Effectiveness of nirmatrelvir–ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(23)00118-4, Mar 2023
Retrospective 7,274 outpatients in the USA treated with paxlovid and matched controls, showing lower combined hospitalization/death with treatment.
With a small percentage of eligible patients receiving treatment, confounding by indication, healthcare seeking behavior, knowledge of COVID-19 treatments, etc. is likely significant. Authors partially address this in their matching procedure. Notably, authors do not appear to address confounding by contraindication, and matching is unable to find a match for patients that seek adjuvant treatments (e.g., paxlovid + vitamin D). Confounding may be more significant for patients that seek care earlier.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending paxlovid also recommended them, or
because the patient seeking out paxlovid is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Malden et al. confirm significant bias in the use of paxlovid, showing that treated
patients are more likely to be from affluent neighborhoods, be more health-conscious, and
have better access to care. Campion et al. also show that female patients were more
likely to receive paxlovid, and studies show that female patients are significantly more
likely to be health-conscious, for example being more likely to take additional
non-prescription treatments.
Therefore, these kind of studies may
overestimate efficacy.
Resistance. Variants may be resistant to paxlovid6-13. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID14. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid15. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid16. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury17 and liver injury18,19. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound20-22.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments23.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
only a fraction of eligible patients received treatment and these patients may be more likely to follow other recommendations, receive additional care, and more more likely to use additional untracked treatments such as vitamin D and nasal/oral hygiene.
|
risk of death, 39.9% higher, RR 1.40, p = 0.34, treatment 10 of 7,274 (0.1%), control 124 of 126,152 (0.1%).
|
|
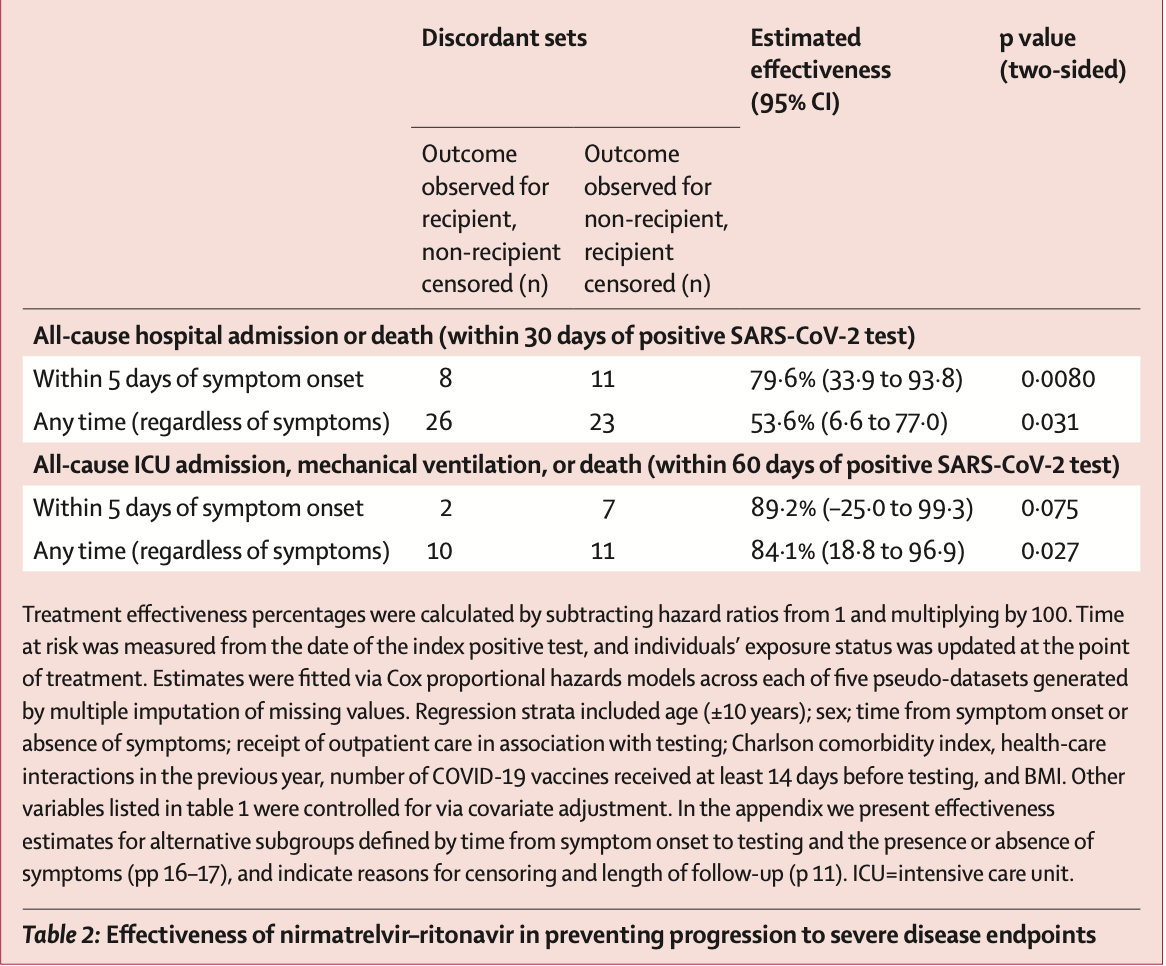
death/mechanical ventilation/ICU, 84.1% lower, HR 0.16, p = 0.03, adjusted per study, multivariable, Cox proportional hazards.
|
|
death/mechanical ventilation/ICU, 89.2% lower, HR 0.11, p = 0.07, adjusted per study, within 5 days, multivariable, Cox proportional hazards.
|
|
risk of death/hospitalization, 53.6% lower, HR 0.46, p = 0.03, adjusted per study, multivariable, Cox proportional hazards.
|
|
risk of death/hospitalization, 79.6% lower, HR 0.20, p = 0.008, adjusted per study, within 5 days, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Malden et al., Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system, Scientific Reports, doi:10.1038/s41598-024-57633-7.
5.
Campion et al., Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study, Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1809.
6.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
7.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
8.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
9.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
10.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
11.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
12.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
13.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
14.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
15.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
16.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
17.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
18.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
19.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
20.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
21.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Lewnard et al., 15 Mar 2023, retrospective, USA, peer-reviewed, 12 authors, study period 31 December, 2021 - 7 April, 2022.
Contact: jlewnard@berkeley.edu.
Effectiveness of nirmatrelvir–ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(23)00118-4
Background In the USA, oral nirmatrelvir-ritonavir is authorised for use in patients aged 12 years or older with mildto-moderate COVID-19 who are at risk of progression to severe disease and hospitalisation. We aimed to establish the effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and death in people with COVID-19 in an outpatient prescribing context in the USA. Methods In this matched observational outpatient cohort study in the Kaiser Permanente Southern California (CA, USA) health-care system, data were extracted from electronic health records of non-hospitalised patients aged 12 years or older who received a positive SARS-CoV-2 PCR test result (their index test) between April 8 and Oct 7, 2022, and had not received another positive test result within the preceding 90 days. We compared outcomes between people who received nirmatrelvir-ritonavir and those who did not receive nirmatrelvir-ritonavir by matching cases by date, age, sex, clinical status (including care received, the presence or absence of acute COVID-19 symptoms at testing, and time from symptom onset to testing), vaccination history, comorbidities, health-care seeking during the previous year, and BMI. Our primary endpoint was the estimated effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions or death within 30 days of a positive test for SARS-CoV-2. Findings 7274 nirmatrelvir-ritonavir recipients and 126 152 non-recipients with positive SARS-CoV-2 tests were included in our study. 5472 (75•2%) treatment recipients and 84 657 (67•1%) non-recipients were tested within 5 days of symptom onset. Nirmatrelvir-ritonavir had an overall estimated effectiveness of 53•6% (95% CI 6•6-77•0) in preventing hospital admission or death within 30 days of a positive test for SARS-CoV-2, which increased to 79•6% (33•9-93•8) when nirmatrelvir-ritonavir was dispensed within 5 days of symptom onset. Within the subgroup of patients tested within 5 days of symptom onset and whose treatment was dispensed on the day of their test, the estimated effectiveness of nirmatrelvir-ritonavir was 89•6% (50•2-97•8). Interpretation In a setting with high levels of COVID-19 vaccine uptake, nirmatrelvir-ritonavir effectively reduced the risk of hospital admission or death within 30 days of a positive outpatient SARS-CoV-2 test.
References
Andrejko, Myers, Fukui, Real-world uptake of COVID-19 vaccination among individuals expressing vaccine hesitancy: a registry-linkage study, medRxiv, doi:10.1101/2022.08.02.22278300
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe COVID-19 outcomes during the omicron surge, N Engl J Med
Bajema, Wang, Hynes, Early adoption of anti-SARS-CoV-2 pharmacotherapies among US veterans with mild to moderate COVID-19, January and February 2022, JAMA Netw Open
Black, (9•5%) 9981 (7•9%) Hispanic 3061, non-Hispanic
Bmi, None, kg/m²
Clarke, Jones, Deng, Seroprevalence of infectioninduced SARS-CoV-2 antibodies-United States, September 2021-February 2022, MMWR Morb Mortal Wkly Rep
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, medRxiv, doi:10.1101/2022.06.14.22276393
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
Huynh, Millan, Quijada, John, Khan et al., Description and early results of the Kaiser Permanente Southern California COVID-19 home monitoring program, Permanente J
Iketani, Liu, Guo, Antibody evasion properties of SARS-CoV-2 omicron sublineages, Nature
Lewnard, Hong, Patel, Kahn, Lipsitch et al., Clinical outcomes associated with SARS-CoV-2 omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California, Nat Med
Lewnard, Patel, Jewell, Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines, Epidemiology
Malden, Hong, Lewin, Hospitalization and emergency department encounters for COVID-19 after Paxlovid treatment-California, December 2021-May 2022, MMWR Morb Mortal Wkly Rep
Malden, Tartof, Ackerson, Natural language processing for improved COVID-19 characterization: evidence from more than 350,000 patients in a large integrated health care system, JMIR Public Health Surveill
Messer, Laraia, Kaufman, The development of a standardized neighborhood deprivation index, J Urban Health
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high-risk patients, Clin Infect Dis
Rubin, Multiple imputation after 18+ years, J Am Stat Assoc
Schoenfeld, Partial residuals for the proportional hazards regression model, Biometrika
Shah, Joyce, Plumb, Paxlovid associated with decreased hospitalization rate among adults with COVID-19-United States, April-September, 2022, MMWR Morb Mortal Wkly Rep
Stowe, Andrews, Kirsebom, Ramsay, Bernal, Effectiveness of COVID-19 vaccines against omicron and delta hospitalization: a test-negative case-control study, Nat Comm
Sundarajan, Henderson, Perry, Muggivan, Quan et al., New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality, J Clin Epidemiol
Tartof, Qian, Hong, Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization, Ann Intern Med
Vasan, Foote, Long, Ensuring widespread and equitable access to treatments for COVID-19, JAMA
Villalobos, Ott, Klett-Tammen, Effect modification of the association between comorbidities and severe course of COVID-19 disease by age of study participants: a systematic review and meta-analysis, Syst Rev
White, None, non-Hispanic
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalized patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet
Yip, Lui, Lai, Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients, Clin Infect Dis
DOI record:
{
"DOI": "10.1016/s1473-3099(23)00118-4",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(23)00118-4",
"alternative-id": [
"S1473309923001184"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effectiveness of nirmatrelvir–ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(23)00118-4"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(23)00180-9"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Lewnard",
"given": "Joseph A",
"sequence": "first"
},
{
"affiliation": [],
"family": "McLaughlin",
"given": "John M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malden",
"given": "Debbie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Vennis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puzniak",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ackerson",
"given": "Bradley K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lewin",
"given": "Bruno J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Jeniffer S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shaw",
"given": "Sally F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takhar",
"given": "Harpreet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jodar",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tartof",
"given": "Sara Y",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
15
]
],
"date-time": "2023-03-15T23:41:11Z",
"timestamp": 1678923671000
},
"deposited": {
"date-parts": [
[
2023,
3,
15
]
],
"date-time": "2023-03-15T23:41:29Z",
"timestamp": 1678923689000
},
"indexed": {
"date-parts": [
[
2023,
3,
29
]
],
"date-time": "2023-03-29T19:30:26Z",
"timestamp": 1680118226334
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2023,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
1
]
],
"date-time": "2023-03-01T00:00:00Z",
"timestamp": 1677628800000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
22
]
],
"date-time": "2023-02-22T00:00:00Z",
"timestamp": 1677024000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309923001184?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309923001184?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
3
]
]
},
"published-print": {
"date-parts": [
[
2023,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41586-022-04594-4",
"article-title": "Antibody evasion properties of SARS-CoV-2 omicron sublineages",
"author": "Iketani",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(23)00118-4_bib2",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(23)00118-4_bib3",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe COVID-19 outcomes during the omicron surge",
"author": "Arbel",
"doi-asserted-by": "crossref",
"first-page": "790",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(23)00118-4_bib4",
"volume": "387",
"year": "2022"
},
{
"article-title": "Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system",
"author": "Dryden-Peterson",
"journal-title": "medRxiv",
"key": "10.1016/S1473-3099(23)00118-4_bib5",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high-risk patients",
"author": "Najjar-Debbiny",
"doi-asserted-by": "crossref",
"first-page": "e342",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S1473-3099(23)00118-4_bib6",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1213",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(23)00118-4_bib7",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac687",
"article-title": "Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients",
"author": "Yip",
"doi-asserted-by": "crossref",
"first-page": "e26",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S1473-3099(23)00118-4_bib8",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"article-title": "Real-world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalized patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1681",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(23)00118-4_bib9",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7148e2",
"article-title": "Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April–September, 2022",
"author": "Shah",
"doi-asserted-by": "crossref",
"first-page": "1531",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/S1473-3099(23)00118-4_bib10",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.13554",
"article-title": "Ensuring widespread and equitable access to treatments for COVID-19",
"author": "Vasan",
"doi-asserted-by": "crossref",
"first-page": "705",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(23)00118-4_bib11",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.41434",
"article-title": "Early adoption of anti-SARS-CoV-2 pharmacotherapies among US veterans with mild to moderate COVID-19, January and February 2022",
"author": "Bajema",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/S1473-3099(23)00118-4_bib12",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7117e3",
"article-title": "Seroprevalence of infection-induced SARS-CoV-2 antibodies—United States, September 2021–February 2022",
"author": "Clarke",
"doi-asserted-by": "crossref",
"first-page": "606",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/S1473-3099(23)00118-4_bib13",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01887-z",
"article-title": "Clinical outcomes associated with SARS-CoV-2 omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California",
"author": "Lewnard",
"doi-asserted-by": "crossref",
"first-page": "1933",
"journal-title": "Nat Med",
"key": "10.1016/S1473-3099(23)00118-4_bib14",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7125e2",
"article-title": "Hospitalization and emergency department encounters for COVID-19 after Paxlovid treatment—California, December 2021–May 2022",
"author": "Malden",
"doi-asserted-by": "crossref",
"first-page": "830",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/S1473-3099(23)00118-4_bib15",
"volume": "71",
"year": "2022"
},
{
"article-title": "Real-world uptake of COVID-19 vaccination among individuals expressing vaccine hesitancy: a registry-linkage study",
"author": "Andrejko",
"journal-title": "medRxiv",
"key": "10.1016/S1473-3099(23)00118-4_bib16",
"year": "2022"
},
{
"DOI": "10.2196/41529",
"article-title": "Natural language processing for improved COVID-19 characterization: evidence from more than 350,000 patients in a large integrated health care system",
"author": "Malden",
"doi-asserted-by": "crossref",
"journal-title": "JMIR Public Health Surveill",
"key": "10.1016/S1473-3099(23)00118-4_bib17",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1007/s11524-006-9094-x",
"article-title": "The development of a standardized neighborhood deprivation index",
"author": "Messer",
"doi-asserted-by": "crossref",
"first-page": "1041",
"journal-title": "J Urban Health",
"key": "10.1016/S1473-3099(23)00118-4_bib18",
"volume": "83",
"year": "2006"
},
{
"DOI": "10.1016/j.jclinepi.2004.03.012",
"article-title": "New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality",
"author": "Sundarajan",
"doi-asserted-by": "crossref",
"first-page": "1288",
"journal-title": "J Clin Epidemiol",
"key": "10.1016/S1473-3099(23)00118-4_bib19",
"volume": "57",
"year": "2004"
},
{
"DOI": "10.7812/TPP/20.281",
"article-title": "Description and early results of the Kaiser Permanente Southern California COVID-19 home monitoring program",
"author": "Huynh",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Permanente J",
"key": "10.1016/S1473-3099(23)00118-4_bib20",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1093/biomet/69.1.239",
"article-title": "Partial residuals for the proportional hazards regression model",
"author": "Schoenfeld",
"doi-asserted-by": "crossref",
"first-page": "239",
"journal-title": "Biometrika",
"key": "10.1016/S1473-3099(23)00118-4_bib21",
"volume": "69",
"year": "1982"
},
{
"DOI": "10.7326/M20-3742",
"article-title": "Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization",
"author": "Tartof",
"doi-asserted-by": "crossref",
"first-page": "773",
"journal-title": "Ann Intern Med",
"key": "10.1016/S1473-3099(23)00118-4_bib22",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1186/s13643-021-01732-3",
"article-title": "Effect modification of the association between comorbidities and severe course of COVID-19 disease by age of study participants: a systematic review and meta-analysis",
"author": "Fernández Villalobos",
"doi-asserted-by": "crossref",
"first-page": "194",
"journal-title": "Syst Rev",
"key": "10.1016/S1473-3099(23)00118-4_bib23",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1080/01621459.1996.10476908",
"article-title": "Multiple imputation after 18+ years",
"author": "Rubin",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "J Am Stat Assoc",
"key": "10.1016/S1473-3099(23)00118-4_bib24",
"volume": "91",
"year": "1996"
},
{
"DOI": "10.1097/EDE.0000000000001366",
"article-title": "Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines",
"author": "Lewnard",
"doi-asserted-by": "crossref",
"first-page": "508",
"journal-title": "Epidemiology",
"key": "10.1016/S1473-3099(23)00118-4_bib26",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-33378-7",
"article-title": "Effectiveness of COVID-19 vaccines against omicron and delta hospitalization: a test-negative case-control study",
"author": "Stowe",
"doi-asserted-by": "crossref",
"journal-title": "Nat Comm",
"key": "10.1016/S1473-3099(23)00118-4_bib27",
"volume": "13",
"year": "2022"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309923001184"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Effectiveness of nirmatrelvir–ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}