short-term viral load results.
|
relative decrease in viral load, 100% better, RR < 0.001, p = 0.50, treatment mean 0.42 (±2.48) n=16, control mean -0.1 (±1.72) n=16, 3 hours, viral load increased in control group.
|
|
relative decrease in viral load, 90.2% better, RR 0.10, p = 0.56, treatment mean 0.51 (±2.39) n=16, control mean 0.05 (±1.96) n=16, 5 min.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sharma et al., 31 Oct 2022, Double Blind Randomized Controlled Trial, placebo-controlled, India, peer-reviewed, mean age 61.3, 6 authors, study period November 2020 - May 2021, trial CTRI/2020/11/029063.
Contact: nilimatakhel@gmail.com.
Effect of 0.5% povidone-iodine on the nasopharyngeal and oropharyngeal viral loads in patients with COVID-19: A double-blind placebo-controlled randomized clinical trial
Journal of Family Medicine and Primary Care, doi:10.4103/jfmpc.jfmpc_446_22
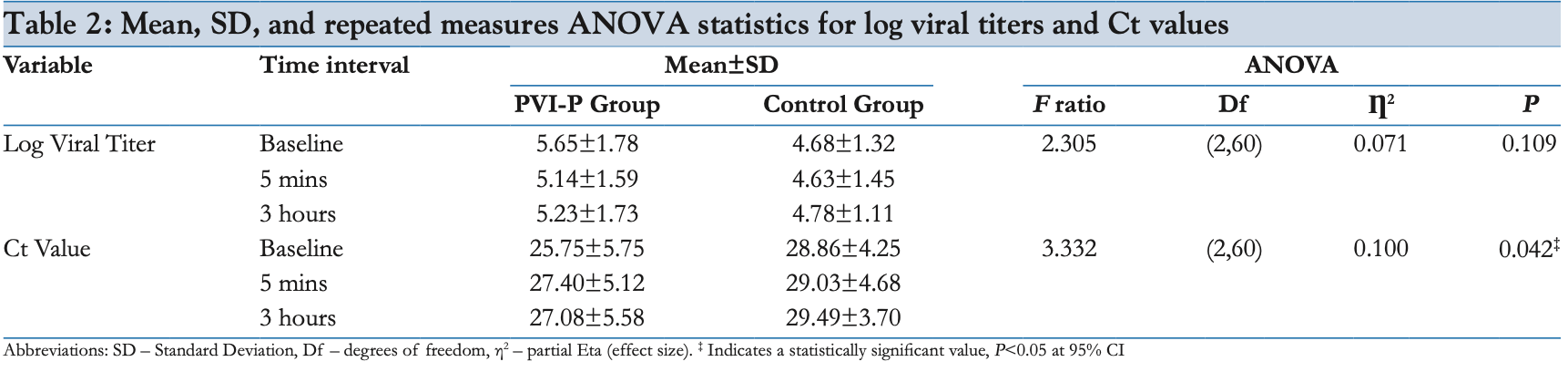
Background and Objective: The povidone-iodine (PvP-I) nasal antiseptic has been shown to completely inactivate the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro at variable concentrations. This study was performed to investigate the effect of 0.5% PvP-I nasal drops and oral gargles on the nasopharyngeal and oropharyngeal viral loads in SARS-CoV-2-positive patients. Methods: This was a double-blind, placebo-controlled, randomized clinical trial among patients aged ≥18 years with reverse-transcriptase polymerase chain reaction confirmed in the mild to moderate category of SARS-CoV-2 infection. A total of 32 patients were randomly assigned to receive either freshly prepared 0.5% PvP-I solution or distilled water in the form of supervised self-administered 4-5 nasal drops, followed by 20 ml for gargling for at least 30 seconds. The main outcome measure was the mean change in viral titer and Ct values in the nasopharyngeal and oropharyngeal samples at baseline, 5 minutes, and 3 hours post intervention. Results: The mean change in viral titers across the time duration for the test group when compared with the control group was not statistically significant (P = 0.109). However, the mean change in Ct value was found to be borderline statistically significant (P = 0.042). Noticeable differences were noted among the mean viral titers and Ct values in the intervention group when plotted against the time of testing as compared to the control group. PvP-I solution at 0.5% dilution was well tolerated, and no evident side effects were reported. Conclusions: This study shows that 0.5% PvP-I has an effect on reducing nasopharyngeal and oropharyngeal viral loads in COVID-19 patients. This can be of substantial aid for the primary care physicians, especially for the practitioners in remote and resource poor areas.
Conflicts of interest There are no conflicts of interest.
References
Association, World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects, JAMA
Berkelman, Holland, Anderson, Increased bactericidal activity of dilute preparations of povidone-iodine solutions, J Clin Microbiol
Domingo, Farrales, Loya, Pura, Uy, The effect of 1% povidone-iodine as a pre-procedural mouthrinse in 20 patients with varying degrees of oral hygiene, J Philipp Dent Assoc
Eggers, Eickmann, Zorn, Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus ankara (MVA), Infect Dis Ther
Eggers, Koburger-Janssen, Eickmann, Zorn, In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens, Infect Dis Ther
Eggers, Koburger-Janssen, Ward, Newby, Müller, Bactericidal and virucidal activity of povidone-iodine and chlorhexidine gluconate cleansers in an in vivo hand hygiene clinical simulation study, Infect Dis Ther
Frank, Brown, Capriotti, Westover, Pelletier et al., In vitro efficacy of a povidone-iodine nasal antiseptic for rapid inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg
Frank, Capriotti, Brown, Tessema, Povidone-iodine use in sinonasal and oral cavities: A review of safety in the COVID-19 Era, Ear Nose Throat J
Guenezan, Garcia, Strasters, Jousselin, Lévêque et al., povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: A randomized clinical trial, JAMA Otolaryngol Head Neck Surg
Hou, Okuda, Edwards, Martinez, Asakura et al., SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell
Khan, Parab, Paranjape, Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic, Am J Otolaryngol
Lamas, Dios, Rodríguez, Pérez, Alvargonzalez et al., Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis
Naqvi, Citardi, Cattano, Ostrosky-Zeichner, Knackstedt et al., Povidone-iodine solution as SARS-CoV-2 prophylaxis for procedures of the upper aerodigestive tract a theoretical framework, J Otolaryngol Head Neck Surg
Rothe, Schunk, Sothmann, Bretzel, Froeschl et al., Transmission of 2019-nCoV infection from an asymptomatic contact in Germany, N Engl J Med
Sungnak, Huang, Bécavin, Berg, Queen et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med
Zou, Ruan, Huang, Liang, Huang et al., SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.4103/jfmpc.jfmpc_446_22",
"ISSN": [
"2249-4863"
],
"URL": "http://dx.doi.org/10.4103/jfmpc.jfmpc_446_22",
"alternative-id": [
"360773"
],
"author": [
{
"affiliation": [],
"family": "Takhelchangbam",
"given": "Nilima",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Pranav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Amit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "NareshPal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Raj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yadav",
"given": "Ramakant",
"sequence": "additional"
}
],
"container-title": "Journal of Family Medicine and Primary Care",
"container-title-short": "J Family Med Prim Care",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
9
]
],
"date-time": "2022-11-09T10:53:22Z",
"timestamp": 1667991202000
},
"deposited": {
"date-parts": [
[
2022,
11,
9
]
],
"date-time": "2022-11-09T10:54:56Z",
"timestamp": 1667991296000
},
"indexed": {
"date-parts": [
[
2022,
11,
10
]
],
"date-time": "2022-11-10T05:35:26Z",
"timestamp": 1668058526170
},
"is-referenced-by-count": 0,
"issue": "10",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"language": "en",
"member": "2581",
"original-title": [],
"page": "6320",
"prefix": "10.4103",
"published": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Medknow",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.4103/jfmpc.jfmpc_446_22"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Materials Science"
],
"subtitle": [],
"title": "Effect of 0.5% povidone-iodine on the nasopharyngeal and oropharyngeal viral loads in patients with COVID-19: A double-blind placebo-controlled randomized clinical trial",
"type": "journal-article",
"volume": "11"
}