Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19
et al., PLOS Medicine, doi:10.1371/journal.pmed.1004120, FLARE, NCT04499677, Feb 2022 (preprint)
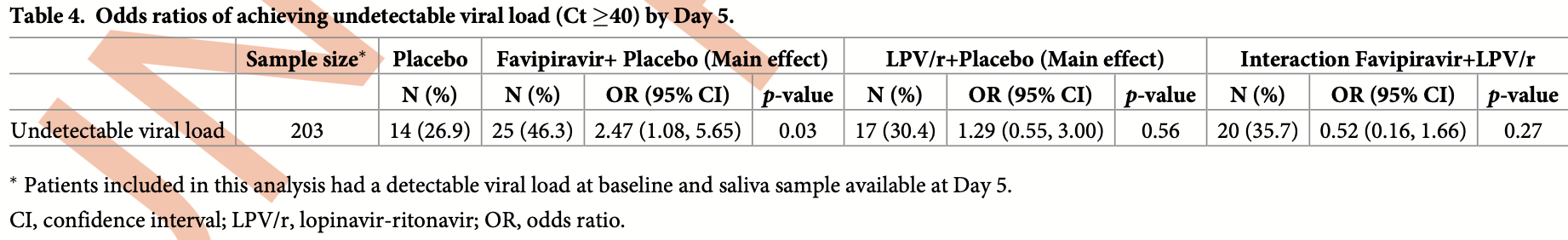
240 patient RCT comparing favipiravir, favipiravir + LPV/r, LPV/r, and placebo, showing improved viral clearance with favipiravir, but no significant difference for LPV/r. Efficacy was lower in the combined favipiravir + LPV/r arm, where plasma levels of favipiravir were lower. Favipiravir 1800mg twice daily on day 1 followed by 400mg four times daily on days 2-7.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments15.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers favipiravir and lopinavir/ritonavir.
|
risk of ICU admission, 201.7% higher, RR 3.02, p = 0.50, treatment 1 of 59 (1.7%), control 0 of 60 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 201.7% higher, RR 3.02, p = 0.50, treatment 1 of 59 (1.7%), control 0 of 60 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of no viral clearance, 28.4% lower, RR 0.72, p = 0.03, treatment 29 of 54 (53.7%), control 38 of 52 (73.1%), NNT 5.2, inverted to make RR<1 favor treatment, odds ratio converted to relative risk, day 5, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Lowe et al., 15 Feb 2022, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, 18 authors, study period 6 October, 2020 - 4 November, 2021, trial NCT04499677 (history) (FLARE).
Contact: d.lowe@ucl.ac.uk.
Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19
PLOS Medicine, doi:10.1371/journal.pmed.1004120
Background Early antiviral treatment is effective for Coronavirus Disease 2019 (COVID-19) but currently available agents are expensive. Favipiravir is routinely used in many countries, but efficacy is unproven. Antiviral combinations have not been systematically studied. We aimed to evaluate the effect of favipiravir, lopinavir-ritonavir or the combination of both agents on Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral load trajectory when administered early.
Methods and findings We conducted a Phase 2, proof of principle, randomised, placebo-controlled, 2 × 2 factorial, double-blind trial of ambulatory outpatients with early COVID-19 (within 7 days of symptom onset) at 2 sites in the United Kingdom. Participants were randomised using a centralised online process to receive: favipiravir (1,800 mg twice daily on Day 1 followed by 400 mg 4 times daily on Days 2 to 7) plus lopinavir-ritonavir (400 mg/100 mg twice daily on Day 1, followed by 200 mg/50 mg 4 times daily on Days 2 to 7), favipiravir plus lopinavir-ritonavir placebo, lopinavir-ritonavir plus favipiravir placebo, or both placebos. The primary outcome
Author summary Why was this study done? � The FLARE trial aimed to discover whether existing oral antiviral drugs could reduce the viral load of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus if given soon after symptoms started.
References
Ader, Peiffer-Smadja, Poissy, Bouscambert-Duchamp, Belhadi et al., An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.05.020
Arshad, Pertinez, Box, Tatham, Rajoli et al., Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics, Clin Pharmacol Ther, doi:10.1002/cpt.1909
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Beyene, Alemu, Kebede, Alemayehu, Seyoum et al., Saliva is superior over nasopharyngeal swab for detecting SARS-CoV2 in COVID-19 patients, Sci Rep, doi:10.1038/s41598-021-02097-2
Brown, Freemantle, Breuer, Dehbi, Chowdhury et al., Early antiviral treatment in outpatients with COVID-19 (FLARE): a structured summary of a study protocol for a randomised controlled trial, Trials, doi:10.1186/s13063-021-05139-2
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering, doi:10.1016/j.eng.2020.03.007
Chan, Lai, Chu, Tsui, Tam et al., Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study, Hong Kong Med J
Chu, Cheng, Hung, Wong, Chan et al., Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings, Thorax, doi:10.1136/thorax.2003.012658
Chuah, Chow, Hor, Cheng, Ker et al., Efficacy of Early Treatment with Favipiravir on Disease Progression among High Risk COVID-19 Patients: A Randomized, Open-Label Clinical Trial, Clin Infect Dis
Cook, Faustini, Williams, Cunningham, Drayson et al., Validation of a combined ELISA to detect IgG, IgA and IgM antibody responses to SARS-CoV-2 in mild or moderate non-hospitalised patients, J Immunol Methods, doi:10.1016/j.jim.2021.113046
Doi, Hibino, Hase, Yamamoto, Kasamatsu et al., Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrob Agents Chemother, doi:10.1128/AAC.01897-20
Fischer, Jj, Holman, Cohen, Fang et al., A Phase 2a clinical trial of Molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Gastine, Pang, Boshier, Carter, Lonsdale et al., Systematic Review and Patient-Level Meta-Analysis of SARS-CoV-2 Viral Dynamics to Model Response to Antiviral Therapies, Clin Pharmacol Ther, doi:10.1002/cpt.2223
Gonc ¸alves, Bertrand, Ke, Comets, De Lamballerie et al., Timing of Antiviral Treatment Initiation is Critical to Reduce SARS-CoV-2 Viral Load, CPT Pharmacometrics Syst Pharmacol, doi:10.1002/psp4.12543
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Herrera, Hidalgo-Miranda, Reynoso-Noveron, Garcia, Mendoza-Vargas, Saliva is a reliable and accessible source for the detection of SARS-CoV-2, Int J Infect Dis, doi:10.1016/j.ijid.2021.02.009
Holubar, Sa, Purington, Hedlin, Bunning et al., Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clin Infect Dis, doi:10.1093/cid/ciaa1176
Kaptein, Jacobs, Langendries, Seldeslachts, Horst et al., Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2014441117
Khamis, Naabi, Lawati, Ambusaidi, Sharji et al., Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.008
Nguyen, Guedj, Anglaret, Laouenan, Madelain et al., Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted, PLoS Negl Trop Dis, doi:10.1371/journal.pntd.0005389
Owen, Allerton, Anderson, Aschenbrenner, Avery et al., An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19, Science, doi:10.1126/science.abl4784
Park, Lee, Son, Ko, Peck et al., Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers, J Hosp Infect, doi:10.1016/j.jhin.2018.09.005
Pertinez, Rajoli, Khoo, Owen, Pharmacokinetic modelling to estimate intracellular favipiravir ribofuranosyl-5'-triphosphate exposure to support posology for SARS-CoV-2, J Antimicrob Chemother, doi:10.1093/jac/dkab135
Ruzhentsova, Oseshnyuk, Soluyanova, Dmitrikova, Mustafaev et al., Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19, Am J Transl Res
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.6019
Shinkai, Tsushima, Tanaka, Hagiwara, Tarumoto et al., Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial, Infect Dis Ther, doi:10.1007/s40121-021-00517-4
Solaymani-Dodaran, Ghanei, Bagheri, Qazvini, Vahedi et al., Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia, Int Immunopharmacol, doi:10.1016/j.intimp.2021.107522
Syed, Ciling, Khalid, Sreekumar, Chen et al., Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Ursic, Kogoj, Sikonja, Roskaric, Virant et al., Performance of nasopharyngeal swab and saliva in detecting Delta and Omicron SARS-CoV-2 variants, J Med Virol, doi:10.1002/jmv.27898
Wang, Zhong, Salam, Tarning, Zhan et al., Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza, EBioMedicine, doi:10.1016/j.ebiom.2020.103125
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
DOI record:
{
"DOI": "10.1371/journal.pmed.1004120",
"ISSN": [
"1549-1676"
],
"URL": "http://dx.doi.org/10.1371/journal.pmed.1004120",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Early antiviral treatment is effective for Coronavirus Disease 2019 (COVID-19) but currently available agents are expensive. Favipiravir is routinely used in many countries, but efficacy is unproven. Antiviral combinations have not been systematically studied. We aimed to evaluate the effect of favipiravir, lopinavir-ritonavir or the combination of both agents on Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral load trajectory when administered early.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and findings</jats:title>\n<jats:p>We conducted a Phase 2, proof of principle, randomised, placebo-controlled, 2 × 2 factorial, double-blind trial of ambulatory outpatients with early COVID-19 (within 7 days of symptom onset) at 2 sites in the United Kingdom. Participants were randomised using a centralised online process to receive: favipiravir (1,800 mg twice daily on Day 1 followed by 400 mg 4 times daily on Days 2 to 7) plus lopinavir-ritonavir (400 mg/100 mg twice daily on Day 1, followed by 200 mg/50 mg 4 times daily on Days 2 to 7), favipiravir plus lopinavir-ritonavir placebo, lopinavir-ritonavir plus favipiravir placebo, or both placebos. The primary outcome was SARS-CoV-2 viral load at Day 5, accounting for baseline viral load. Between 6 October 2020 and 4 November 2021, we recruited 240 participants. For the favipiravir+lopinavir-ritonavir, favipiravir+placebo, lopinavir-ritonavir+placebo, and placebo-only arms, we recruited 61, 59, 60, and 60 participants and analysed 55, 56, 55, and 58 participants, respectively, who provided viral load measures at Day 1 and Day 5. In the primary analysis, the mean viral load in the favipiravir+placebo arm had changed by −0.57 log<jats:sub>10</jats:sub> (95% CI −1.21 to 0.07, <jats:italic>p</jats:italic> = 0.08) and in the lopinavir-ritonavir+placebo arm by −0.18 log<jats:sub>10</jats:sub> (95% CI −0.82 to 0.46, <jats:italic>p</jats:italic> = 0.58) compared to the placebo arm at Day 5. There was no significant interaction between favipiravir and lopinavir-ritonavir (interaction coefficient term: 0.59 log<jats:sub>10</jats:sub>, 95% CI −0.32 to 1.50, <jats:italic>p</jats:italic> = 0.20). More participants had undetectable virus at Day 5 in the favipiravir+placebo arm compared to placebo only (46.3% versus 26.9%, odds ratio (OR): 2.47, 95% CI 1.08 to 5.65; <jats:italic>p</jats:italic> = 0.03). Adverse events were observed more frequently with lopinavir-ritonavir, mainly gastrointestinal disturbance. Favipiravir drug levels were lower in the combination arm than the favipiravir monotherapy arm, possibly due to poor absorption. The major limitation was that the study population was relatively young and healthy compared to those most affected by the COVID-19 pandemic.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Conclusions</jats:title>\n<jats:p>At the current doses, no treatment significantly reduced viral load in the primary analysis. Favipiravir requires further evaluation with consideration of dose escalation. Lopinavir-ritonavir administration was associated with lower plasma favipiravir concentrations.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Trial registration</jats:title>\n<jats:p>Clinicaltrials.gov <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04499677\" xlink:type=\"simple\">NCT04499677</jats:ext-link></jats:p>\n<jats:p>EudraCT: 2020-002106-68</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6102-2375",
"affiliation": [],
"authenticated-orcid": true,
"family": "Lowe",
"given": "David M.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8261-0288",
"affiliation": [],
"authenticated-orcid": true,
"family": "Brown",
"given": "Li-An K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8185-5152",
"affiliation": [],
"authenticated-orcid": true,
"family": "Chowdhury",
"given": "Kashfia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9479-6473",
"affiliation": [],
"authenticated-orcid": true,
"family": "Davey",
"given": "Stephanie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3426-9257",
"affiliation": [],
"authenticated-orcid": true,
"family": "Yee",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ikeji",
"given": "Felicia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0982-1222",
"affiliation": [],
"authenticated-orcid": true,
"family": "Ndoutoumou",
"given": "Amalia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7190-8257",
"affiliation": [],
"authenticated-orcid": true,
"family": "Shah",
"given": "Divya",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3363-0637",
"affiliation": [],
"authenticated-orcid": true,
"family": "Lennon",
"given": "Alexander",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9464-7013",
"affiliation": [],
"authenticated-orcid": true,
"family": "Rai",
"given": "Abhulya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agyeman",
"given": "Akosua A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Checkley",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Longley",
"given": "Nicola",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0816-0178",
"affiliation": [],
"authenticated-orcid": true,
"family": "Dehbi",
"given": "Hakim-Moulay",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5807-5740",
"affiliation": [],
"authenticated-orcid": true,
"family": "Freemantle",
"given": "Nick",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4792-5903",
"affiliation": [],
"authenticated-orcid": true,
"family": "Breuer",
"given": "Judith",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4561-7173",
"affiliation": [],
"authenticated-orcid": true,
"family": "Standing",
"given": "Joseph F.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "FLARE Investigators",
"sequence": "additional"
}
],
"container-title": "PLOS Medicine",
"container-title-short": "PLoS Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosmedicine.org"
]
},
"created": {
"date-parts": [
[
2022,
10,
19
]
],
"date-time": "2022-10-19T17:42:13Z",
"timestamp": 1666201333000
},
"deposited": {
"date-parts": [
[
2022,
10,
19
]
],
"date-time": "2022-10-19T17:42:53Z",
"timestamp": 1666201373000
},
"funder": [
{
"DOI": "10.13039/100012357",
"award": [
"COVID0005"
],
"doi-asserted-by": "publisher",
"name": "LifeArc"
},
{
"DOI": "10.13039/501100000265",
"award": [
"MR/M008665/"
],
"doi-asserted-by": "publisher",
"name": "Medical Research Council"
},
{
"DOI": "10.13039/501100000265",
"award": [
"MR/W015560/1"
],
"doi-asserted-by": "publisher",
"name": "Medical Research Council"
}
],
"indexed": {
"date-parts": [
[
2022,
10,
20
]
],
"date-time": "2022-10-20T05:13:45Z",
"timestamp": 1666242825324
},
"is-referenced-by-count": 0,
"issue": "10",
"issued": {
"date-parts": [
[
2022,
10,
19
]
]
},
"journal-issue": {
"issue": "10",
"published-online": {
"date-parts": [
[
2022,
10,
19
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
19
]
],
"date-time": "2022-10-19T00:00:00Z",
"timestamp": 1666137600000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pmed.1004120",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e1004120",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2022,
10,
19
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
19
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"author": "A Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "pmed.1004120.ref001",
"volume": "385",
"year": "2021"
},
{
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "A Jayk Bernal",
"journal-title": "N Engl J Med",
"key": "pmed.1004120.ref002",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19",
"author": "DM Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "pmed.1004120.ref003",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1126/science.abl4784",
"article-title": "An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19",
"author": "DR Owen",
"doi-asserted-by": "crossref",
"first-page": "1586",
"journal-title": "Science",
"key": "pmed.1004120.ref004",
"volume": "374",
"year": "2021"
},
{
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19",
"author": "EPIC-HR Investigators",
"journal-title": "N Engl J Med",
"key": "pmed.1004120.ref005",
"year": "2022"
},
{
"article-title": "Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles",
"author": "AM Syed",
"journal-title": "medRxiv",
"key": "pmed.1004120.ref006",
"year": "2022"
},
{
"article-title": "Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review",
"author": "JM Sanders",
"first-page": "1824",
"journal-title": "JAMA",
"key": "pmed.1004120.ref007",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1136/thorax.2003.012658",
"article-title": "Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings",
"author": "CM Chu",
"doi-asserted-by": "crossref",
"first-page": "252",
"journal-title": "Thorax",
"key": "pmed.1004120.ref008",
"volume": "59",
"year": "2004"
},
{
"article-title": "Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study",
"author": "KS Chan",
"first-page": "399",
"journal-title": "Hong Kong Med J",
"key": "pmed.1004120.ref009",
"volume": "9",
"year": "2003"
},
{
"DOI": "10.1016/j.jhin.2018.09.005",
"article-title": "Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers",
"author": "SY Park",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "J Hosp Infect",
"key": "pmed.1004120.ref010",
"volume": "101",
"year": "2019"
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics",
"author": "U Arshad",
"doi-asserted-by": "crossref",
"first-page": "775",
"journal-title": "Clin Pharmacol Ther",
"key": "pmed.1004120.ref011",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2014441117",
"article-title": "Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity",
"author": "SJF Kaptein",
"doi-asserted-by": "crossref",
"first-page": "26955",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "pmed.1004120.ref012",
"volume": "117",
"year": "2020"
},
{
"article-title": "Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study",
"author": "Q Cai",
"first-page": "1192",
"journal-title": "Engineering (Beijing)",
"key": "pmed.1004120.ref013",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1176",
"article-title": "AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial",
"author": "AA Ivashchenko",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Clin Infect Dis",
"key": "pmed.1004120.ref014",
"volume": "73",
"year": "2021"
},
{
"article-title": "Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial",
"author": "A SA Holubar",
"journal-title": "medRxiv",
"key": "pmed.1004120.ref015",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2021.05.020",
"article-title": "An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19",
"author": "F Ader",
"doi-asserted-by": "crossref",
"first-page": "1826",
"journal-title": "Clin Microbiol Infect",
"key": "pmed.1004120.ref016",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)32013-4",
"article-title": "Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "1345",
"journal-title": "Lancet",
"key": "pmed.1004120.ref017",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1002/psp4.12543",
"article-title": "Timing of Antiviral Treatment Initiation is Critical to Reduce SARS-CoV-2 Viral Load",
"author": "A Gonçalves",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "CPT Pharmacometrics Syst Pharmacol",
"key": "pmed.1004120.ref018",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1002/cpt.2223",
"article-title": "Systematic Review and Patient-Level Meta-Analysis of SARS-CoV-2 Viral Dynamics to Model Response to Antiviral Therapies",
"author": "S Gastine",
"doi-asserted-by": "crossref",
"first-page": "321",
"journal-title": "Clin Pharmacol Ther",
"key": "pmed.1004120.ref019",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1186/s13063-021-05139-2",
"article-title": "Early antiviral treatment in outpatients with COVID-19 (FLARE): a structured summary of a study protocol for a randomised controlled trial",
"author": "LK Brown",
"doi-asserted-by": "crossref",
"first-page": "193",
"journal-title": "Trials",
"key": "pmed.1004120.ref020",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.jim.2021.113046",
"article-title": "Validation of a combined ELISA to detect IgG, IgA and IgM antibody responses to SARS-CoV-2 in mild or moderate non-hospitalised patients",
"author": "AM Cook",
"doi-asserted-by": "crossref",
"first-page": "113046",
"journal-title": "J Immunol Methods",
"key": "pmed.1004120.ref021",
"volume": "494",
"year": "2021"
},
{
"article-title": "A Phase 2a clinical trial of Molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus",
"author": "WA, 2nd Fischer",
"journal-title": "Sci Transl Med",
"key": "pmed.1004120.ref022",
"year": "2021"
},
{
"DOI": "10.1371/journal.pntd.0005389",
"article-title": "Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted",
"author": "TH Nguyen",
"doi-asserted-by": "crossref",
"first-page": "e0005389",
"journal-title": "PLoS Negl Trop Dis",
"key": "pmed.1004120.ref023",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.1016/j.ebiom.2020.103125",
"article-title": "Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza",
"author": "Y Wang",
"doi-asserted-by": "crossref",
"first-page": "103125",
"journal-title": "EBioMedicine",
"key": "pmed.1004120.ref024",
"volume": "62",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkab135",
"article-title": "Pharmacokinetic modelling to estimate intracellular favipiravir ribofuranosyl-5’-triphosphate exposure to support posology for SARS-CoV-2",
"author": "H Pertinez",
"doi-asserted-by": "crossref",
"first-page": "2121",
"journal-title": "J Antimicrob Chemother",
"key": "pmed.1004120.ref025",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"article-title": "Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial",
"author": "M Shinkai",
"doi-asserted-by": "crossref",
"first-page": "2489",
"journal-title": "Infect Dis Ther",
"key": "pmed.1004120.ref026",
"volume": "10",
"year": "2021"
},
{
"article-title": "Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19",
"author": "TA Ruzhentsova",
"first-page": "12575",
"journal-title": "Am J Transl Res",
"key": "pmed.1004120.ref027",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "ZF Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "pmed.1004120.ref028",
"volume": "103",
"year": "2021"
},
{
"article-title": "A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19",
"author": "Y Doi",
"first-page": "64",
"journal-title": "Antimicrob Agents Chemother",
"key": "pmed.1004120.ref029",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.008",
"article-title": "Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia",
"author": "F Khamis",
"doi-asserted-by": "crossref",
"first-page": "538",
"journal-title": "Int J Infect Dis",
"key": "pmed.1004120.ref030",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2021.107522",
"article-title": "Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia",
"author": "M Solaymani-Dodaran",
"doi-asserted-by": "crossref",
"first-page": "107522",
"journal-title": "Int Immunopharmacol",
"key": "pmed.1004120.ref031",
"volume": "95",
"year": "2021"
},
{
"article-title": "Efficacy of Early Treatment with Favipiravir on Disease Progression among High Risk COVID-19 Patients: A Randomized, Open-Label Clinical Trial",
"author": "CH Chuah",
"journal-title": "Clin Infect Dis",
"key": "pmed.1004120.ref032",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-02097-2",
"article-title": "Saliva is superior over nasopharyngeal swab for detecting SARS-CoV2 in COVID-19 patients",
"author": "GT Beyene",
"doi-asserted-by": "crossref",
"first-page": "22640",
"journal-title": "Sci Rep",
"key": "pmed.1004120.ref033",
"volume": "11",
"year": "2021"
},
{
"article-title": "Performance of nasopharyngeal swab and saliva in detecting Delta and Omicron SARS-CoV-2 variants",
"author": "T Ursic",
"journal-title": "J Med Virol",
"key": "pmed.1004120.ref034",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2021.02.009",
"article-title": "Saliva is a reliable and accessible source for the detection of SARS-CoV-2",
"author": "LA Herrera",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Int J Infect Dis",
"key": "pmed.1004120.ref035",
"volume": "105",
"year": "2021"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pmed.1004120"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pmed.corrections_policy",
"volume": "19"
}