Comparative Analysis of Neuropsychiatric Adverse Reactions Associated with Remdesivir and Nirmatrelvir/Ritonavir in COVID-19 Treatment: Insights from EudraVigilance Data
et al., Journal of Clinical Medicine, doi:10.3390/jcm14061886, Mar 2025
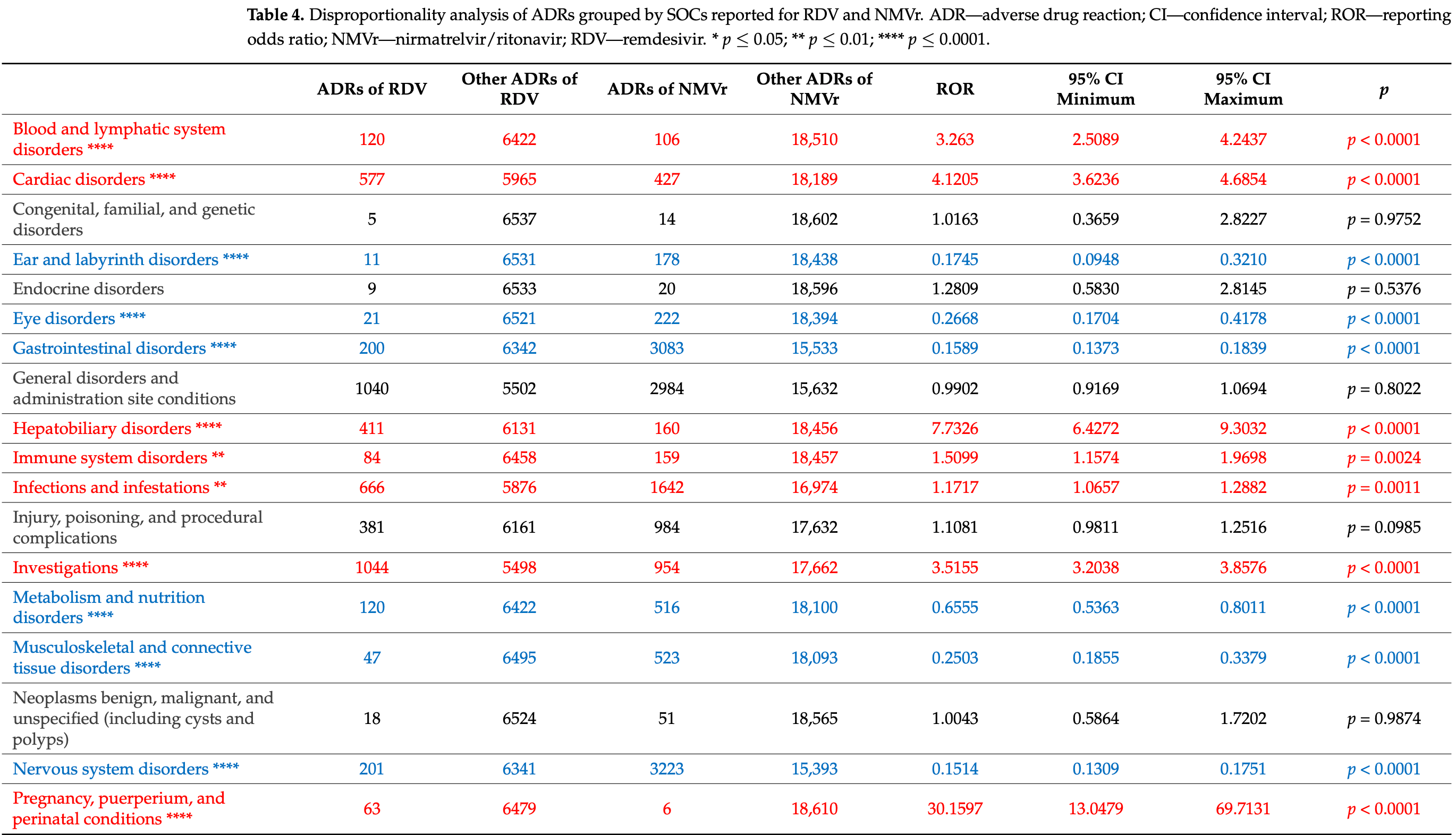
Pharmacovigilance analysis of 8,078 paxlovid 3,934 remdesivir case safety reports from the EudraVigilance database, showing paxlovid associated with significantly higher incidence of neuropsychiatric adverse drug reactions. Paxlovid reports included higher rates of nervous system disorders (17.31% vs 3.07%) and psychiatric disorders (3.61% vs 0.92%). Remdesivir was associated with higher rates of hepatobiliary disorders, renal disorders, and movement disorders, and a greater proportion of serious ADRs (84.2% vs 59.9%).
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Study covers remdesivir and paxlovid.
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Pacnejer et al., 11 Mar 2025, retrospective, peer-reviewed, 9 authors.
Contact: mihaelaprodea@umft.ro (corresponding author), aliteia.pacnejer@umft.ro, cadehelean@umft.ro, felicia.gligor@ulbsibiu.ro, claudiu.morgovan@ulbsibiu.ro, anca.butuca@ulbsibiu.ro, trandafirescu.cristina@umft.ro, oancea@umft.ro, anca.arseniu@ulbsibiu.ro.
Comparative Analysis of Neuropsychiatric Adverse Reactions Associated with Remdesivir and Nirmatrelvir/Ritonavir in COVID-19 Treatment: Insights from EudraVigilance Data
Journal of Clinical Medicine, doi:10.3390/jcm14061886
Remdesivir (RDV) and nirmatrelvir/ritonavir (NMVr) are among the most widely used antivirals in the treatment of COVID-19, aiming to reduce disease severity and progression. Adverse neuropsychiatric effects, such as anxiety, sleep disturbances, and movement disorders, have emerged as significant concerns associated with these treatments. To better understand the safety profiles of RDV and NMVr, this study performs a pharmacovigilance analysis of individual case safety reports (ICSRs) from the EudraVigilance (EV) database. Objectives: This study evaluates the risk of neuropsychiatric adverse events associated with RDV and NMVr. Comparisons with other antiviral drugs, including darunavir, sofosbuvir, ribavirin, tenofovir, ritonavir, and sotrovimab, are also performed to develop a comprehensive understanding of the safety profiles. Methods: A retrospective analysis of ICSRs submitted to EV until 7 July 2024, with data extraction on 12 July 2024, was conducted. Demographic characteristics (age, sex, geographic region, and reporter type) and case severity were included in the descriptive analysis. Disproportionality analysis using reporting odds ratio (ROR) and 95% confidence intervals (CI) was performed to compare adverse drug reaction (ADRs) frequencies across 27 system organ classes (SOCs), with emphasis on "Nervous system disorders" and "Psychiatric disorders. Results: The total number of ICSRs was significantly higher for NMVr (n = 8078) compared to RDV (n = 3934). Nervous system disorders accounted for 3.07% of the total RDV reports and for 17.31% of NMVr reports, while psychiatric disorders represented 0.92% of the total ADRs reported for RDV (n = 60) and 3.61% for NMVr (n = 672). On the other hand, RDV showed a significantly lower frequency of reporting headache compared to NMVr (ROR: 0.1057; 95% CI: 0.0676-0.1653). Conclusions: NMVr presents a higher risk of neuropsychiatric ADRs than RDV, underscoring the need for enhanced monitoring, particularly in patients with preexisting central nervous system (CNS) conditions. These findings contribute to optimizing antiviral safety and informing clinical decision making.
Funding: We would like to acknowledge the Victor Babes University of Medicine and Pharmacy Timis , oara for their support in covering the costs of publication for this research paper. Institutional Review Board Statement: Ethical review and approval were waived for this study, due to all reports in the EV portal are anonymized and do not include personal data.
Informed Consent Statement: Not applicable. Data Availability Statement: Data are contained within the article.
Conflicts of Interest: The authors declare no conflicts of interest.
Abbreviations The
References
Adamsick, Gandhi, Bidell, Elshaboury, Bhattacharyya et al., Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19, J. Am. Soc. Nephrol, doi:10.1681/ASN.2020050589
Aggarwal, Beaty, Bennett, Fish, Jacobs et al., Real-World Use of Nirmatrelvir-Ritonavir in COVID-19 Outpatients during BQ.1, BQ.1.1., and XBB.1.5 Predominant Omicron Variants in Three U.S. Health Systems: A Retrospective Cohort Study, Lancet Reg. Health Am, doi:10.1016/j.lana.2024.100693
Allavena, Le Moal, Michau, Chiffoleau, Raffi, Neuropsychiatric Adverse Events after Switching from an Antiretroviral Regimen Containing Efavirenz without Tenofovir to an Efavirenz Regimen Containing Tenofovir: A Report of Nine Cases, Antivir. Ther, doi:10.1177/135965350601100214
Amani, Amani, Efficacy and Safety of Sotrovimab in Patients with COVID-19: A Rapid Review and Meta-analysis, Rev. Med. Virol, doi:10.1002/rmv.2402
Back, Sekar, Hoetelmans, Darunavir, Pharmacokinetics and Drug Interactions, Antivir. Ther, doi:10.1177/135965350801300101
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of COVID-19-Final Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Bilbul, Paparone, Kim, Mutalik, Ernst, Psychopharmacology of COVID-19, Psychosomatics
Brown, Benjamin, Lunn, Bharucha, Zandi et al., Diagnosis, and Management of Neuroinflammation in COVID-19, BMJ
Carothers, Birrer, Vo, Acetylcysteine for the Treatment of Suspected Remdesivir-Associated Acute Liver Failure in COVID-19: A Case Series, Pharmacother. J. Hum. Pharmacol. Drug Ther, doi:10.1002/phar.2464
Ferrer, Rakhmanina, Neuropsychiatric Effects of Tenofovir in Comparison with Other Antiretroviral Drugs, Neurobehav. HIV Med, doi:10.2147/NBHIV.S24888
Focosi, Casadevall, Franchini, Maggi, Sotrovimab: A Review of Its Efficacy against SARS-CoV-2 Variants, Viruses, doi:10.3390/v16020217
García, Sánchez, Huerta, Gómez-Arnau, Covid-19 Treatment-Induced Neuropsychiatric Adverse Effects, Gen. Hosp. Psychiatry, doi:10.1016/j.genhosppsych.2020.06.001
Goldman, Lye, Hui, Marks, Bruno et al., Remdesivir for 5 or 10 Days in Patients with Severe COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2015301
Gonçalves De Andrade, Šimončičová, Carrier, Vecchiarelli, Robert et al., Microglia Fighting for Neurological and Mental Health: On the Central Nervous System Frontline of COVID-19 Pandemic, Front. Cell. Neurosci, doi:10.3389/fncel.2021.647378
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients, N. Engl. J. Med, doi:10.1056/NEJMoa2116846
Grein, Ohmagari, Shin, Diaz, Asperges et al., Compassionate Use of Remdesivir for Patients with Severe COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2007016
Gupta, Gonzalez-Rojas, Juarez, Crespo Casal, Moya et al., Effect of Sotrovimab on Hospitalization or Death Among High-Risk Patients with Mild to Moderate COVID-19, JAMA, doi:10.1001/jama.2022.2832
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2118542
Hu, Chang, Yang, Wang, Xie et al., Pharmacokinetics and Tissue Distribution of Remdesivir and Its Metabolites Nucleotide Monophosphate, Nucleotide Triphosphate, and Nucleoside in Mice, Acta Pharmacol. Sin, doi:10.1038/s41401-020-00537-9
Huang, Wang, Li, Ren, Zhao et al., Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Iriana, Curry, Afdhal, Neurologic Manifestations of Hepatitis C Virus Infection, Clin. Liver Dis, doi:10.1016/j.cld.2017.03.008
Lee, Yang, Jung, Kim, Yon et al., Neuropsychological Adverse Drug Reactions of Remdesivir: Analysis Using VigiBase, the WHO Global Database of Individual Case Safety Reports, Eur. Rev. Med. Pharmacol. Sci, doi:10.26355/eurrev_202112_27435
Li, Zhang, Liu, Wang, Liu, Adverse Events Associated with Nirmatrelvir/Ritonavir: A Pharmacovigilance Analysis Based on FAERS, Pharmaceuticals, doi:10.3390/ph15121455
Lin, Fadel, Huang, Milinovich, Sacha et al., Nirmatrelvir or Molnupiravir Use and Severe Outcomes from Omicron Infections, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2023.35077
Lory, Combret, Michot, Veyrac, Chouchana et al., Safety Profile of the Lopinavir/Ritonavir Combination before and during the SARS-CoV-2 Pandemic, Therapies, doi:10.1016/j.therap.2022.10.066
Majerová, Konvalinka, Viral Proteases as Therapeutic Targets, Mol. Asp. Med, doi:10.1016/j.mam.2022.101159
Marin, Behl, Negrut, Bungau, Management of Antiretroviral Therapy with Boosted Protease Inhibitors-Darunavir/Ritonavir or Darunavir/Cobicistat, Biomedicines, doi:10.3390/biomedicines9030313
Marinho, Novais, Marques, Bragança, Efavirenz and Neuropsychiatric Effects-When the Treatment Complicates Matter Further, Eur. Psychiatry, doi:10.1016/j.eurpsy.2017.01.2255
Matt, Gaskill, Dopaminergic Impact of CART and Anti-Depressants on HIV Neuropathogenesis in Older Adults, Brain Res, doi:10.1016/j.brainres.2019.146398
Moltó, Valle, Clotet, Interacciones Medicamentosas de Darunavir, Enferm. Infecc. Microbiol. Clin, doi:10.1016/S0213-005X(08)76553-4
Morgovan, Dobrea, Chis, Juncan, Arseniu et al., A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database, Pharmaceuticals, doi:10.3390/ph16030455
Ohl, Miller, Lund, Kobayashi, Richardson Miell et al., Association of Remdesivir Treatment with Survival and Length of Hospital Stay Among US Veterans Hospitalized with COVID-19, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.14741
Pacnejer, Butuca, Dobrea, Arseniu, Frum et al., Neuropsychiatric Burden of SARS-CoV-2: A Review of Its Physiopathology, Underlying Mechanisms, and Management Strategies, Viruses, doi:10.3390/v16121811
Panahi, Gorabi, Talaei, Beiraghdar, Akbarzadeh et al., An Overview on the Treatments and Prevention against COVID-19, Virol. J, doi:10.1186/s12985-023-01973-9
Patone, Snelling, Tibble, Coupland, Sheikh et al., Uptake and Safety of Sotrovimab for Prevention of Severe COVID-19 in a Cohort and Self-Controlled Case Series Study, Commun. Med, doi:10.1038/s43856-024-00720-7
Plasencia-García, Rodríguez-Menéndez, Rico-Rangel, Rubio-García, Torelló-Iserte et al., Drug-Drug Interactions between COVID-19 Treatments and Antipsychotics Drugs: Integrated Evidence from 4 Databases and a Systematic Review, Psychopharmacology, doi:10.1007/s00213-020-05716-4
Punekar, Kshirsagar, Tellapragada, Patil, Repurposing of Antiviral Drugs for COVID-19 and Impact of Repurposed Drugs on the Nervous System, Microb. Pathog, doi:10.1016/j.micpath.2022.105608
Rochette, Ghibu, Mechanics Insights of Alpha-Lipoic Acid against Cardiovascular Diseases during COVID-19 Infection, Int. J. Mol. Sci, doi:10.3390/ijms22157979
Rubin, Chan-Tack, Farley, Sherwat, FDA Approval of Remdesivir-A Step in the Right Direction, N. Engl. J. Med, doi:10.1056/NEJMp2032369
Sakamaki, Kamimura, Fukui, Watanabe, Sakai et al., A Case Report of Psychiatric Symptoms Following Direct-Acting Antiviral and Ribavirin Combination Therapy for Chronic Hepatitis C in a Patient with Innate Anxiety, BMC Gastroenterol, doi:10.1186/s12876-019-1013-1
Scavone, Mascolo, Rafaniello, Sportiello, Trama et al., Therapeutic Strategies to Fight COVID-19: Which Is the Status Artis?, Br. J. Pharmacol, doi:10.1111/bph.15452
Spinner, Gottlieb, Criner, Arribas López, Cattelan et al., Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19, JAMA, doi:10.1001/jama.2020.16349
Strohbehn, Ouyang, Lee, Zhao, Harden et al., The Effect of Nirmatrelvir-Ritonavir on Short-and Long-Term Adverse Outcomes from COVID-19 Among Patients with Kidney Disease: A Propensity Score-Matched Study, Open Forum Infect. Dis, doi:10.1093/ofid/ofae756
Sun, Deng, Huang, He, Huang, Data Mining of Adverse Drug Event Signals with Nirmatrelvir/Ritonavir from FAERS, PLoS ONE, doi:10.1371/journal.pone.0316573
Tang, Masur, Sims, Nelson, Osinusi et al., Safe and Effective Sofosbuvir-Based Therapy in Patients with Mental Health Disease on Hepatitis C Virus Treatment, World J. Hepatol, doi:10.4254/wjh.v8.i31.1318
Van Laar, De Boer, Gombert-Handoko, Guchelaar, Zwaveling, LUMC-Covid-19 research group Liver and Kidney Function in Patients with Covid-19 Treated with Remdesivir, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14831
Vintila, Arseniu, Morgovan, Butuca, Sava et al., A Pharmacovigilance Study Regarding the Risk of Antibiotic-Associated Clostridioides Difficile Infection Based on Reports from the EudraVigilance Database: Analysis of Some of the Most Used Antibiotics in Intensive Care Units, Pharmaceuticals, doi:10.3390/ph16111585
Wang, Vashistha, Kaur, Houchens, Serotonin Syndrome: Preventing, Recognizing, and Treating It, Cleve Clin. J. Med, doi:10.3949/ccjm.83a.15129
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Wang, Zhou, Zhang, Zhao, Du et al., Evaluation of the Efficacy and Safety of Intravenous Remdesivir in Adult Patients with Severe COVID-19: Study Protocol for a Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multicentre Trial, Trials, doi:10.1186/s13063-020-04352-9
Watson, Caster, Rochon, Den Ruijter, Reported Adverse Drug Reactions in Women and Men: Aggregated Evidence from Globally Collected Individual Case Reports during Half a Century, EClinicalMedicine, doi:10.1016/j.eclinm.2019.10.001
Winston, Fätkenheuer, Arribas, Hill, Van Delft et al., Neuropsychiatric Adverse Events with Ritonavir-Boosted Darunavir Monotherapy in HIV-Infected Individuals: A Randomised Prospective Study, HIV Clin. Trials, doi:10.1310/hct1103-163
Wong, Au, Cheng, Man, Lau et al., Remdesivir Use and Risks of Acute Kidney Injury and Acute Liver Injury among Patients Hospitalised with COVID-19: A Self-Controlled Case Series Study, Aliment. Pharmacol. Ther, doi:10.1111/apt.16894
Zareifopoulos, Lagadinou, Karela, Kyriakopoulou, Velissaris, Neuropsychiatric Effects of Antiviral Drugs, Cureus, doi:10.7759/cureus.9536
Zhou, Yu, Du, Fan, Liu et al., Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.3390/jcm14061886",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm14061886",
"abstract": "<jats:p>Remdesivir (RDV) and nirmatrelvir/ritonavir (NMVr) are among the most widely used antivirals in the treatment of COVID-19, aiming to reduce disease severity and progression. Adverse neuropsychiatric effects, such as anxiety, sleep disturbances, and movement disorders, have emerged as significant concerns associated with these treatments. To better understand the safety profiles of RDV and NMVr, this study performs a pharmacovigilance analysis of individual case safety reports (ICSRs) from the EudraVigilance (EV) database. Objectives: This study evaluates the risk of neuropsychiatric adverse events associated with RDV and NMVr. Comparisons with other antiviral drugs, including darunavir, sofosbuvir, ribavirin, tenofovir, ritonavir, and sotrovimab, are also performed to develop a comprehensive understanding of the safety profiles. Methods: A retrospective analysis of ICSRs submitted to EV until 7 July 2024, with data extraction on 12 July 2024, was conducted. Demographic characteristics (age, sex, geographic region, and reporter type) and case severity were included in the descriptive analysis. Disproportionality analysis using reporting odds ratio (ROR) and 95% confidence intervals (CI) was performed to compare adverse drug reaction (ADRs) frequencies across 27 system organ classes (SOCs), with emphasis on “Nervous system disorders” and “Psychiatric disorders. Results: The total number of ICSRs was significantly higher for NMVr (n = 8078) compared to RDV (n = 3934). Nervous system disorders accounted for 3.07% of the total RDV reports and for 17.31% of NMVr reports, while psychiatric disorders represented 0.92% of the total ADRs reported for RDV (n = 60) and 3.61% for NMVr (n = 672). On the other hand, RDV showed a significantly lower frequency of reporting headache compared to NMVr (ROR: 0.1057; 95% CI: 0.0676–0.1653). Conclusions: NMVr presents a higher risk of neuropsychiatric ADRs than RDV, underscoring the need for enhanced monitoring, particularly in patients with preexisting central nervous system (CNS) conditions. These findings contribute to optimizing antiviral safety and informing clinical decision making.</jats:p>",
"alternative-id": [
"jcm14061886"
],
"author": [
{
"affiliation": [
{
"name": "Department of Toxicology, Drug Industry, Management and Legislation, Faculty of Pharmacy, “Victor Babeș” University of Medicine and Pharmacy, 2nd Eftimie Murgu Square, 300041 Timișoara, Romania"
},
{
"name": "Preclinical Department, Faculty of Medicine, “Lucian Blaga” University of Sibiu, 550169 Sibiu, Romania"
}
],
"family": "Pacnejer",
"given": "Aliteia-Maria",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-3002-7656",
"affiliation": [
{
"name": "Department of ENT, “Victor Babeș” University of Medicine and Pharmacy, Eftimie Murgu Square No. 2, 300041 Timișoara, Romania"
}
],
"authenticated-orcid": false,
"family": "Negru",
"given": "Mihaela Cristina",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9801-0060",
"affiliation": [
{
"name": "Preclinical Department, Faculty of Medicine, “Lucian Blaga” University of Sibiu, 550169 Sibiu, Romania"
}
],
"authenticated-orcid": false,
"family": "Arseniu",
"given": "Anca Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Discipline of Pharmaceutical Chemistry, Faculty of Pharmacy, “Victor Babeș” University of Medicine and Pharmacy, 2nd Eftimie Murgu Square, 300041 Timișoara, Romania"
}
],
"family": "Trandafirescu",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonology, Center for Research and Innovation in Personalized Medicine of Respiratory Diseases, “Victor Babeș” University of Medicine and Pharmacy, 300041 Timișoara, Romania"
}
],
"family": "Oancea",
"given": "Cristian",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3167-9864",
"affiliation": [
{
"name": "Preclinical Department, Faculty of Medicine, “Lucian Blaga” University of Sibiu, 550169 Sibiu, Romania"
}
],
"authenticated-orcid": false,
"family": "Gligor",
"given": "Felicia Gabriela",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2730-8729",
"affiliation": [
{
"name": "Preclinical Department, Faculty of Medicine, “Lucian Blaga” University of Sibiu, 550169 Sibiu, Romania"
}
],
"authenticated-orcid": false,
"family": "Morgovan",
"given": "Claudiu",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1540-641X",
"affiliation": [
{
"name": "Preclinical Department, Faculty of Medicine, “Lucian Blaga” University of Sibiu, 550169 Sibiu, Romania"
}
],
"authenticated-orcid": false,
"family": "Butuca",
"given": "Anca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Toxicology, Drug Industry, Management and Legislation, Faculty of Pharmacy, “Victor Babeș” University of Medicine and Pharmacy, 2nd Eftimie Murgu Square, 300041 Timișoara, Romania"
},
{
"name": "Research Center for Pharmaco-Toxicological Evaluations, Faculty of Pharmacy, “Victor Babeș” University of Medicine and Pharmacy, Eftimie Murgu Square No. 2, 300041 Timișoara, Romania"
}
],
"family": "Dehelean",
"given": "Cristina Adriana",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
3,
11
]
],
"date-time": "2025-03-11T12:59:52Z",
"timestamp": 1741697992000
},
"deposited": {
"date-parts": [
[
2025,
3,
12
]
],
"date-time": "2025-03-12T17:35:19Z",
"timestamp": 1741800919000
},
"funder": [
{
"name": "Victor Babes University of Medicine and Pharmacy Timișoara"
}
],
"indexed": {
"date-parts": [
[
2025,
3,
13
]
],
"date-time": "2025-03-13T04:21:34Z",
"timestamp": 1741839694182,
"version": "3.38.0"
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2025,
3,
11
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2025,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
11
]
],
"date-time": "2025-03-11T00:00:00Z",
"timestamp": 1741651200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/14/6/1886/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1886",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
3,
11
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
11
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "ref_1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref_2",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.3390/ijms22157979",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Rochette, L., and Ghibu, S. (2021). Mechanics Insights of Alpha-Lipoic Acid against Cardiovascular Diseases during COVID-19 Infection. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1016/j.micpath.2022.105608",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Punekar, M., Kshirsagar, M., Tellapragada, C., and Patil, K. (2022). Repurposing of Antiviral Drugs for COVID-19 and Impact of Repurposed Drugs on the Nervous System. Microb. Pathog., 168."
},
{
"DOI": "10.3390/v16121811",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Pacnejer, A.-M., Butuca, A., Dobrea, C.M., Arseniu, A.M., Frum, A., Gligor, F.G., Arseniu, R., Vonica, R.C., Vonica-Tincu, A.L., and Oancea, C. (2024). Neuropsychiatric Burden of SARS-CoV-2: A Review of Its Physiopathology, Underlying Mechanisms, and Management Strategies. Viruses, 16."
},
{
"DOI": "10.1056/NEJMoa2015301",
"article-title": "Remdesivir for 5 or 10 Days in Patients with Severe COVID-19",
"author": "Goldman",
"doi-asserted-by": "crossref",
"first-page": "1827",
"journal-title": "N. Engl. J. Med.",
"key": "ref_6",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of COVID-19—Final Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N. Engl. J. Med.",
"key": "ref_7",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007016",
"article-title": "Compassionate Use of Remdesivir for Patients with Severe COVID-19",
"author": "Grein",
"doi-asserted-by": "crossref",
"first-page": "2327",
"journal-title": "N. Engl. J. Med.",
"key": "ref_8",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "ref_9",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1111/bph.15452",
"article-title": "Therapeutic Strategies to Fight COVID-19: Which Is the Status Artis?",
"author": "Scavone",
"doi-asserted-by": "crossref",
"first-page": "2128",
"journal-title": "Br. J. Pharmacol.",
"key": "ref_10",
"volume": "179",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "ref_11",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "ref_12",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04352-9",
"article-title": "Evaluation of the Efficacy and Safety of Intravenous Remdesivir in Adult Patients with Severe COVID-19: Study Protocol for a Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multicentre Trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "422",
"journal-title": "Trials",
"key": "ref_13",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19",
"author": "Spinner",
"doi-asserted-by": "crossref",
"first-page": "1048",
"journal-title": "JAMA",
"key": "ref_14",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1002/phar.2464",
"article-title": "Acetylcysteine for the Treatment of Suspected Remdesivir-Associated Acute Liver Failure in COVID-19: A Case Series",
"author": "Carothers",
"doi-asserted-by": "crossref",
"first-page": "1166",
"journal-title": "Pharmacother. J. Hum. Pharmacol. Drug Ther.",
"key": "ref_15",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1007/s00213-020-05716-4",
"article-title": "Drug-Drug Interactions between COVID-19 Treatments and Antipsychotics Drugs: Integrated Evidence from 4 Databases and a Systematic Review",
"doi-asserted-by": "crossref",
"first-page": "329",
"journal-title": "Psychopharmacology",
"key": "ref_16",
"volume": "238",
"year": "2021"
},
{
"DOI": "10.1310/hct1103-163",
"article-title": "Neuropsychiatric Adverse Events with Ritonavir-Boosted Darunavir Monotherapy in HIV-Infected Individuals: A Randomised Prospective Study",
"author": "Winston",
"doi-asserted-by": "crossref",
"first-page": "163",
"journal-title": "HIV Clin. Trials",
"key": "ref_17",
"volume": "11",
"year": "2010"
},
{
"DOI": "10.26226/morressier.5885d719d462b8028d892917",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Marinho, M., Novais, C., Marques, J., and Bragança, M. (2017). Efavirenz and Neuropsychiatric Effects–When the Treatment Complicates Matter Further. Eur. Psychiatry, 41."
},
{
"DOI": "10.1016/j.therap.2022.10.066",
"article-title": "Safety Profile of the Lopinavir/Ritonavir Combination before and during the SARS-CoV-2 Pandemic",
"author": "Lory",
"doi-asserted-by": "crossref",
"first-page": "419",
"journal-title": "Therapies",
"key": "ref_19",
"volume": "78",
"year": "2023"
},
{
"DOI": "10.1016/j.genhosppsych.2020.06.001",
"article-title": "Covid-19 Treatment-Induced Neuropsychiatric Adverse Effects",
"author": "Huerta",
"doi-asserted-by": "crossref",
"first-page": "163",
"journal-title": "Gen. Hosp. Psychiatry",
"key": "ref_20",
"volume": "67",
"year": "2020"
},
{
"DOI": "10.1016/j.psym.2020.05.006",
"article-title": "Psychopharmacology of COVID-19",
"author": "Bilbul",
"doi-asserted-by": "crossref",
"first-page": "411",
"journal-title": "Psychosomatics",
"key": "ref_21",
"volume": "61",
"year": "2020"
},
{
"key": "ref_22",
"unstructured": "International Council for Harmonisation of Technical (2021). Requirements for Pharmaceuticals for Human Use. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 24.1, International Council for Harmonisation of Technical."
},
{
"DOI": "10.3390/ph16030455",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Morgovan, C., Dobrea, C.M., Chis, A.A., Juncan, A.M., Arseniu, A.M., Rus, L.L., Gligor, F.G., Ardelean, S.A., Stoicescu, L., and Ghibu, S. (2023). A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals, 16."
},
{
"DOI": "10.3390/ph16111585",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Vintila, B.I., Arseniu, A.M., Morgovan, C., Butuca, A., Sava, M., Bîrluțiu, V., Rus, L.L., Ghibu, S., Bereanu, A.S., and Roxana Codru, I. (2023). A Pharmacovigilance Study Regarding the Risk of Antibiotic-Associated Clostridioides Difficile Infection Based on Reports from the EudraVigilance Database: Analysis of Some of the Most Used Antibiotics in Intensive Care Units. Pharmaceuticals, 16."
},
{
"key": "ref_25",
"unstructured": "MedCalc Software Ltd. (2025, January 18). Odds Ratio Calculator. Version 23.1.3. Available online: https://www.medcalc.org/calc/odds_ratio.php."
},
{
"key": "ref_26",
"unstructured": "European Medicines Agency (2025, January 18). Screening for Adverse Reactions in EudraVigilance. Available online: https://www.ema.europa.eu/en/documents/other/screening-adverse-reactions-eudravigilance_en.pdf."
},
{
"DOI": "10.1056/NEJMp2032369",
"article-title": "FDA Approval of Remdesivir—A Step in the Right Direction",
"author": "Rubin",
"doi-asserted-by": "crossref",
"first-page": "2598",
"journal-title": "N. Engl. J. Med.",
"key": "ref_27",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.14741",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Ohl, M.E., Miller, D.R., Lund, B.C., Kobayashi, T., Richardson Miell, K., Beck, B.F., Alexander, B., Crothers, K., and Vaughan Sarrazin, M.S. (2021). Association of Remdesivir Treatment with Survival and Length of Hospital Stay Among US Veterans Hospitalized with COVID-19. JAMA Netw. Open, 4."
},
{
"DOI": "10.1001/jamanetworkopen.2023.35077",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Lin, D.-Y., Abi Fadel, F., Huang, S., Milinovich, A.T., Sacha, G.L., Bartley, P., Duggal, A., and Wang, X. (2023). Nirmatrelvir or Molnupiravir Use and Severe Outcomes from Omicron Infections. JAMA Netw. Open, 6."
},
{
"DOI": "10.1016/j.lana.2024.100693",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Aggarwal, N.R., Beaty, L.E., Bennett, T.D., Fish, L.E., Jacobs, J.R., Mayer, D.A., Molina, K.C., Peers, J.L., Richardson, D.B., and Russell, S. (2024). Real-World Use of Nirmatrelvir-Ritonavir in COVID-19 Outpatients during BQ.1, BQ.1.1., and XBB.1.5 Predominant Omicron Variants in Three U.S. Health Systems: A Retrospective Cohort Study. Lancet Reg. Health Am., 31."
},
{
"article-title": "Neuropsychiatric Effects of Tenofovir in Comparison with Other Antiretroviral Drugs",
"author": "Ferrer",
"first-page": "1",
"journal-title": "Neurobehav. HIV Med.",
"key": "ref_31",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.1177/135965350601100214",
"article-title": "Neuropsychiatric Adverse Events after Switching from an Antiretroviral Regimen Containing Efavirenz without Tenofovir to an Efavirenz Regimen Containing Tenofovir: A Report of Nine Cases",
"author": "Allavena",
"doi-asserted-by": "crossref",
"first-page": "263",
"journal-title": "Antivir. Ther.",
"key": "ref_32",
"volume": "11",
"year": "2006"
},
{
"DOI": "10.4254/wjh.v8.i31.1318",
"article-title": "Safe and Effective Sofosbuvir-Based Therapy in Patients with Mental Health Disease on Hepatitis C Virus Treatment",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "1318",
"journal-title": "World J. Hepatol.",
"key": "ref_33",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1016/j.cld.2017.03.008",
"article-title": "Neurologic Manifestations of Hepatitis C Virus Infection",
"author": "Iriana",
"doi-asserted-by": "crossref",
"first-page": "535",
"journal-title": "Clin. Liver Dis.",
"key": "ref_34",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.7759/cureus.9536",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Zareifopoulos, N., Lagadinou, M., Karela, A., Kyriakopoulou, O., and Velissaris, D. (2020). Neuropsychiatric Effects of Antiviral Drugs. Cureus, 12."
},
{
"DOI": "10.1177/135965350801300101",
"article-title": "Darunavir: Pharmacokinetics and Drug Interactions",
"author": "Back",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Antivir. Ther.",
"key": "ref_36",
"volume": "13",
"year": "2008"
},
{
"DOI": "10.1016/S0213-005X(08)76553-4",
"article-title": "Interacciones Medicamentosas de Darunavir",
"author": "Valle",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Enferm. Infecc. Microbiol. Clin.",
"key": "ref_37",
"volume": "26",
"year": "2008"
},
{
"DOI": "10.3390/biomedicines9030313",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Marin, R.-C., Behl, T., Negrut, N., and Bungau, S. (2021). Management of Antiretroviral Therapy with Boosted Protease Inhibitors—Darunavir/Ritonavir or Darunavir/Cobicistat. Biomedicines, 9."
},
{
"DOI": "10.3390/ph15121455",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Li, M., Zhang, Q.-S., Liu, X.-L., Wang, H.-L., and Liu, W. (2022). Adverse Events Associated with Nirmatrelvir/Ritonavir: A Pharmacovigilance Analysis Based on FAERS. Pharmaceuticals, 15."
},
{
"DOI": "10.1038/s41401-020-00537-9",
"article-title": "Pharmacokinetics and Tissue Distribution of Remdesivir and Its Metabolites Nucleotide Monophosphate, Nucleotide Triphosphate, and Nucleoside in Mice",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "1195",
"journal-title": "Acta Pharmacol. Sin.",
"key": "ref_40",
"volume": "42",
"year": "2021"
},
{
"DOI": "10.1111/apt.16894",
"article-title": "Remdesivir Use and Risks of Acute Kidney Injury and Acute Liver Injury among Patients Hospitalised with COVID-19: A Self-Controlled Case Series Study",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "121",
"journal-title": "Aliment. Pharmacol. Ther.",
"key": "ref_41",
"volume": "56",
"year": "2022"
},
{
"DOI": "10.1111/bcp.14831",
"article-title": "LUMC-Covid-19 research group Liver and Kidney Function in Patients with Covid-19 Treated with Remdesivir",
"author": "Guchelaar",
"doi-asserted-by": "crossref",
"first-page": "4450",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_42",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1681/ASN.2020050589",
"article-title": "Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19",
"author": "Adamsick",
"doi-asserted-by": "crossref",
"first-page": "1384",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "ref_43",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1186/s12985-023-01973-9",
"article-title": "An Overview on the Treatments and Prevention against COVID-19",
"author": "Panahi",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Virol. J.",
"key": "ref_44",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1186/s12876-019-1013-1",
"doi-asserted-by": "crossref",
"key": "ref_45",
"unstructured": "Sakamaki, A., Kamimura, K., Fukui, N., Watanabe, H., Sakai, N., Tominaga, K., Mizuno, K., Takamura, M., Kawai, H., and Sugai, T. (2019). A Case Report of Psychiatric Symptoms Following Direct-Acting Antiviral and Ribavirin Combination Therapy for Chronic Hepatitis C in a Patient with Innate Anxiety. BMC Gastroenterol., 19."
},
{
"DOI": "10.3390/v16020217",
"doi-asserted-by": "crossref",
"key": "ref_46",
"unstructured": "Focosi, D., Casadevall, A., Franchini, M., and Maggi, F. (2024). Sotrovimab: A Review of Its Efficacy against SARS-CoV-2 Variants. Viruses, 16."
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of Sotrovimab on Hospitalization or Death Among High-Risk Patients with Mild to Moderate COVID-19",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "JAMA",
"key": "ref_47",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1038/s43856-024-00720-7",
"article-title": "Uptake and Safety of Sotrovimab for Prevention of Severe COVID-19 in a Cohort and Self-Controlled Case Series Study",
"author": "Patone",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "Commun. Med.",
"key": "ref_48",
"volume": "5",
"year": "2025"
},
{
"DOI": "10.1002/rmv.2402",
"doi-asserted-by": "crossref",
"key": "ref_49",
"unstructured": "Amani, B., and Amani, B. (2022). Efficacy and Safety of Sotrovimab in Patients with COVID-19: A Rapid Review and Meta-analysis. Rev. Med. Virol., 32."
},
{
"DOI": "10.3949/ccjm.83a.15129",
"article-title": "Serotonin Syndrome: Preventing, Recognizing, and Treating It",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "810",
"journal-title": "Cleve Clin. J. Med.",
"key": "ref_50",
"volume": "83",
"year": "2016"
},
{
"DOI": "10.1016/j.mam.2022.101159",
"doi-asserted-by": "crossref",
"key": "ref_51",
"unstructured": "Majerová, T., and Konvalinka, J. (2022). Viral Proteases as Therapeutic Targets. Mol. Asp. Med., 88."
},
{
"DOI": "10.1016/j.brainres.2019.146398",
"doi-asserted-by": "crossref",
"key": "ref_52",
"unstructured": "Matt, S.M., and Gaskill, P.J. (2019). Dopaminergic Impact of CART and Anti-Depressants on HIV Neuropathogenesis in Older Adults. Brain Res., 1723."
},
{
"DOI": "10.1016/j.eclinm.2019.10.001",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Watson, S., Caster, O., Rochon, P.A., and den Ruijter, H. (2019). Reported Adverse Drug Reactions in Women and Men: Aggregated Evidence from Globally Collected Individual Case Reports during Half a Century. EClinicalMedicine, 17."
},
{
"article-title": "Neuropsychological Adverse Drug Reactions of Remdesivir: Analysis Using VigiBase, the WHO Global Database of Individual Case Safety Reports",
"author": "Lee",
"first-page": "7390",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref_54",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofae756",
"doi-asserted-by": "crossref",
"key": "ref_55",
"unstructured": "Strohbehn, I.A., Ouyang, T., Lee, M.D., Zhao, S., Harden, D., Mejia, S.M., Cao, A., Bhattacharyya, R.P., and Sise, M.E. (2024). The Effect of Nirmatrelvir-Ritonavir on Short- and Long-Term Adverse Outcomes from COVID-19 Among Patients with Kidney Disease: A Propensity Score–Matched Study. Open Forum Infect. Dis., 12."
},
{
"DOI": "10.1371/journal.pone.0316573",
"doi-asserted-by": "crossref",
"key": "ref_56",
"unstructured": "Sun, J., Deng, X., Huang, J., He, G., and Huang, S. (2024). Data Mining of Adverse Drug Event Signals with Nirmatrelvir/Ritonavir from FAERS. PLoS ONE, 19."
},
{
"DOI": "10.3389/fncel.2021.647378",
"doi-asserted-by": "crossref",
"key": "ref_57",
"unstructured": "Gonçalves de Andrade, E., Šimončičová, E., Carrier, M., Vecchiarelli, H.A., Robert, M.-È., and Tremblay, M.-È. (2021). Microglia Fighting for Neurological and Mental Health: On the Central Nervous System Frontline of COVID-19 Pandemic. Front. Cell. Neurosci., 15."
},
{
"DOI": "10.1136/bmj-2022-073923",
"doi-asserted-by": "crossref",
"key": "ref_58",
"unstructured": "Brown, R.L., Benjamin, L., Lunn, M.P., Bharucha, T., Zandi, M.S., Hoskote, C., McNamara, P., and Manji, H. (2023). Pathophysiology, Diagnosis, and Management of Neuroinflammation in COVID-19. BMJ, 382."
}
],
"reference-count": 58,
"references-count": 58,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/14/6/1886"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Comparative Analysis of Neuropsychiatric Adverse Reactions Associated with Remdesivir and Nirmatrelvir/Ritonavir in COVID-19 Treatment: Insights from EudraVigilance Data",
"type": "journal-article",
"volume": "14"
}
pacnejer