Administration of antivirals, IL-6 inhibitors, monoclonal neutralizing antibodies and systemic corticosteroids in acute SARS-CoV-2 infection do not reduce the subsequent burden of Long-COVID symptoms
et al., Le Infezioni in Medicina, doi:10.53854/liim-3304-4, Dec 2025
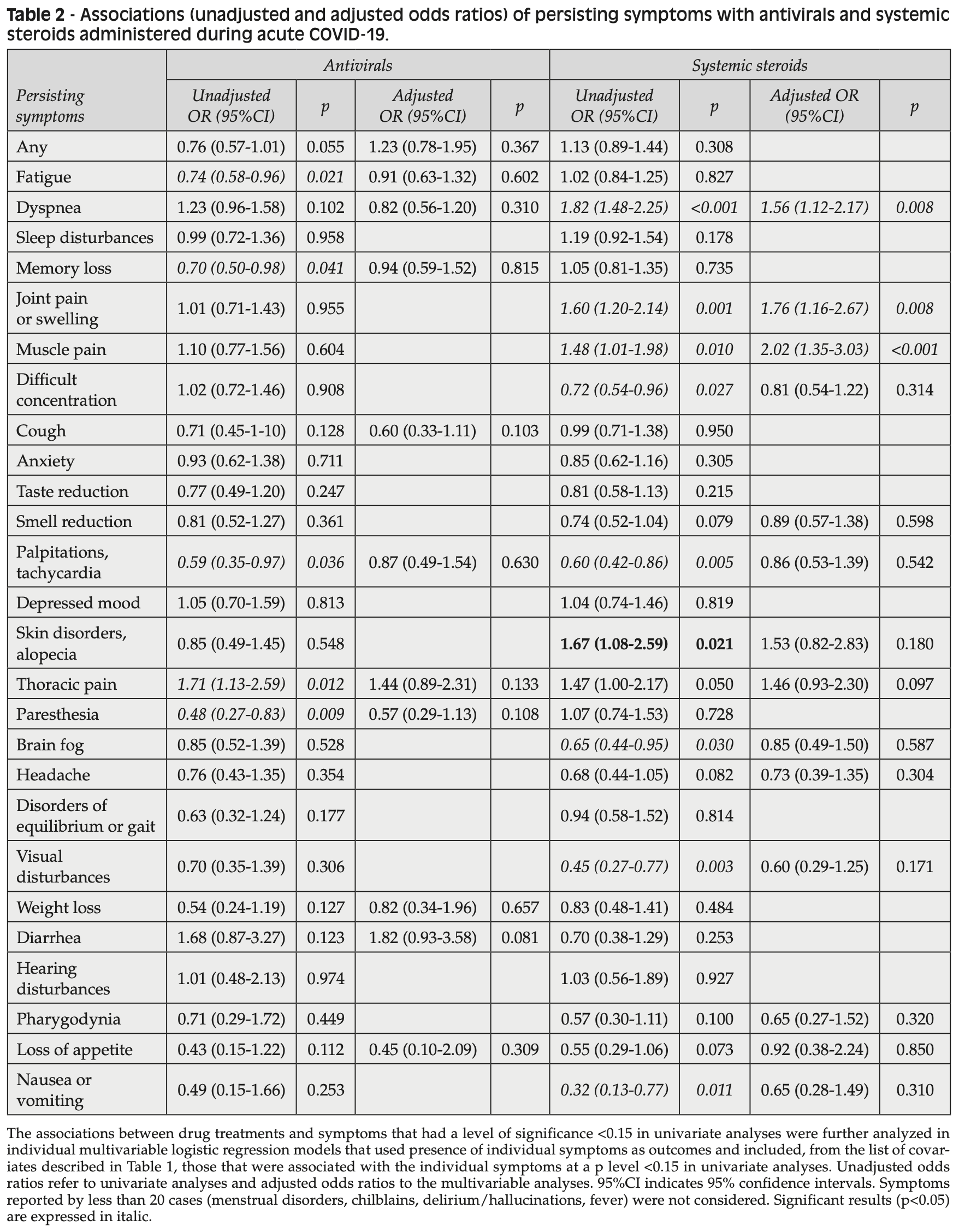
Retrospective 1,534 patients (67% hospitalized) accessing long-COVID centers in Italy showing higher persistent long-COVID symptoms with antiviral treatment (97% remdesivir) after adjustment for confounders, without statistical significance. The mean interval between acute SARS-CoV-2 infection and symptom evaluation was of 338 days.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
|
risk of long COVID, 23.0% higher, OR 1.23, p = 0.37, treatment 318, control 1,216, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Floridia et al., 1 Dec 2025, retrospective, Italy, peer-reviewed, mean age 60.3, 22 authors, study period January 2023 - March 2024.
Contact: marco.floridia@iss.it.
Abstract: Le Infezioni in Medicina, n. 4, 391-403, 2025
doi: 10.53854/liim-3304-4
ORIGINAL ARTICLES
Administration of antivirals,
IL-6 inhibitors, monoclonal
neutralizing antibodies and systemic
corticosteroids in acute SARS-CoV-2
infection do not reduce the
subsequent burden of Long-COVID
symptoms
Marco Floridia1, Liliana Elena Weimer1, Aldo Lo Forte2, Paolo Palange3,
Maria Rosa Ciardi3, Patrizia Rovere-Querini4, Piergiuseppe Agostoni5,6, Emanuela Barisione7,
Silvia Zucco8, Paola Andreozzi9, Paolo Bonfanti10,11, Stefano Figliozzi12,13, Matteo Tosato14,
Donato Lacedonia15, Kwelusukila Loso16, Paola Gnerre17, Maria Antonietta di Rosolini18,
Domenico Maurizio Toraldo19, Giuseppe Pio Martino20, Guido Vagheggini21,22,
Gianfranco Parati23,24, Graziano Onder14, and the ISS Long-COVID Study Group.
National Center for Global Health, Istituto Superiore di Sanità, Rome, Italy;
Department of Multidimensional Medicine, Internal Medicine Unit, San Giovanni di Dio Hospital, Florence, Italy;
3
Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy;
4
IRCCS Ospedale S. Raffaele and Vita-Salute San Raffaele University, Milan, Italy;
5
Centro Cardiologico Monzino, IRCCS, Milan, Italy;
6
Department of Clinical Science and Community Medicine, University of Milan, Milan, Italy;
7
IRCCS Ospedale Policlinico San Martino, Genova, Italy;
8
Infectious Diseases Unit, Ospedale Amedeo di Savoia, Turin, Italy;
9
Predictive Medicine Unit, Department of Internal Medicine, Endocrine-Metabolic Sciences and Infectious
Diseases, Azienda Ospedaliero Universitaria Policlinico Umberto I, Rome, Italy;
10
Infectious Diseases Unit, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy;
11
University of Milano-Bicocca, School of Medicine and Surgery, Monza, Italy;
12
IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy;
13
Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (MI), Italy.;
14
Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy;
15
Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy;
16
Infectious Diseases and Hepatology Unit, Manduria Hospital, Taranto, Italy;
17
Internal Medicine Department, ASL 2 Savonese, Savona, Italy;
18
UOC Malattie Infettive, ASP Ragusa, Ragusa, Italy;
19
Respiratory Care Unit, Rehabilitation Department, “V. Fazzi” Hospital, Azienda Sanitaria Locale,
San Cesario, Lecce, Italy;
20
Internal Medicine Unit, Hospital Augusto Murri, Fermo, Italy;
21
Department of Internal Medicine and Medical Specialties, Respiratory Failure Pathway,
Azienda USL Toscana Nordovest, Pisa, Italy;
22
Fondazione Volterra Ricerche ONLUS, Volterra (Pisa), Italy;
23
Department of Clinical Sciences and Community Medicine, University of Milan, Milan, Italy;
24
IRCCS Istituto Auxologico Italiano, Milan, Italy.
1
2
Article received 21 July 2025 and accepted 30 September 2025
Corresponding author
Marco Floridia
E-mail: marco.floridia@iss.it
391
392 M. Floridia, L.E. Weimer, A. Lo Forte, et al.
SUMMARY
Purpose: Some studies have suggested that therapeutic
interventions able to mitigate the acute phase of COVID-19 can also reduce the risk of Long-COVID and its
severity, but the issue is still controversial.
Methods: We examined in a national cohort of patients
followed in Long-COVID centers the risk of persistent
symptoms according to administration in acute COVID-19 of four drug classes: antivirals, IL-6 inhibitors,
monoclonal..