Effectiveness of Nirmatrelvir–Ritonavir Against the Development of Post–COVID-19 Conditions Among U.S. Veterans
et al., Annals of Internal Medicine, doi:10.7326/M23-1394, Oct 2023
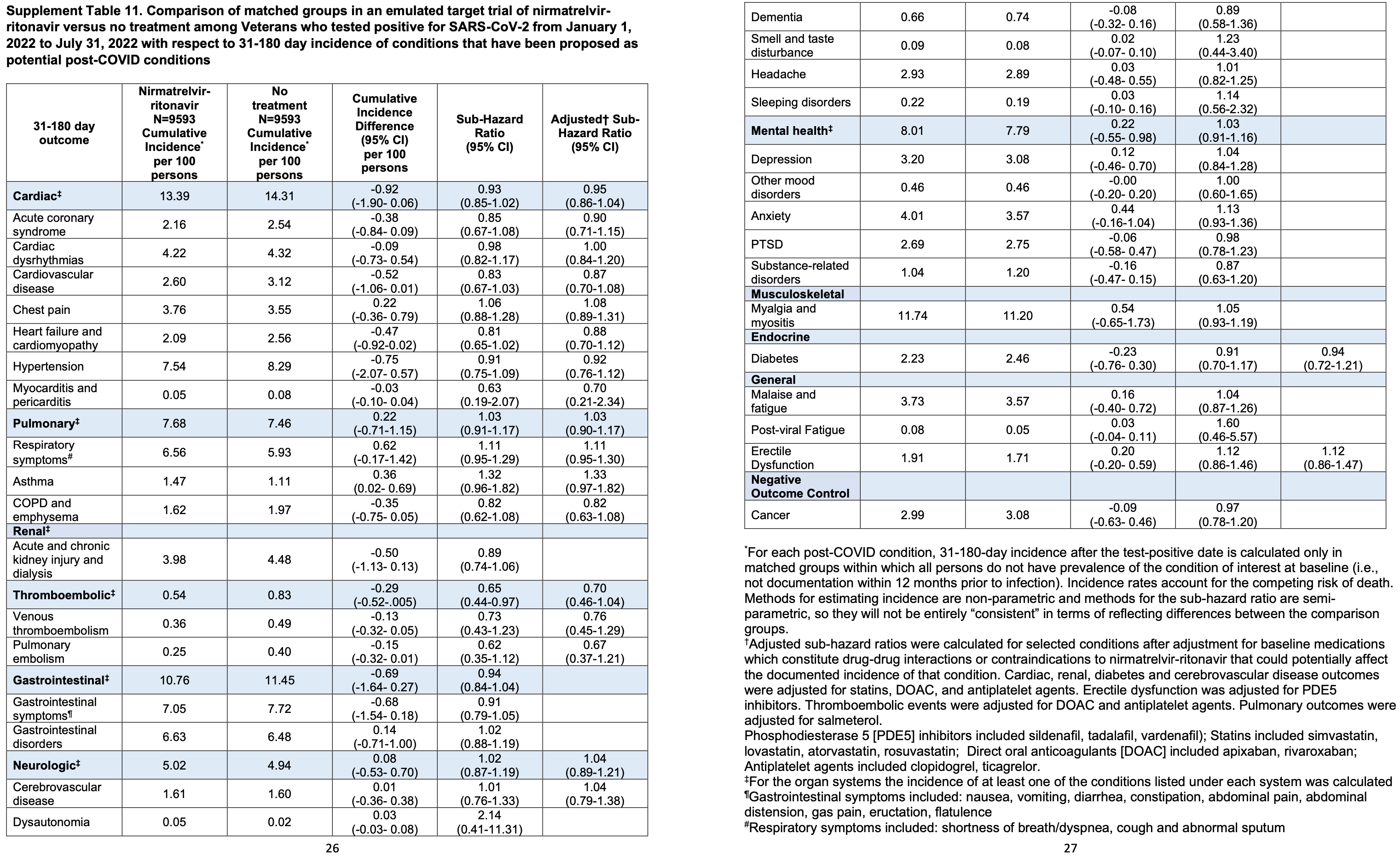
Retrospective 9,593 veterans in the USA treated with paxlovid, matched to 9,593 untreated controls, showing no significant difference in post-COVID conditions across 31 different conditions. There was lower risk for the combination of 2 specific conditions (venous thromboembolism and pulmonary embolism) however this was not significant after adjustment for baseline medications which constitute drug-drug interactions or contraindications to nirmatrelvir-ritonavir.
This analysis should be more accurate than many paxlovid retrospective studies. Many studies are biased due to inclusion of patients with contraindications to paxlovid, and due to confounding by treatment propensity, as below. Authors account for contraindications (though not all would be identified) and partially account for treatment propensity by including the frequency of health care encounters in matching.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending paxlovid also recommended them, or
because the patient seeking out paxlovid is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Malden et al. confirm significant bias in the use of paxlovid, showing that treated
patients are more likely to be from affluent neighborhoods, be more health-conscious, and
have better access to care. Campion et al. also show that female patients were more
likely to receive paxlovid, and studies show that female patients are significantly more
likely to be health-conscious, for example being more likely to take additional
non-prescription treatments.
Therefore, these kind of studies may
overestimate efficacy.
Resistance. Variants may be resistant to paxlovid6-13. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID14. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid15. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid16. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury17 and liver injury18,19. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound20-22.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments23.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of long COVID, 0.7% lower, HR 0.99, p = 0.75, treatment 9,593, control 9,593, adjusted per study, all 31 conditions combined, propensity score matching.
|
|
risk of long COVID, 5.0% lower, HR 0.95, p = 0.29, treatment 9,593, control 9,593, adjusted per study, cardiac, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 3.0% higher, HR 1.03, p = 0.67, treatment 9,593, control 9,593, adjusted per study, pulmonary, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 11.0% lower, HR 0.89, p = 0.21, treatment 9,593, control 9,593, renal, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 30.0% lower, HR 0.70, p = 0.09, treatment 9,593, control 9,593, adjusted per study, thromboembolic, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 6.0% lower, HR 0.94, p = 0.26, treatment 9,593, control 9,593, gastrointestinal, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 4.0% higher, HR 1.04, p = 0.63, treatment 9,593, control 9,593, adjusted per study, neurologic, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 3.0% higher, HR 1.03, p = 0.65, treatment 9,593, control 9,593, mental health, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 5.0% higher, HR 1.05, p = 0.45, treatment 9,593, control 9,593, musculoskeletal, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 6.0% lower, HR 0.94, p = 0.65, treatment 9,593, control 9,593, adjusted per study, endocrine, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 4.0% higher, HR 1.04, p = 0.69, treatment 9,593, control 9,593, general - malaise, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 60.0% higher, HR 1.60, p = 0.47, treatment 9,593, control 9,593, general - post-viral fatigue, propensity score matching, supplemental table 11.
|
|
risk of long COVID, 12.0% higher, HR 1.12, p = 0.41, treatment 9,593, control 9,593, general - ED, propensity score matching, supplemental table 11.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Malden et al., Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system, Scientific Reports, doi:10.1038/s41598-024-57633-7.
5.
Campion et al., Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study, Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1809.
6.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
7.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
8.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
9.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
10.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
11.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
12.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
13.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
14.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
15.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
16.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
17.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
18.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
19.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
20.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
21.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Ioannou et al., 31 Oct 2023, retrospective, propensity score matching, USA, peer-reviewed, 19 authors.
Effectiveness of Nirmatrelvir-Ritonavir Against the Development of Post-COVID-19 Conditions Among U.S. Veterans A Target Trial Emulation
doi:10.7326/M23-1394
Background: COVID-19 has been linked to the development of many post-COVID-19 conditions (PCCs) after acute infection. Limited information is available on the effectiveness of oral antivirals used to treat acute COVID-19 in preventing the development of PCCs. Objective: To measure the effectiveness of outpatient treatment of COVID-19 with nirmatrelvir-ritonavir in preventing PCCs. Design: Retrospective target trial emulation study comparing matched cohorts receiving nirmatrelvir-ritonavir versus no treatment. Setting: Veterans Health Administration (VHA). Participants: Nonhospitalized veterans in VHA care who were at risk for severe COVID-19 and tested positive for SARS-CoV-2 during January through July 2022. Intervention: Nirmatrelvir-ritonavir treatment for acute COVID-19. Measurements: Cumulative incidence of 31 potential PCCs at 31 to 180 days after treatment or a matched index date, including cardiac, pulmonary, renal, thromboembolic, gastrointestinal, neurologic, mental health, musculoskeletal, endocrine, and general conditions and symptoms. Results: Eighty-six percent of the participants were male, with a median age of 66 years, and 17.5% were unvaccinated. Baseline characteristics were well balanced between participants treated with nirmatrelvir-ritonavir and matched untreated comparators. No differences were observed between participants treated with nirmatrelvir-ritonavir (n ¼ 9593) and their matched untreated comparators in the incidence of most PCCs examined individually or grouped by organ system, except for lower combined risk for venous thromboembolism and pulmonary embolism (subhazard ratio, 0.65 [95% CI, 0.44 to 0.97]; cumulative incidence difference, À0.29 percentage points [CI, À0.52 to À0.05 percentage points]). Limitations: Ascertainment of PCCs using International Classification of Diseases, 10th Revision codes may be inaccurate. Evaluation of many outcomes could have resulted in spurious associations with combined thromboembolic events by chance.
Conclusion: Out of 31 potential PCCs, only combined thromboembolic events seemed to be reduced by nirmatrelvir-ritonavir.
Author contributions are available at Annals.org.
References
Aggarwal, Molina, Beaty, Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of Omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00011-7
Al-Aly, Xie, Bowe, High-dimensional characterization of post-acute sequelae of COVID-19, Nature, doi:10.1038/s41586-021-03553-9
Altman, Andersen, Calculating the number needed to treat for trials where the outcome is time to an event, BMJ, doi:10.1136/bmj.319.7223.1492
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Austin, Cafri, Variance estimation when using propensity-score matching with replacement with survival or time-to-event outcomes, Stat Med, doi:10.1002/sim.8502
Ayoubkhani, Khunti, Nafilyan, Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study, BMJ, doi:10.1136/bmj.n693
Bajema, Berry, Streja, Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. veterans: target trial emulation studies with one-month and six-month outcomes, Ann Intern Med, doi:10.7326/M22-3565
Bernal, Da Silva, Musungaie, Study Group. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bull-Otterson, Baca, Saydah, Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥65 years-United States, March 2020-November 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7121e1
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Carfi, Bernabei, Landi, Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19, JAMA, doi:10.1001/jama.2020.12603
Cohen, Ren, Heath, Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study, BMJ, doi:10.1136/bmj-2021-068414
Crook, Raza, Nowell, Long covid-mechanisms, risk factors, and management, BMJ, doi:10.1136/bmj.n1648
Daugherty, Guo, Heath, Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study, BMJ, doi:10.1136/bmj.n1098
Davis, Mccorkell, Vogel, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol, doi:10.1038/s41579-022-00846-2
Dickerman, Gerlovin, Madenci, Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans, N Engl J Med, doi:10.1056/NEJMoa2115463
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. health system: a populationbased cohort study, Ann Intern Med, doi:10.7326/M22-2141
Food, Administration, Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers
Gupta, Madhavan, Sehgal, Extrapulmonary manifestations of COVID-19, Nat Med, doi:10.1038/s41591-020-0968-3
Hammond, Leister-Tebbe, Gardner, EPIC-HR Investigators. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hernán, Robins, Using big data to emulate a target trial when a randomized trial is not available, Am J Epidemiol, doi:10.1093/aje/kwv254
Huang, Huang, Wang, 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study, Lancet, doi:10.1016/S0140-6736(20)32656-8
Ioannou, Locke, Green, Comparison of Moderna versus Pfizer-BioNTech COVID-19 vaccine outcomes: a target trial emulation study in the U.S. Veterans Affairs healthcare system, EClinicalMedicine, doi:10.1016/j.eclinm.2022.101326
Ioannou, Locke, Hare, COVID-19 vaccination effectiveness against infection or death in a national U.S. health care system: a target trial emulation study, Ann Intern Med, doi:10.7326/M21-3256
Katsoularis, Fonseca-Rodríguez, Farrington, Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study, Lancet, doi:10.1016/S0140-6736(21)00896-5
Labrecque, Swanson, Target trial emulation: teaching epidemiology and beyond, Eur J Epidemiol, doi:10.1007/s10654-017-0293-4
Lewnard, Mclaughlin, Malden, Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00118-4
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Nalbandian, Sehgal, Gupta, Post-acute COVID-19 syndrome, Nat Med, doi:10.1038/s41591-021-01283-z
Robins, Hernán, Brumback, Marginal structural models and causal inference in epidemiology, Epidemiology, doi:10.1097/00001648-200009000-00011
Rosenbaum, Db, Reducing bias in observational studies using subclassification on the propensity score, J Am Stat Assoc, doi:10.2307/2288398
Statacorp, Chapter 11: Language syntax, Stata Manual
Wang, Porter, Maynard, Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration, Med Care, doi:10.1097/MLR.0b013e31827da95a
Wong, Au, Lau, Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the Omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Xie, Bowe, Al-Aly, Berry, Aslan et al., Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records, BMJ, doi:10.1136/bmj-2022-072705
Xie, Bowe, Al-Aly, Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status, Nat Commun, doi:10.1038/s41467-021-26513-3
Xie, Choi, Al-Aly, Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition, JAMA Intern Med, doi:10.1001/jamainternmed.2023.0743
Xie, Choi, Al-Aly, Molnupiravir and risk of post-acute sequelae of covid-19: cohort study, BMJ, doi:10.1136/bmj-2022-074572
Xie, Xu, Al-Aly, Risks of mental health outcomes in people with covid-19: cohort study, BMJ, doi:10.1136/bmj-2021-068993
Xie, Xu, Bowe, Long-term cardiovascular outcomes of COVID-19, Nat Med, doi:10.1038/s41591-022-01689-3
Yan, Streja, Li, Anti-SARS-CoV-2 pharmacotherapies among nonhospitalized US veterans, January 2022 to January 2023, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.31249
Yip, Lui, Lai, Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciac687
DOI record:
{
"DOI": "10.7326/m23-1394",
"ISSN": [
"0003-4819",
"1539-3704"
],
"URL": "http://dx.doi.org/10.7326/M23-1394",
"alternative-id": [
"10.7326/M23-1394"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1796-8977",
"affiliation": [
{
"name": "Research and Development and Division of Gastroenterology, Veterans Affairs Puget Sound Health Care System, and Division of Gastroenterology, University of Washington, Seattle, Washington (G.N.I.)"
}
],
"authenticated-orcid": false,
"family": "Ioannou",
"given": "George N.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Research and Development, Veterans Affairs Puget Sound Health Care System, Seattle, Washington (K.B.)"
}
],
"family": "Berry",
"given": "Kristin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2045-0872",
"affiliation": [
{
"name": "Veterans Affairs Cooperative Studies Program Clinical Epidemiology Research Center (CSP-CERC), Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut, and Yale Center for Medical Informatics, Yale School of Medicine, New Haven, Connecticut (N.R., P.M.)"
}
],
"authenticated-orcid": false,
"family": "Rajeevan",
"given": "Nallakkandi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veterans Affairs Cooperative Studies Program Clinical Epidemiology Research Center (CSP-CERC), Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut (Y.L.)"
}
],
"family": "Li",
"given": "Yuli",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0002-4573-4617",
"affiliation": [
{
"name": "Veterans Affairs Cooperative Studies Program Clinical Epidemiology Research Center (CSP-CERC), Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut, and Yale Center for Medical Informatics, Yale School of Medicine, New Haven, Connecticut (N.R., P.M.)"
}
],
"authenticated-orcid": false,
"family": "Mutalik",
"given": "Pradeep",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4779-2688",
"affiliation": [
{
"name": "Veterans Affairs Cooperative Studies Program Clinical Epidemiology Research Center (CSP-CERC), Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut, and Department of Biostatistics, Yale School of Public Health, New Haven, Connecticut (L.Y.)"
}
],
"authenticated-orcid": false,
"family": "Yan",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veterans Affairs Portland Health Care System, Portland, Oregon (D.B.)"
}
],
"family": "Bui",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veterans Affairs Center for Medication Safety - Pharmacy Benefit Management (PBM) Services, Hines, Illinois (F.C.)"
}
],
"family": "Cunningham",
"given": "Francesca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6436-7157",
"affiliation": [
{
"name": "Center of Innovation to Improve Veteran Involvement in Care (CIVIC), Veterans Affairs Portland Healthcare System, Portland, Oregon; Health Management and Policy, School of Social and Behavioral Health Sciences, College of Public Health and Human Sciences, Oregon State University, Corvallis, Oregon; and Health Data and Informatics Program, Center for Quantitative Life Sciences, Oregon State University, Corvallis, Oregon (D.M.H.)"
}
],
"authenticated-orcid": false,
"family": "Hynes",
"given": "Denise M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center of Innovation to Improve Veteran Involvement in Care (CIVIC), Veterans Affairs Portland Healthcare System, Portland, Oregon (M.R.)"
}
],
"family": "Rowneki",
"given": "Mazhgan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Clinical Management Research, Veterans Affairs Ann Arbor Healthcare System, and Department of Anesthesiology, University of Michigan, Ann Arbor, Michigan (A.B.)"
}
],
"family": "Bohnert",
"given": "Amy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3695-192X",
"affiliation": [
{
"name": "Seattle Epidemiologic Research and Information Center, Veterans Affairs Puget Sound Health Care System, Seattle, Washington (E.J.B.)"
}
],
"authenticated-orcid": false,
"family": "Boyko",
"given": "Edward J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Clinical Management Research, Veterans Affairs Ann Arbor Healthcare System, Ann Arbor, Michigan, and Schools of Medicine and Public Health, Johns Hopkins University, Baltimore, Maryland (T.J.I.)"
}
],
"family": "Iwashyna",
"given": "Theodore J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1765-5938",
"affiliation": [
{
"name": "Center of Innovation to Accelerate Discovery and Practice Transformation, Durham Veterans Affairs Medical Center; Department of Population Health Sciences, Duke University School of Medicine; and Duke-Margolis Center for Health Policy, Duke University, Durham, North Carolina (M.L.M.)"
}
],
"authenticated-orcid": false,
"family": "Maciejewski",
"given": "Matthew L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8896-2487",
"affiliation": [
{
"name": "Veterans Affairs Palo Alto Health Care System, Palo Alto, California, and Department of Radiology, Stanford University School of Medicine, Stanford, California (T.F.O.)"
}
],
"authenticated-orcid": false,
"family": "Osborne",
"given": "Thomas F.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7439-6322",
"affiliation": [
{
"name": "Center for Clinical Management Research, Veterans Affairs Ann Arbor Healthcare System, and Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan (E.M.V.)"
}
],
"authenticated-orcid": false,
"family": "Viglianti",
"given": "Elizabeth M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veterans Affairs Cooperative Studies Program Clinical Epidemiology Research Center (CSP-CERC), Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut, and Department of Medicine, Yale School of Medicine, New Haven, Connecticut (M.A.)"
}
],
"family": "Aslan",
"given": "Mihaela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1217-0002",
"affiliation": [
{
"name": "Office of Research and Development, Veterans Health Administration, Washington, DC (G.D.H.)"
}
],
"authenticated-orcid": false,
"family": "Huang",
"given": "Grant D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3229-5590",
"affiliation": [
{
"name": "Veterans Affairs Portland Health Care System, and Division of Infectious Diseases, Department of Medicine, Oregon Health & Science University, Portland, Oregon (K.L.B.)."
}
],
"authenticated-orcid": false,
"family": "Bajema",
"given": "Kristina L.",
"sequence": "additional"
}
],
"container-title": "Annals of Internal Medicine",
"container-title-short": "Ann Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
30
]
],
"date-time": "2023-10-30T21:01:52Z",
"timestamp": 1698699712000
},
"deposited": {
"date-parts": [
[
2023,
11,
20
]
],
"date-time": "2023-11-20T22:05:57Z",
"timestamp": 1700517957000
},
"funder": [
{
"DOI": "10.13039/100000738",
"award": [
"CSP #2038",
"C19 21-278",
"C19 21-279",
"RCS 10-391",
"RCS 21-136"
],
"doi-asserted-by": "publisher",
"name": "U.S. Department of Veterans Affairs"
}
],
"indexed": {
"date-parts": [
[
2023,
11,
21
]
],
"date-time": "2023-11-21T00:31:08Z",
"timestamp": 1700526668994
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"member": "4285",
"original-title": [],
"page": "1486-1497",
"prefix": "10.7326",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "American College of Physicians",
"reference": [
{
"DOI": "10.1136/bmj.n1648",
"doi-asserted-by": "publisher",
"key": "r1-M231394"
},
{
"DOI": "10.1038/s41467-021-26513-3",
"doi-asserted-by": "publisher",
"key": "r2-M231394"
},
{
"DOI": "10.1038/s41586-021-03553-9",
"doi-asserted-by": "publisher",
"key": "r3-M231394"
},
{
"DOI": "10.1038/s41591-022-01689-3",
"doi-asserted-by": "publisher",
"key": "r4-M231394"
},
{
"DOI": "10.1136/bmj.n693",
"doi-asserted-by": "publisher",
"key": "r5-M231394"
},
{
"DOI": "10.1016/S0140-6736(20)32656-8",
"doi-asserted-by": "publisher",
"key": "r6-M231394"
},
{
"DOI": "10.1001/jama.2020.12603",
"doi-asserted-by": "publisher",
"key": "r7-M231394"
},
{
"DOI": "10.1136/bmj.n1098",
"doi-asserted-by": "publisher",
"key": "r8-M231394"
},
{
"DOI": "10.1016/S0140-6736(21)00896-5",
"doi-asserted-by": "publisher",
"key": "r9-M231394"
},
{
"DOI": "10.1038/s41591-020-0968-3",
"doi-asserted-by": "publisher",
"key": "r10-M231394"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"doi-asserted-by": "publisher",
"key": "r11-M231394"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"doi-asserted-by": "publisher",
"key": "r12-M231394"
},
{
"DOI": "10.15585/mmwr.mm7121e1",
"doi-asserted-by": "publisher",
"key": "r13-M231394"
},
{
"DOI": "10.7326/M22-3565",
"doi-asserted-by": "publisher",
"key": "r15-M231394"
},
{
"DOI": "10.1001/jamanetworkopen.2023.31249",
"doi-asserted-by": "publisher",
"key": "r16-M231394"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "r18-M231394"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"doi-asserted-by": "publisher",
"key": "r19-M231394"
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"doi-asserted-by": "publisher",
"key": "r20-M231394"
},
{
"DOI": "10.1136/bmj-2022-074572",
"doi-asserted-by": "publisher",
"key": "r21-M231394"
},
{
"DOI": "10.1093/aje/kwv254",
"doi-asserted-by": "publisher",
"key": "r22-M231394"
},
{
"DOI": "10.1056/NEJMoa2115463",
"doi-asserted-by": "publisher",
"key": "r23-M231394"
},
{
"DOI": "10.7326/M21-3256",
"doi-asserted-by": "publisher",
"key": "r24-M231394"
},
{
"DOI": "10.1016/j.eclinm.2022.101326",
"doi-asserted-by": "publisher",
"key": "r25-M231394"
},
{
"DOI": "10.1007/s10654-017-0293-4",
"doi-asserted-by": "publisher",
"key": "r26-M231394"
},
{
"DOI": "10.2307/2288398",
"doi-asserted-by": "publisher",
"key": "r32-M231394"
},
{
"DOI": "10.1136/bmj-2021-068993",
"doi-asserted-by": "publisher",
"key": "r33-M231394"
},
{
"DOI": "10.1136/bmj-2021-068414",
"doi-asserted-by": "publisher",
"key": "r34-M231394"
},
{
"DOI": "10.1136/bmj.319.7223.1492",
"doi-asserted-by": "publisher",
"key": "r35-M231394"
},
{
"DOI": "10.1002/sim.8502",
"doi-asserted-by": "publisher",
"key": "r37-M231394"
},
{
"DOI": "10.1097/00001648-200009000-00011",
"doi-asserted-by": "publisher",
"key": "r38-M231394"
},
{
"DOI": "10.1097/MLR.0b013e31827da95a",
"doi-asserted-by": "publisher",
"key": "r39-M231394"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "r40-M231394"
},
{
"DOI": "10.1016/S1473-3099(23)00011-7",
"doi-asserted-by": "publisher",
"key": "r41-M231394"
},
{
"DOI": "10.1056/NEJMoa2204919",
"doi-asserted-by": "publisher",
"key": "r42-M231394"
},
{
"DOI": "10.7326/M22-2141",
"doi-asserted-by": "publisher",
"key": "r43-M231394"
},
{
"DOI": "10.1093/cid/ciac443",
"doi-asserted-by": "publisher",
"key": "r44-M231394"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"doi-asserted-by": "publisher",
"key": "r45-M231394"
},
{
"DOI": "10.1093/cid/ciac687",
"doi-asserted-by": "publisher",
"key": "r46-M231394"
},
{
"DOI": "10.1016/S1473-3099(23)00118-4",
"doi-asserted-by": "publisher",
"key": "r47-M231394"
},
{
"DOI": "10.1136/bmj-2022-072705",
"doi-asserted-by": "publisher",
"key": "r48-M231394"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.acpjournals.org/doi/10.7326/M23-1394"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Internal Medicine"
],
"subtitle": [
"A Target Trial Emulation"
],
"title": "Effectiveness of Nirmatrelvir–Ritonavir Against the Development of Post–COVID-19 Conditions Among U.S. Veterans",
"type": "journal-article",
"volume": "176"
}