Favipiravir efficacy and safety for the treatment of severe coronavirus 2019: a retrospective study
et al., Journal of Ayub Medical College Abbottabad, doi:10.55519/JAMC-03-10305, Jun 2022
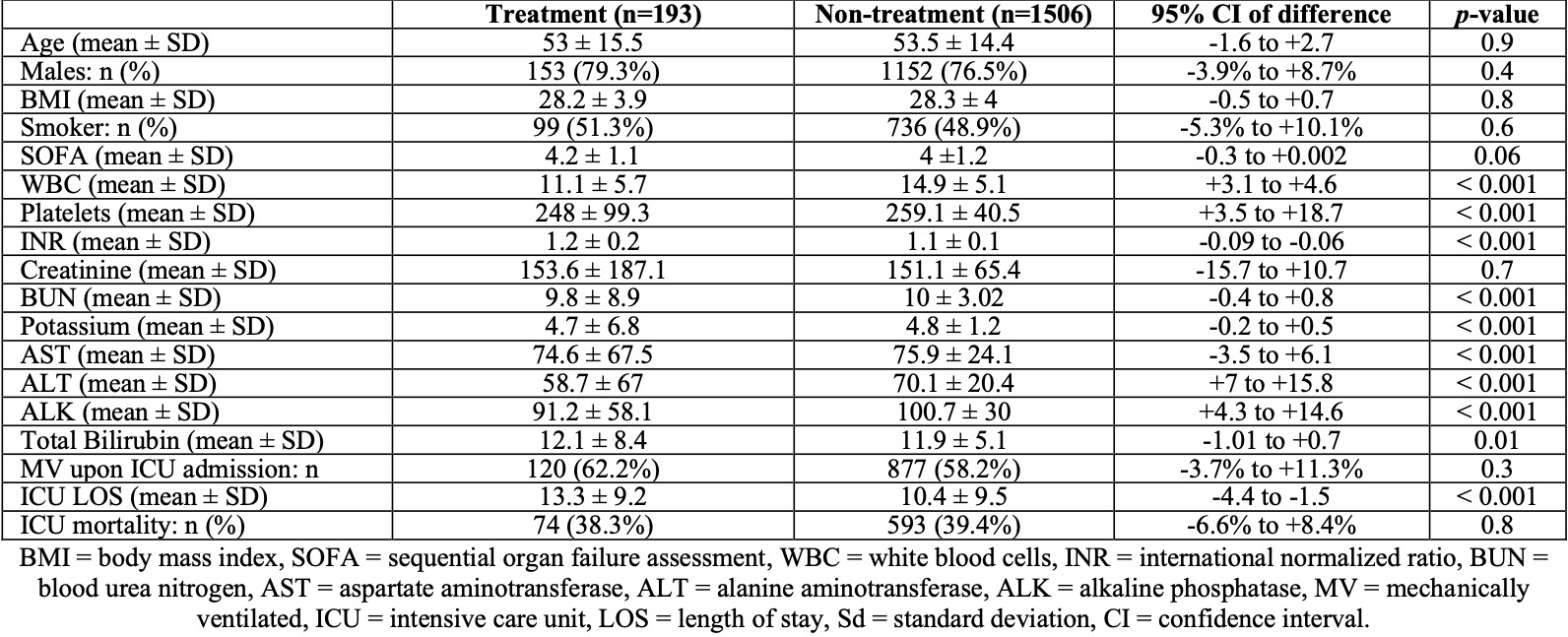
Retrospective 1,699 ICU patients in Saudi Arabia, 193 treated with favipiravir, showing no significant difference in mortality.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
|
risk of death, 2.6% lower, RR 0.97, p = 0.81, treatment 74 of 193 (38.3%), control 593 of 1,506 (39.4%), NNT 97.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Abdulrahman et al., 21 Jun 2022, retrospective, Saudi Arabia, peer-reviewed, 15 authors, study period June 2020 - August 2020.
FAVIPIRAVIR EFFICACY AND SAFETY FOR THE TREATMENT OF SEVERE CORONAVIRUS 2019. A RETROSPECTIVE STUDY.
Journal of Ayub Medical College Abbottabad, doi:10.55519/jamc-03-10305
Background: Corona virus disease is caused by the enveloped, single stranded RNA virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) becoming the deadliest disease of the century. Its global outbreak has led researchers to develop drugs or vaccines to prevent the spread of the disease. Favipiravir is an approved orally administered antiviral drug that selectively inhibits RNAdependent RNA polymerase, used off-label to treat COVID-19. Objectives: The purpose of this study was to assess the efficacy and safety of this drug for severe COVID-19 infection. Methods: This was an observational retrospective study, carried out at the ICU of King Saud Medical City (KSMC) from June 2020 to August 2020. Including a total of one thousand six hundred and ninety-nine patients (n=1699). Categorized into a treatment group (193 patients) who received Favipiravir along with standard care, and non-treatment group (1506 patients) who received standard care only. Results: ICU all-cause mortality was similar in both groups i.e., (Treated group 38.3% Vs Untreated group 39.4%, 95% CI of difference: -6.6% to +8.4%; p = 0.8). The subgroup analysis of survivors as compared to deceased in the treatment group showed that survivors had significantly lower age, international normalising ratio (INR), blood urea nitrogen (BUN), and creatinine. The mean ICU length of stay (LOS) was shorter for survivors compared to deceased (11.2± 8.03 Vs 16.7±9.8 days respectively), while hospital LOS was almost similar between the two groups. Advanced age (OR 1.03 [95% CI: 1.01-1.06]; p=0.004), higher INR and BUN were significantly associated with increased odds of mortality. Comparison of lab investigations at day 1 and day 10 in the treatment group (regardless of outcome) showed that there was a significant increase in Alanine transaminase (ALT), alkaline phosphatase (ALK), and Bilirubin, while an insignificant trend of increase in Aspartate transaminase (AST) and creatinine was recorded. Conclusion: In this study, Favipiravir showed better therapeutic responses in patients with severe COVID-19 infection, in terms of average duration of stay in the intensive care unit and was well tolerated in the younger age, but showed no mortality benefit. However, elevated levels of inflammatory markers, including increased ALT, AST, BUN, bilirubin, and creatinine, needs to be carefully examined.
AUTHORS' CONTRIBUTION
References
Aletreby, Alharthy, Faqihi, Mady, Ramadan et al., Dynamics of SARS-CoV-2 outbreak in the Kingdom of Saudi Arabia: A predictive model, Saudi Crit Care J
Alharthy, Aletreby, Faqihi, Balhamar, Alaklobi et al., Clinical Characteristics and Predictors of 28-Day Mortality in 352 Critically Ill Patients with COVID-19: A Retrospective Study, J Epidemiol Glob Health
Alhazzani, Møller, Arabi, Loeb, Gong, Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Intensive Care Med
Bergman, COVID-19 treatment: investigational drugs and other therapies, Medscape
Bhimraj, Morgan, Shumaker, Lavergne, Baden et al., Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19, Clin Infect Dis
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Dabbous, Abd-Elsalam, El-Sayed, Sherief, Ebeid et al., Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study, Arch Virol
Dabbous, El-Sayed, Assal, Elghazaly, Ebeid et al., Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: A randomised controlled trial, Sci Rep
Food, Administration, FDA approves first treatment for COVID-19
Food, Administration, Fact sheet for health care providers Emergency Use Authorization
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Gallegos, WHO declares public health emergency for novel coronavirus
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials, Sci Rep
Idda, Soru, Floris, Overview of the first 6 months of clinical trials for COVID-19 pharmacotherapy: the most studied drugs, Front Public Health
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clin Infect Dis
Jomah, Asdaq, Mj, Clinical efficacy of antivirals against novel coronavirus (COVID-19): A review, J Infect Public Health
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, Int J Infect Dis
Kaur, Charan, Dutta, Sharma, Bhardwaj et al., Favipiravir use in COVID-19: Analysis of suspected adverse drug events reported in the WHO database, Infect Drug Resist
Marks, Controlled Substance Regulation for the COVID-19 Mental Health Crisis
Mumtaz, Shahzad, Ahmed, Alodat, Gharba et al., External validation of 4C ISARIC mortality score in the setting of a Saudi Arabian ICU. Retrospective study, medRxiv
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review, JAMA
Talukdar, Jain, Balaramnavar, Srivastava, Sivanandy et al., Potential Drugs for Treatment Management With Their Contraindications and Drug-Drug Interaction
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-tomoderate COVID-19: A randomized, comparative, openlabel, multicenter, phase 3 clinical trial, Int J Infect Dis
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
Wee, Mcneil, Hernández, WHO declares global emergency as Wuhan coronavirus spreads
Zhao, Zhang, Zhu, Chen, Chen et al., Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: A multicenter, openlabel, randomized trial, Int Immunopharmacol
DOI record:
{
"DOI": "10.55519/jamc-03-10305",
"ISSN": [
"1819-2718",
"1025-9589"
],
"URL": "http://dx.doi.org/10.55519/JAMC-03-10305",
"abstract": "<jats:p>Background: Corona virus disease is caused by the enveloped, single stranded RNA virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) becoming the deadliest disease of the century. Its global outbreak has led researchers to develop drugs or vaccines to prevent the spread of the disease. Favipiravir is an approved orally administered antiviral drug that selectively inhibits RNA-dependent RNA polymerase, used off-label to treat COVID-19. Objectives: The purpose of this study was to assess the efficacy and safety of this drug for severe COVID-19 infection. Methods: This was an observational retrospective study, carried out at the ICU of King Saud Medical City (KSMC) from June 2020 to August 2020. Including a total of one thousand six hundred and ninety-nine patients (n=1699). Categorized into a treatment group (193 patients) who received Favipiravir along with standard care, and non-treatment group (1506 patients) who received standard care only. Results: ICU all-cause mortality was similar in both groups i.e., (Treated group 38.3% Vs Untreated group 39.4%, 95% CI of difference: -6.6% to +8.4%; p = 0.8). The subgroup analysis of survivors as compared to deceased in the treatment group showed that survivors had significantly lower age, international normalising ratio (INR), blood urea nitrogen (BUN), and creatinine. The mean ICU length of stay (LOS) was shorter for survivors compared to deceased (11.2± 8.03 Vs 16.7±9.8 days respectively), while hospital LOS was almost similar between the two groups. Advanced age (OR 1.03 [95% CI: 1.01–1.06]; p=0.004), higher INR and BUN were significantly associated with increased odds of mortality. Comparison of lab investigations at day 1 and day 10 in the treatment group (regardless of outcome) showed that there was a significant increase in Alanine transaminase (ALT), alkaline phosphatase (ALK), and Bilirubin, while an insignificant trend of increase in Aspartate transaminase (AST) and creatinine was recorded. Conclusion: In this study, Favipiravir showed better therapeutic responses in patients with severe COVID-19 infection, in terms of average duration of stay in the intensive care unit and was well tolerated in the younger age, but showed no mortality benefit. However, elevated levels of inflammatory markers, including increased ALT, AST, BUN, bilirubin, and creatinine, needs to be carefully examined.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Aletreby",
"given": "Waleed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Abdulrahman",
"given": "Basheer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mady",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al-Odat",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Tayar",
"given": "Ashraf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rana",
"given": "Muhammad Asim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alharthy",
"given": "Abdulrahman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alhazmi",
"given": "Alyaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdelmoaty",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hafeez",
"given": "Muhammad Mansoor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kuhail",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noor",
"given": "Alfateh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haddad",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mady",
"given": "Anas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Noor",
"sequence": "additional"
}
],
"container-title": "Journal of Ayub Medical College Abbottabad",
"container-title-short": "J Ayub Med Coll Abbottabad",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
17
]
],
"date-time": "2022-10-17T18:09:25Z",
"timestamp": 1666030165000
},
"deposited": {
"date-parts": [
[
2022,
10,
17
]
],
"date-time": "2022-10-17T18:09:29Z",
"timestamp": 1666030169000
},
"indexed": {
"date-parts": [
[
2022,
10,
18
]
],
"date-time": "2022-10-18T10:44:15Z",
"timestamp": 1666089855031
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
6,
21
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
6,
21
]
]
}
},
"link": [
{
"URL": "https://jamc.ayubmed.edu.pk/jamc/index.php/jamc/article/download/10305/3313",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://jamc.ayubmed.edu.pk/jamc/index.php/jamc/article/download/10305/3313",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "34325",
"original-title": [],
"page": "397-402",
"prefix": "10.55519",
"published": {
"date-parts": [
[
2022,
6,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
21
]
]
},
"publisher": "Ayub Medical College, Abbottabad Pakistan",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamc.ayubmed.edu.pk/jamc/index.php/jamc/article/view/10305"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "FAVIPIRAVIR EFFICACY AND SAFETY FOR THE TREATMENT OF SEVERE CORONAVIRUS 2019. A RETROSPECTIVE STUDY.",
"type": "journal-article",
"volume": "34"
}