Efficacy of Favipiravir in the Treatment of Moderate COVID-19 Patients: A Randomized, Open-label, Controlled Clinical Trial
et al., Mediterranean Journal of Infection Microbes and Antimicrobials, doi:10.4274/mjima.galenos.2022.2022.30, IRCT20211004052664N1, Jun 2022
RCT 78 patients in Iran, showing improved recovery with favipiravir treatment.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
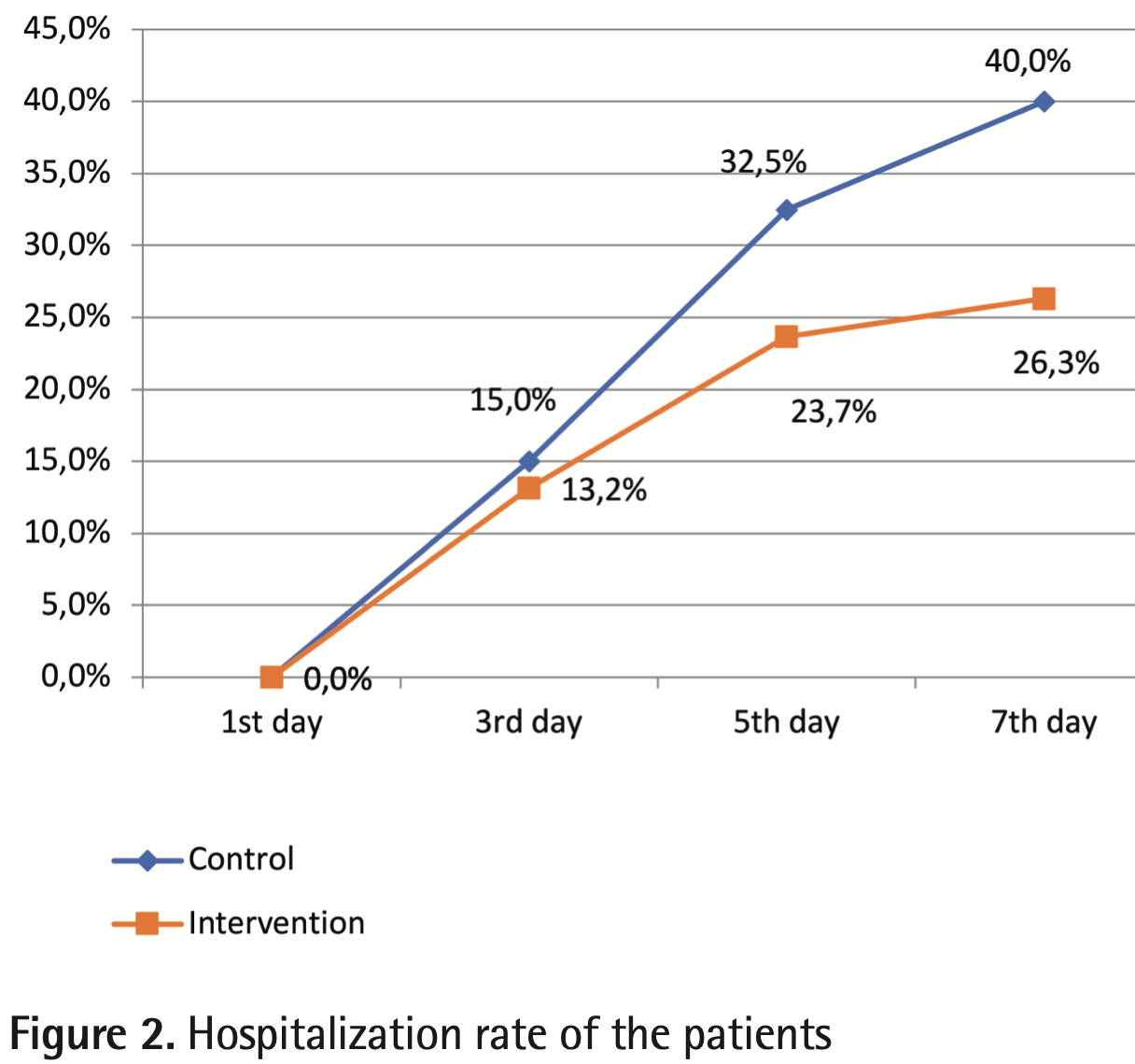
risk of hospitalization, 34.2% lower, RR 0.66, p = 0.24, treatment 10 of 38 (26.3%), control 16 of 40 (40.0%), NNT 7.3.
|
|
risk of no recovery, 79.6% lower, RR 0.20, p = 0.49, treatment 0 of 38 (0.0%), control 2 of 40 (5.0%), NNT 20, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 7, dyspnea.
|
|
risk of no recovery, 57.9% lower, RR 0.42, p = 0.010, treatment 8 of 38 (21.1%), control 20 of 40 (50.0%), NNT 3.5, day 5, dyspnea.
|
|
risk of no recovery, 47.4% lower, RR 0.53, p = 1.00, treatment 1 of 38 (2.6%), control 2 of 40 (5.0%), NNT 42, day 7, fever.
|
|
risk of no recovery, 47.4% lower, RR 0.53, p = 0.25, treatment 5 of 38 (13.2%), control 10 of 40 (25.0%), NNT 8.4, day 5, fever.
|
|
risk of no recovery, 66.1% lower, RR 0.34, p = 1.00, treatment 0 of 38 (0.0%), control 1 of 40 (2.5%), NNT 40, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 7, sore throat.
|
|
risk of no recovery, 47.4% lower, RR 0.53, p = 0.68, treatment 2 of 38 (5.3%), control 4 of 40 (10.0%), NNT 21, day 5, sore throat.
|
|
risk of no recovery, 29.8% lower, RR 0.70, p = 0.17, treatment 16 of 38 (42.1%), control 24 of 40 (60.0%), NNT 5.6, day 7, cough.
|
|
risk of no recovery, 7.1% lower, RR 0.93, p = 0.56, treatment 30 of 38 (78.9%), control 34 of 40 (85.0%), NNT 17, day 5, cough.
|
|
risk of no recovery, 21.1% lower, RR 0.79, p = 0.77, treatment 6 of 38 (15.8%), control 8 of 40 (20.0%), NNT 24, day 7, myalgia.
|
|
risk of no recovery, 38.1% lower, RR 0.62, p = 0.16, treatment 10 of 38 (26.3%), control 17 of 40 (42.5%), NNT 6.2, day 5, myalgia.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Tehrani et al., 15 Jun 2022, Randomized Controlled Trial, Iran, peer-reviewed, mean age 52.5, 5 authors, study period April 2021 - September 2021, average treatment delay 5.29 days, trial IRCT20211004052664N1.
Contact: amirrezakeyvanfar@yahoo.com.
Efficacy of Favipiravir in the Treatment of Moderate COVID-19 Patients: A Randomized, Open-label, Controlled Clinical Trial
Mediterranean Journal of Infection Microbes and Antimicrobials, doi:10.4274/mjima.galenos.2022.2022.30
Since the beginning of the Coronavirus disease-2019 (COVID-19) pandemic, scientists have studied many drugs to treat it, but none of them have been approved as a complete cure. Favipiravir is one of those drugs that effectively clears the body from the virus by interfering with the process of replication. This study aimed to determine the efficacy of favipiravir compared with supportive medication to treat moderate COVID-19 patients. Materials and Methods: In this randomized, open-label, controlled clinical trial, we examined the efficacy of favipiravir to treat moderate COVID-19 patients. The study was conducted in Labbafinejad Hospital (Tehran, Iran) from April to September 2021. A 1:1 ratio of eligible patients were assigned to the intervention and control groups. The control group received supportive medication. In addition to supportive medication, the intervention group received favipiravir. The primary endpoint was the hospitalization rate during the seven-day follow-up. And the secondary endpoints were symptoms, signs, and laboratory tests of the patients. Results: Out of 78 patients who were included in the study, 40 patients were assigned to the control group and 38 patients were assigned to the intervention group. At the beginning of treatment, the respiratory rate was higher in the intervention group (p=0.001), however, on the fifth (p=0.001) and seventh (p<0.001) days, it was significantly lower in the intervention group. In addition, oxygen saturation at the beginning of treatment was lower in the intervention group (p<0.001); however, on the fifth (p=0.016) and seventh (p<0.001) days, it was significantly higher in the intervention group. Furthermore, the consumption of favipiravir was not associated with the hospitalization rate (p=0.200). Conclusion: Favipiravir enhances respiratory manifestations in patients with moderate COVID-19 when compared to supportive medication alone.
Ethics Ethics Committee Approval: The Ethics Committee of the School of Medicine, of Shahid Beheshti University of Medical Sciences, approved this study on March 3, 2021 (approval ID: IR.SBMU.MSP.REC.1399.750).
Informed Consent: Consent form was filled out by all participants. Peer-review: Externally peer-reviewed. Financial Disclosure: The authors declared that this study received no financial support.
Authorship Contributions
Concept
References
Al-Muhsen, Sharif-Askari, Basamh, Alyounes, Jabaan et al., Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia, Front Med
Alamer, Alrashed, Alfaifi, Alosaimi, Alhassar et al., Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis, Curr Med Res Opin
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial, Front Pharmacol
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial, Front Pharmacol
Gandhi, Lynch, Rio, Mild or Moderate Covid-19, N Engl J Med
Han, Ren, Li, Yan, Ma et al., Advances and challenges in the prevention and treatment of COVID-19, Int J Med Sci
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clin Infect Dis
Jean, Lee, Hsueh, Treatment options for COVID-19: The reality and challenges, J Microbiol Immunol Infect
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, Int J Infect Dis
Lou, Liu, Yao, Hu, Su et al., Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial, Eur J Pharm Sci
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis
Marra, Kobayashi, Suzuki, Alsuhaibani, Tofaneto et al., Short-term effectiveness of COVID-19 vaccines in immunocompromised patients: A systematic literature review and metaanalysis, J Infect
Mckee, Sternberg, Stange, Laufer, Naujokat, Candidate drugs against SARS-CoV-2 and COVID-19, Pharmacol Res
Mortaz, Bassir, Roofchayee, Dezfuli, Jamaati et al., Serum cytokine levels of COVID-19 patients after 7 days of treatment with Favipiravir or Kaletra, Int Immunopharmacol
Samudrala, Kumar, Choudhary, Thakur, Wadekar et al., Virology, pathogenesis, diagnosis and in-line treatment of COVID-19, Eur J Pharmacol
Shinkai, Tsushima, Tanaka, Hagiwara, Tarumoto et al., Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial, Infect Dis Ther
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virol J
Solaymani-Dodaran, Ghanei, Bagheri, Qazvini, Vahedi et al., Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia, Int Immunopharmacol
Szabo, Lenart, Petrik, Gaspar, Kiss-Dala et al., Favipiravir treatment does not influence disease progression among adult patients hospitalized with moderate-to-severe COVID-19: a prospective, sequential cohort study from Hungary, Geroscience
Tehrani, Khabiri, Moradi, Mosavat, Khabiri, Evaluation of vitamin D levels in COVID-19 patients referred to Labafinejad hospital in Tehran and its relationship with disease severity and mortality, Clin Nutr ESPEN
Tu, Chien, Yarmishyn, Lin, Luo et al., A Review of SARS-CoV-2 and the Ongoing Clinical Trials, Int J Mol Sci
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Wang, Li, Liu, An overview of the safety, clinical application and antiviral research of the COVID-19 therapeutics, J Infect Public Health
Wu, Mcgoogan, Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention, Jama
Zhao, Zhang, Zhu, Chen, Chen et al., Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: A multicenter, openlabel, randomized trial, Int Immunopharmacol
DOI record:
{
"DOI": "10.4274/mjima.galenos.2022.2022.30",
"ISSN": [
"2147-673X"
],
"URL": "http://dx.doi.org/10.4274/mjima.galenos.2022.2022.30",
"author": [
{
"affiliation": [],
"family": "TEHRANI",
"given": "Shabnam",
"sequence": "first"
},
{
"affiliation": [],
"family": "YADEGARYNIA",
"given": "Davood",
"sequence": "additional"
},
{
"affiliation": [],
"family": "BAGHERZADE",
"given": "Afshin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "GACHKAR",
"given": "Latif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "KEYVANFAR",
"given": "Amirreza",
"sequence": "additional"
}
],
"container-title": "Mediterranean Journal of Infection Microbes and Antimicrobials",
"container-title-short": "mjima",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
6,
28
]
],
"date-time": "2022-06-28T12:48:26Z",
"timestamp": 1656420506000
},
"deposited": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T10:54:33Z",
"timestamp": 1660128873000
},
"indexed": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T11:11:51Z",
"timestamp": 1660129911967
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
8,
10
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
8,
10
]
]
}
},
"member": "2811",
"original-title": [],
"prefix": "10.4274",
"published": {
"date-parts": [
[
2022,
8,
10
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
10
]
]
},
"publisher": "Galenos Yayinevi",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://mjima.org/pdf.php?&id=340"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Immunology and Microbiology"
],
"subtitle": [],
"title": "Efficacy of Favipiravir in the Treatment of Moderate COVID-19 Patients: A Randomized, Open-label, Controlled Clinical Trial",
"type": "journal-article",
"volume": "11"
}