Cardiovascular Outcomes in COVID-19 Patients Treated with Paxlovid: A Multicenter Retrospective Study
et al., Acta Cardiol Sin, doi:10.6515/ACS.202601_42(1).20250726A, Jan 2026
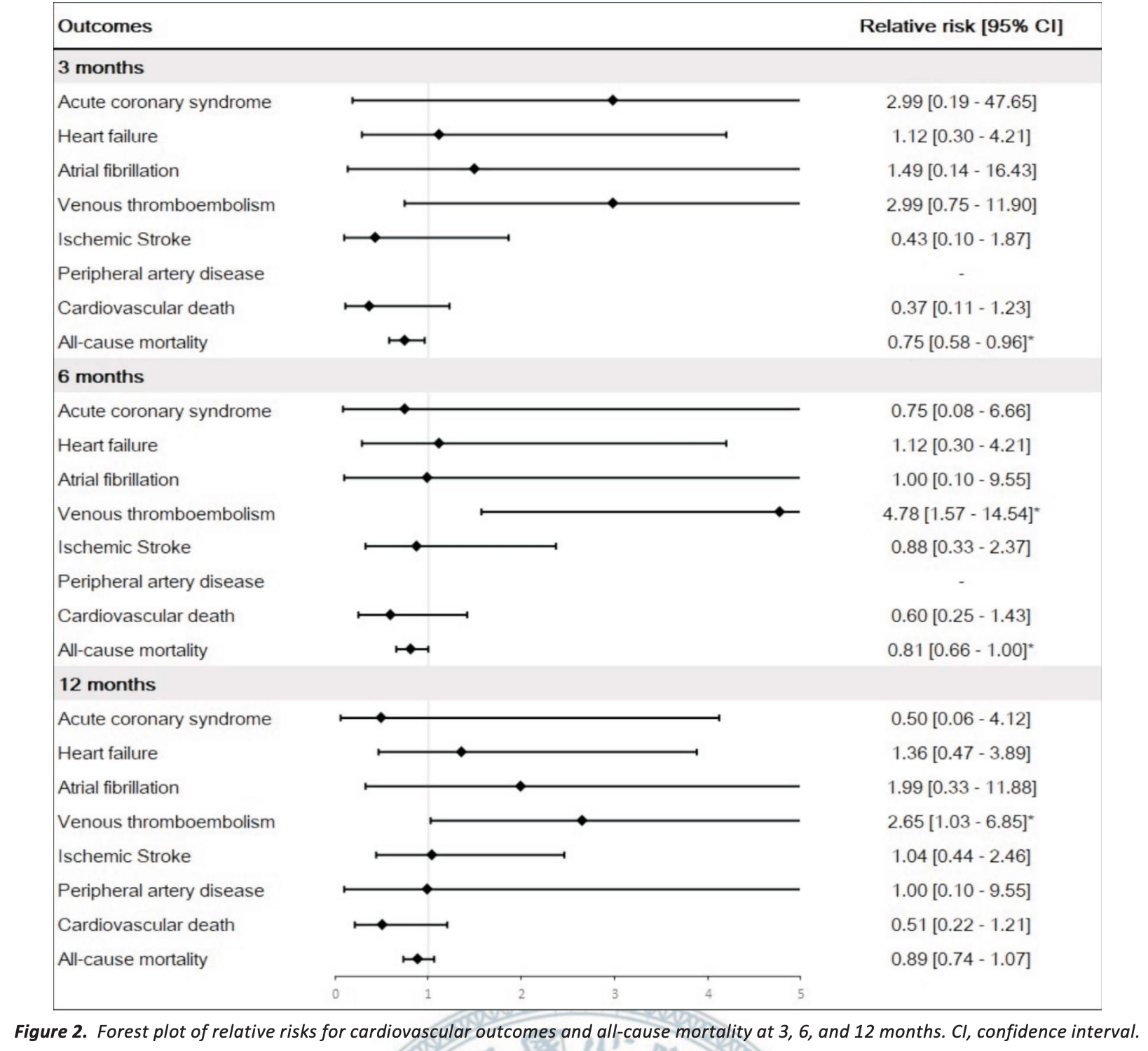
Retrospective 606 COVID-19 patients treated with paxlovid and 1,809 propensity score-matched controls in Taiwan, showing short-term mortality benefits at 3 months, but reduced benefit at 6 months, and no significant benefit at 12 months. The study also found significantly higher risk of venous thromboembolism (VTE) at 6 months and 12 months. Authors excluded patients with pre-existing cardiovascular conditions (potentially at higher risk of increased issues with paxlovid use).
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
|
risk of death, 11.0% lower, RR 0.89, p = 0.21, treatment 117 of 606 (19.3%), control 393 of 1,809 (21.7%), NNT 41, propensity score matching, day 365.
|
|
risk of death, 19.0% lower, RR 0.81, p = 0.049, treatment 94 of 606 (15.5%), control 345 of 1,809 (19.1%), NNT 28, propensity score matching, day 180.
|
|
risk of death, 25.0% lower, RR 0.75, p = 0.02, treatment 66 of 606 (10.9%), control 264 of 1,809 (14.6%), NNT 27, propensity score matching, day 90.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Chen et al., 30 Jan 2026, retrospective, Taiwan, peer-reviewed, 11 authors.
DOI record:
{
"DOI": "10.6515/ACS.202601_42(1).20250726A",
"URL": "",
"abstract": "",
"author": [
{
"literal": "Wei-Lun Chen"
},
{
"literal": "Victor Chien-Chia Wu"
},
{
"literal": "Chun-Li Wang"
},
{
"literal": "Yu-Ching Wang"
},
{
"literal": "Yu-Tung Huang"
},
{
"literal": "Chien-Hao Huang"
},
{
"literal": "Chih-Hsiang Chang"
},
{
"literal": "Shao-Wei Chen"
},
{
"literal": "Shang-Hung Chang"
},
{
"literal": "Cheng-Hsun Chiu"
},
{
"literal": "Pao-Hsien Chu"
}
],
"container-title": "Acta Cardiologica Sinica",
"issue": "1",
"issued": {
"date-parts": [
[
"2026",
"1",
"31"
]
]
},
"language": "en",
"page-first": "87",
"title": "Cardiovascular Outcomes in COVID-19 Patients Treated with Paxlovid: A Multicenter Retrospective Study",
"type": "article-journal",
"volume": "42"
}