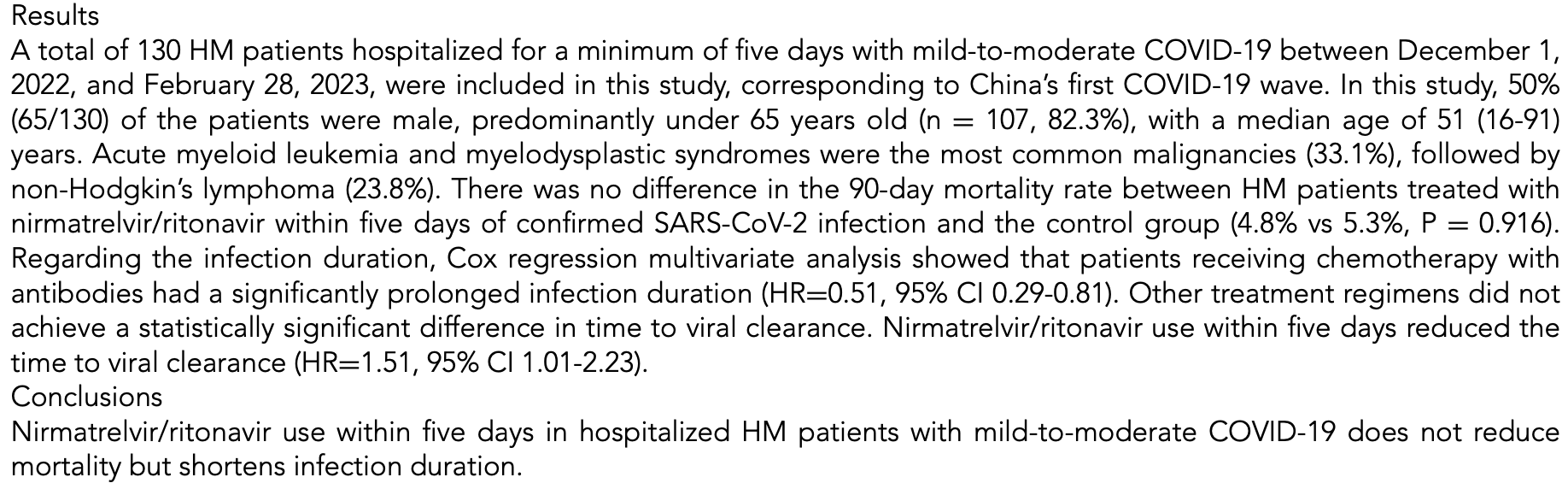
Nirmatrelvir/Ritonavir Reduces Infection Duration in Hospitalized Hematological Malignancies Patients with Mild-to-Moderate COVID-19: A Retrospective Study
et al., Blood, doi:10.1182/blood-2024-207488, ChiCTR2300069374, Nov 2024
Retrospective 130 hematological malignancy patients with mild-to-moderate COVID-19 in China showing no significant difference in 90-day mortality, but shorter infection duration with paxlovid treatment within 5 days.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
|
risk of death, 9.4% lower, RR 0.91, p = 0.92.
|
|
risk of no recovery, 33.8% lower, HR 0.66, p = 0.04, adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Yu et al., 5 Nov 2024, retrospective, China, peer-reviewed, median age 51.0, 5 authors, study period 1 December, 2022 - 28 February, 2023, trial ChiCTR2300069374.
Abstract: Blood 144 (2024) 5955–5956
The 66th ASH Annual Meeting Abstracts
ONLINE PUBLICATION ONLY
615.ACUTE MYELOID LEUKEMIAS: CLINICAL AND EPIDEMIOLOGICAL
Nirmatrelvir/Ritonavir Reduces Infection Duration in Hospitalized Hematological Malignancies Patients with
Mild-to-Moderate COVID-19: A Retrospective Study
Hongbin Yu, MD 1, Tian Chen 2, Jiawei Li, MPH 3, Xin Zhang, MMed 4, Yu Wu, MD 5
1
Department of Hematology, West China Hospital, Sichuan University, Chengdu, China
West China Hospital, Sichuan University, Chengdu, China
3
West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, CHN
4
West China Hospital, Sichuan University, Chengdu, China
5
Department of Hematology and Hematology Research Laboratory, West China Hospital, Sichuan University, Chengdu,
China
2
Background
Since the COVID-19 outbreak in 2020, the virus has significantly impacted global health, particularly affecting patients with
hematological malignancies (HM) due to their immunocompromised status. These patients experience higher infection rates,
prolonged virus clearance, and more severe outcomes. Immunocompromised individuals with persistent infections may also
serve as reservoirs for viral variants, posing public health risks due to the rapid evolution of SARS-CoV-2. Nirmatrelvir/ritonavir,
an antiviral agent approved for treating COVID-19, has demonstrated efficacy in reducing severity and mortality in high-risk,
non-hospitalized patients. While some reported cases support the potential benefits of nirmatrelvir/ritonavir for hospitalized
patients with mild-to-moderate COVID-19 and HM, its effectiveness in this specific patient population remains undetermined.
To address this knowledge gap, we conducted a single-center retrospective study to assess the efficacy of nirmatrelvir/ritonavir
in hospitalized patients with mild-to-moderate COVID-19 and HM.
Methods
We conducted a single-center, retrospective, real-world cohort study at West China Hospital of Sichuan University to evaluate the outcomes of hospitalized patients with HM and SARS-CoV-2 infection treated with nirmatrelvir/ritonavir. Patients
hospitalized for a minimum of five days in the Department of Hematology between December 1, 2022, and February 28,
2023, were included. Eligibility criteria included an active HM diagnosis within the preceding three years and SARS-CoV-2
antigen or nucleic acid positivity. Patients diagnosed with severe COVID-19, or treated with convalescent plasma, azvudine,
or molnupiravir, or with unknown vaccination status, were excluded. The severity levels for COVID-19 infections in this study
followed the definitions provided in the China Diagnosis and Treatment Protocol for COVID-19 Patients (Tentative 10th Version). The Institutional Review Board approved the study protocol. The study, registered with the Chinese Clinical Trial Registry
(ChiCTR2300069374), was conducted following the Helsinki Declaration.
Results
A total of 130 HM patients hospitalized for a minimum of five days with mild-to-moderate COVID-19 between December 1,
2022, and February 28, 2023, were included in this study, corresponding to China’s first COVID-19 wave. In this study, 50%
(65/130) of the patients were male, predominantly under 65 years old (n = 107, 82.3%), with a median age of 51 (16-91)
years. Acute myeloid leukemia and myelodysplastic syndromes were the most common malignancies (33.1%), followed by
non-Hodgkin’s lymphoma (23.8%). There was no difference in the 90-day..
DOI record:
{
"DOI": "10.1182/blood-2024-207488",
"ISSN": [
"0006-4971",
"1528-0020"
],
"URL": "http://dx.doi.org/10.1182/blood-2024-207488",
"abstract": "<jats:sec>\n <jats:title/>\n <jats:p>Background</jats:p>\n <jats:p>Since the COVID-19 outbreak in 2020, the virus has significantly impacted global health, particularly affecting patients with hematological malignancies (HM) due to their immunocompromised status. These patients experience higher infection rates, prolonged virus clearance, and more severe outcomes. Immunocompromised individuals with persistent infections may also serve as reservoirs for viral variants, posing public health risks due to the rapid evolution of SARS-CoV-2. Nirmatrelvir/ritonavir, an antiviral agent approved for treating COVID-19, has demonstrated efficacy in reducing severity and mortality in high-risk, non-hospitalized patients. While some reported cases support the potential benefits of nirmatrelvir/ritonavir for hospitalized patients with mild-to-moderate COVID-19 and HM, its effectiveness in this specific patient population remains undetermined. To address this knowledge gap, we conducted a single-center retrospective study to assess the efficacy of nirmatrelvir/ritonavir in hospitalized patients with mild-to-moderate COVID-19 and HM.</jats:p>\n <jats:p>Methods</jats:p>\n <jats:p>We conducted a single-center, retrospective, real-world cohort study at West China Hospital of Sichuan University to evaluate the outcomes of hospitalized patients with HM and SARS-CoV-2 infection treated with nirmatrelvir/ritonavir. Patients hospitalized for a minimum of five days in the Department of Hematology between December 1, 2022, and February 28, 2023, were included. Eligibility criteria included an active HM diagnosis within the preceding three years and SARS-CoV-2 antigen or nucleic acid positivity. Patients diagnosed with severe COVID-19, or treated with convalescent plasma, azvudine, or molnupiravir, or with unknown vaccination status, were excluded. The severity levels for COVID-19 infections in this study followed the definitions provided in the China Diagnosis and Treatment Protocol for COVID-19 Patients (Tentative 10th Version). The Institutional Review Board approved the study protocol. The study, registered with the Chinese Clinical Trial Registry (ChiCTR2300069374), was conducted following the Helsinki Declaration.</jats:p>\n <jats:p>Results</jats:p>\n <jats:p>A total of 130 HM patients hospitalized for a minimum of five days with mild-to-moderate COVID-19 between December 1, 2022, and February 28, 2023, were included in this study, corresponding to China's first COVID-19 wave. In this study, 50% (65/130) of the patients were male, predominantly under 65 years old (n = 107, 82.3%), with a median age of 51 (16-91) years. Acute myeloid leukemia and myelodysplastic syndromes were the most common malignancies (33.1%), followed by non-Hodgkin's lymphoma (23.8%). There was no difference in the 90-day mortality rate between HM patients treated with nirmatrelvir/ritonavir within five days of confirmed SARS-CoV-2 infection and the control group (4.8% vs 5.3%, P = 0.916). Regarding the infection duration, Cox regression multivariate analysis showed that patients receiving chemotherapy with antibodies had a significantly prolonged infection duration (HR=0.51, 95% CI 0.29-0.81). Other treatment regimens did not achieve a statistically significant difference in time to viral clearance. Nirmatrelvir/ritonavir use within five days reduced the time to viral clearance (HR=1.51, 95% CI 1.01-2.23).</jats:p>\n <jats:p>Conclusions</jats:p>\n <jats:p>Nirmatrelvir/ritonavir use within five days in hospitalized HM patients with mild-to-moderate COVID-19 does not reduce mortality but shortens infection duration.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "1Department of Hematology, West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Yu",
"given": "Hongbin",
"sequence": "first"
},
{
"affiliation": [
{
"name": "2West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Chen",
"given": "Tian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "3West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, CHN"
}
],
"family": "Li",
"given": "Jiawei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "4West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Zhang",
"given": "Xin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "5Department of Hematology and Hematology Research Laboratory, West China Hospital, Sichuan University, Chengdu, China"
}
],
"family": "Wu",
"given": "Yu",
"sequence": "additional"
}
],
"container-title": "Blood",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ashpublications.org"
]
},
"created": {
"date-parts": [
[
2024,
12,
5
]
],
"date-time": "2024-12-05T20:58:08Z",
"timestamp": 1733432288000
},
"deposited": {
"date-parts": [
[
2024,
12,
5
]
],
"date-time": "2024-12-05T20:58:09Z",
"timestamp": 1733432289000
},
"indexed": {
"date-parts": [
[
2024,
12,
6
]
],
"date-time": "2024-12-06T05:21:30Z",
"timestamp": 1733462490080,
"version": "3.30.1"
},
"is-referenced-by-count": 0,
"issue": "Supplement 1",
"issued": {
"date-parts": [
[
2024,
11,
5
]
]
},
"journal-issue": {
"issue": "Supplement 1",
"published-print": {
"date-parts": [
[
2024,
11,
5
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://ashpublications.org/blood/article-pdf/144/Supplement%201/5955/2321528/blood-8784-main.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://ashpublications.org/blood/article-pdf/144/Supplement%201/5955/2321528/blood-8784-main.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "234",
"original-title": [],
"page": "5955-5955",
"prefix": "10.1182",
"published": {
"date-parts": [
[
2024,
11,
5
]
]
},
"published-print": {
"date-parts": [
[
2024,
11,
5
]
]
},
"publisher": "American Society of Hematology",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://ashpublications.org/blood/article/144/Supplement%201/5955/527513/Nirmatrelvir-Ritonavir-Reduces-Infection-Duration"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Nirmatrelvir/Ritonavir Reduces Infection Duration in Hospitalized Hematological Malignancies Patients with Mild-to-Moderate COVID-19: A Retrospective Study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1182/blood.2019cm0000",
"volume": "144"
}