Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System
et al., Annals of Internal Medicine, doi:10.7326/M22-2141, Dec 2022 (preprint)
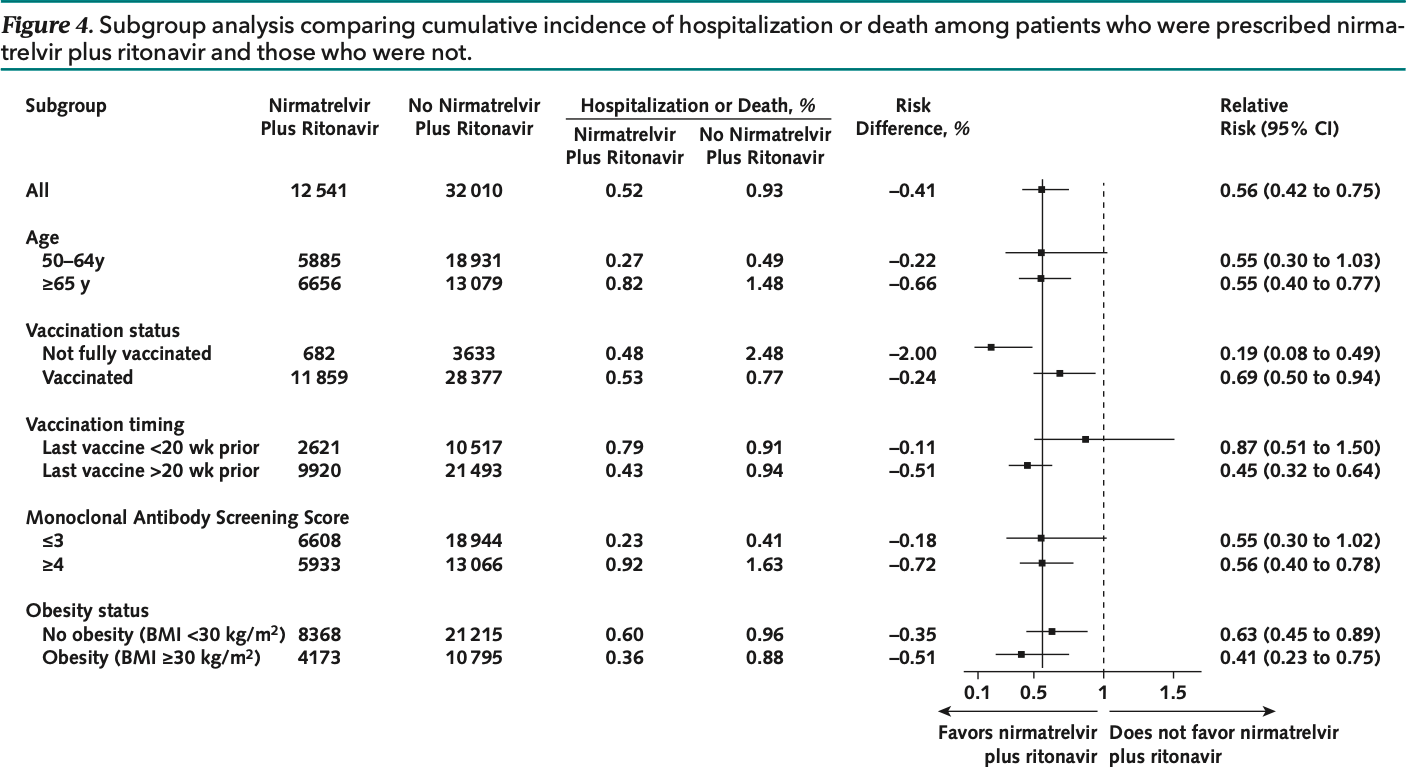
IPW retrospective 44,551 outpatients age 50+ in the USA, showing lower mortality and hospitalization with paxlovid treatment.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments18.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
only a fraction of eligible patients received treatment and these patients may be more likely to follow other recommendations, receive additional care, and more more likely to use additional untracked treatments such as vitamin D and nasal/oral hygiene.
|
risk of death, 71.0% lower, RR 0.29, p = 0.006, treatment 11,797, control 32,248, propensity score weighting.
|
|
risk of death/hospitalization, 44.0% lower, RR 0.56, p < 0.001, treatment 69 of 11,797 (0.6%), control 310 of 32,248 (1.0%), NNT 266, propensity score weighting.
|
|
risk of hospitalization, 40.0% lower, RR 0.60, p = 0.001, treatment 11,797, control 32,248, propensity score weighting.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Dryden-Peterson et al., 13 Dec 2022, retrospective, USA, peer-reviewed, 12 authors, study period 1 January, 2022 - 17 July, 2022.
Contact: sldrydenpeterson@bwh.harvard.edu.
Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System
Annals of Internal Medicine, doi:10.7326/m22-2141
Background: In the EPIC-HR (Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients) trial, nirmatrelvir plus ritonavir led to an 89% reduction in hospitalization or death among unvaccinated outpatients with early COVID-19. The clinical impact of nirmatrelvir plus ritonavir among vaccinated populations is uncertain. Objective: To assess whether nirmatrelvir plus ritonavir reduces risk for hospitalization or death among outpatients with early COVID-19 in the setting of prevalent SARS-CoV-2 immunity and immune-evasive SARS-CoV-2 lineages. Design: Population-based cohort study analyzed to emulate a clinical trial using inverse probability-weighted models to account for anticipated bias in treatment. Setting: A large health care system providing care for 1.5 million patients in Massachusetts and New Hampshire during the Omicron wave (1 January to 17 July 2022). Patients: 44 551 nonhospitalized adults (90.3% with ≥3 vaccine doses) aged 50 years or older with COVID-19 and no contraindications for nirmatrelvir plus ritonavir.
Measurements: The primary outcome was a composite of hospitalization within 14 days or death within 28 days of a COVID-19 diagnosis. Results: During the study period, 12 541 (28.1%) patients were prescribed nirmatrelvir plus ritonavir, and 32 010 (71.9%) were not. Patients prescribed nirmatrelvir plus ritonavir were more likely to be older, have more comorbidities, and be vaccinated. The composite outcome of hospitalization or death occurred in 69 (0.55%) patients who were prescribed nirmatrelvir plus ritonavir and 310 (0.97%) who were not (adjusted risk ratio, 0.56 [95% CI, 0.42 to 0.75]). Recipients of nirmatrelvir plus ritonavir had lower risk for hospitalization (adjusted risk ratio, 0.60 [CI, 0.44 to 0.81]) and death (adjusted risk ratio, 0.29 [CI, 0.12 to 0.71]). Limitation: Potential residual confounding due to differential access to COVID-19 vaccines, diagnostic tests, and treatment.
Conclusion: The overall risk for hospitalization or death was already low (1%) after an outpatient diagnosis of COVID-19, but nirmatrelvir plus ritonavir reduced this risk further.
Author contributions are available at Annals.org. Previous Posting: This manuscript was posted as a preprint on medRxiv on 17 June 2022. doi:10.1101/2022.06. 14.22276393
References
Administrative, Kim, Lennes, Patel, Gainer et al., logistic support
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Bar-On, Goldberg, Mandel, Protection of BNT162b2 vaccine booster against Covid-19 in Israel, N Engl J Med, doi:10.1056/NEJMoa2114255
Cole, Hernán, Constructing inverse probability weights for marginal structural models, Am J Epidemiol, doi:10.1093/aje/kwn164
Denz, Klaaßen-Mielke, Timmesfeld, Boucau, Uddin et al., Virologic characterization of symptom rebound following nirmatrelvir-ritonavir treatment for COVID-19. medRxiv, doi:10.1101/2022.05.24.22275326
Dryden-Peterson, Kim, Caniglia, Patel, Dutton et al., ORIGINAL RESEARCH Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System Author Contributions: Conception and design
Ganatra, Dani, Ahmad, Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19, Clin Infect Dis, doi:10.1093/cid/ciac673
Ganesh, Philpot, Bierle, Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019, J Infect Dis, doi:10.1093/infdis/jiab377
Hammond, Leister-Tebbe, Gardner, EPIC-HR Investigators. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
House, National COVID-19 Preparedness Plan
House, National COVID-19 Preparedness Plan
Højsgaard, Halekoh, Yan, The R package geepack for generalized estimating equations, J Stat Softw, doi:10.18637/jss.v015.i02
Kind, Jencks, Brock, Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study, Ann Intern Med, doi:10.7326/M13-2946
Lambrou, Shirk, Steele, Genomic surveillance for SARS-CoV-2 variants: predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) variants-United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7106a4
Liang, Zeger, Cole, Hernán, Longitudinal data analysis using generalized linear models, Comput Methods Programs Biomed, doi:10.1093/biomet/73.1.13
O'horo, Challener, Speicher, Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the Delta variant, Mayo Clin Proc, doi:10.1056/NEJMp1802313
Zou, A modified Poisson regression approach to prospective studies with binary data, Am J Epidemiol
DOI record:
{
"DOI": "10.7326/m22-2141",
"ISSN": [
"0003-4819",
"1539-3704"
],
"URL": "http://dx.doi.org/10.7326/M22-2141",
"alternative-id": [
"10.7326/M22-2141"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8487-9731",
"affiliation": [
{
"name": "Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health, Boston, Massachusetts, and Botswana Harvard AIDS Institute, Gaborone, Botswana (S.D.)"
}
],
"authenticated-orcid": false,
"family": "Dryden-Peterson",
"given": "Scott",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-6551-2881",
"affiliation": [
{
"name": "Brigham and Women's Hospital, Boston, Massachusetts (A.K., L.D., E.D., L.R.B., A.E.W.)"
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Andy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4712-2657",
"affiliation": [
{
"name": "Massachusetts General Hospital, Boston, Massachusetts (A.Y.K., I.T.L., R.T.G.)"
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Arthur Y.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania (E.C.C.)"
}
],
"family": "Caniglia",
"given": "Ellen C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0783-3411",
"affiliation": [
{
"name": "Massachusetts General Hospital, Boston, Massachusetts (A.Y.K., I.T.L., R.T.G.)"
}
],
"authenticated-orcid": false,
"family": "Lennes",
"given": "Inga T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beth Israel Lahey Health, Cambridge, Massachusetts (R.P.)"
}
],
"family": "Patel",
"given": "Rajesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mass General Brigham Integrated Care, Somerville, Massachusetts (L.G.)."
}
],
"family": "Gainer",
"given": "Lindsay",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1174-0760",
"affiliation": [
{
"name": "Brigham and Women's Hospital, Boston, Massachusetts (A.K., L.D., E.D., L.R.B., A.E.W.)"
}
],
"authenticated-orcid": false,
"family": "Dutton",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brigham and Women's Hospital, Boston, Massachusetts (A.K., L.D., E.D., L.R.B., A.E.W.)"
}
],
"family": "Donahue",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Massachusetts General Hospital, Boston, Massachusetts (A.Y.K., I.T.L., R.T.G.)"
}
],
"family": "Gandhi",
"given": "Rajesh T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brigham and Women's Hospital, Boston, Massachusetts (A.K., L.D., E.D., L.R.B., A.E.W.)"
}
],
"family": "Baden",
"given": "Lindsey R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2810-1618",
"affiliation": [
{
"name": "Brigham and Women's Hospital, Boston, Massachusetts (A.K., L.D., E.D., L.R.B., A.E.W.)"
}
],
"authenticated-orcid": false,
"family": "Woolley",
"given": "Ann E.",
"sequence": "additional"
}
],
"container-title": "Annals of Internal Medicine",
"container-title-short": "Ann Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
12
]
],
"date-time": "2022-12-12T22:00:19Z",
"timestamp": 1670882419000
},
"deposited": {
"date-parts": [
[
2022,
12,
12
]
],
"date-time": "2022-12-12T22:00:41Z",
"timestamp": 1670882441000
},
"indexed": {
"date-parts": [
[
2022,
12,
12
]
],
"date-time": "2022-12-12T22:41:33Z",
"timestamp": 1670884893614
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
12,
13
]
]
},
"language": "en",
"member": "4285",
"original-title": [],
"prefix": "10.7326",
"published": {
"date-parts": [
[
2022,
12,
13
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
13
]
]
},
"publisher": "American College of Physicians",
"reference": [
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "r1-M222141"
},
{
"DOI": "10.1056/NEJMoa2204919",
"doi-asserted-by": "publisher",
"key": "r5-M222141"
},
{
"DOI": "10.1093/cid/ciac673",
"doi-asserted-by": "publisher",
"key": "r6-M222141"
},
{
"DOI": "10.15585/mmwr.mm7106a4",
"doi-asserted-by": "publisher",
"key": "r7-M222141"
},
{
"DOI": "10.1093/infdis/jiab377",
"doi-asserted-by": "publisher",
"key": "r8-M222141"
},
{
"DOI": "10.1016/j.mayocp.2021.12.002",
"doi-asserted-by": "publisher",
"key": "r9-M222141"
},
{
"DOI": "10.1056/NEJMp1802313",
"doi-asserted-by": "publisher",
"key": "r10-M222141"
},
{
"DOI": "10.7326/M13-2946",
"doi-asserted-by": "publisher",
"key": "r11-M222141"
},
{
"DOI": "10.1056/NEJMoa2114255",
"doi-asserted-by": "publisher",
"key": "r12-M222141"
},
{
"DOI": "10.1093/aje/kwn164",
"doi-asserted-by": "publisher",
"key": "r13-M222141"
},
{
"DOI": "10.1093/aje/kwh090",
"doi-asserted-by": "publisher",
"key": "r14-M222141"
},
{
"DOI": "10.18637/jss.v015.i02",
"doi-asserted-by": "crossref",
"key": "r15-M222141",
"unstructured": "Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2005;15:1-11. doi:10.18637/jss.v015.i02"
},
{
"DOI": "10.1093/biomet/73.1.13",
"doi-asserted-by": "crossref",
"key": "r16-M222141",
"unstructured": "Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13-22. doi:10.1093/biomet/73.1.13"
},
{
"DOI": "10.1016/j.cmpb.2003.10.004",
"doi-asserted-by": "publisher",
"key": "r17-M222141"
},
{
"key": "r18-M222141",
"unstructured": "Denz R, Klaaßen-Mielke R, Timmesfeld N. A comparison of different methods to adjust survival curves for confounders. arXiv. Preprint posted online 18 March 2022. doi:10.48550/arXiv.2203.10002"
},
{
"DOI": "10.1101/2022.05.24.22275326",
"doi-asserted-by": "crossref",
"key": "r19-M222141",
"unstructured": "Boucau J, Uddin R, Marino C, et al. Virologic characterization of symptom rebound following nirmatrelvir-ritonavir treatment for COVID-19. medRxiv. Preprint posted online 26 May 2022. doi:10.1101/2022.05.24.22275326"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.acpjournals.org/doi/10.7326/M22-2141"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Internal Medicine"
],
"subtitle": [
"A Population-Based Cohort Study"
],
"title": "Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System",
"type": "journal-article"
}