Quercetin for COVID-19
36th treatment shown to reduce risk in
January 2022, now with p = 0.0018 from 9 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of the biological and medicinal properties of quercetin, particularly its anti-inflammatory, antioxidant, anti-tumor, and antiviral effects. Quercetin demonstrates promise against SARS-CoV-2 as evidenced by its binding interactions with key viral targets like the spike protein, though its poor bioavailability requires advanced formulations. Researchers have successfully complexed quercetin with cyclodextrins, polymers, liposomes, and nanomaterials to form stable compounds with enhanced solubility and bioavailability. Combination therapy synergizing quercetin with other drugs also shows promising results, with multiple studies showing augmented inhibition of viruses, cancers, cardiovascular diseases, and more. Overall, authors find that complexed and synergized formulations of quercetin have significant potential as inexpensive, safe therapeutics, especially as adjunct treatments for viral infections like COVID-19 where oxidative damage contributes to pathogenesis.
1.
Ricke, D., Mast cells and histamine receptor-targeted adjunctive treatments for COVID-19: A literature review, Innovative Medicines & Omics, doi:10.36922/IMO025440058.
2.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
3.
Sanduzzi Zamparelli et al., Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective, Life, doi:10.3390/life15010113.
4.
Duan et al., Bioactive compounds,quercetin, curcumin and β-glucan,regulate innate immunity via the gut-liver-brain axis, Trends in Food Science & Technology, doi:10.1016/j.tifs.2024.104864.
5.
Beşler et al., Investigation of potential effects of quercetin on COVID-19 treatment: a systematic review of randomized controlled trials, Clinical Science of Nutrition, doi:10.62210/ClinSciNutr.2024.86.
6.
Ho et al., Therapeutic Implications of Quercetin and its Derived-products in COVID-19 Protection and Prophylactic, Heliyon, doi:10.1016/j.heliyon.2024.e30080.
7.
Vajdi et al., Effect of polyphenols against complications of COVID-19: current evidence and potential efficacy, Pharmacological Reports, doi:10.1007/s43440-024-00585-6.
8.
Chen et al., Effect and mechanism of quercetin or quercetin‐containing formulas against COVID‐19: From bench to bedside, Phytotherapy Research, doi:10.1002/ptr.8175.
9.
Yong et al., Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases, International Journal of Nanomedicine, doi:10.2147/ijn.s451206.
10.
Agrawal et al., Antiviral Significance of Isoquercetin (Quercetin-3-O-Glucoside) With Special Reference to its Anti-Coronaviral Potential, Natural Product Communications, doi:10.1177/1934578X231219560.
11.
Matías-Pérez et al., Relationship of quercetin intake and oxidative stress in persistent Covid, Frontiers in Nutrition, doi:10.3389/fnut.2023.1278039.
12.
Georgiou et al., Quercetin: A Potential Polydynamic Drug, Molecules, doi:10.3390/molecules28248141.
13.
Donzelli, A., Neglected Effective Early Therapies against COVID-19: Focus on Functional Foods and Related Active Substances. A Review, MDPI AG, doi:10.20944/preprints202312.1178.v1.
14.
Dinda et al., Anti-SARS-CoV-2, antioxidant and immunomodulatory potential of dietary flavonol quercetin: Focus on molecular targets and clinical efficacy, European Journal of Medicinal Chemistry Reports, doi:10.1016/j.ejmcr.2023.100125.
15.
Mirza et al., Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications—A Review, Pharmaceuticals, doi:10.3390/ph16111631.
16.
Massimo Magro et al., Use of Quercetin for Therapeutic Purposes in COVID-19 Infections: The Opinion of the Geriatrician Doctor, Journal of Modern Biology and Drug Discovery, doi:10.53964/jmbdd.2023004.
17.
Shorobi et al., Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions, Molecules, doi:10.3390/molecules28030938.
18.
Gasmi et al., Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2, Pharmaceuticals, doi:10.3390/ph15091049.
19.
Rizky et al., The pharmacological mechanism of quercetin as adjuvant therapy of COVID-19, Life Research, doi:10.53388/life2022-0205-302.
20.
Imran et al., The Therapeutic and Prophylactic Potential of Quercetin against COVID-19: An Outlook on the Clinical Studies, Inventive Compositions, and Patent Literature, Antioxidants, doi:10.3390/antiox11050876.
Georgiou et al., 17 Dec 2023, peer-reviewed, 10 authors.
Contact: tmavrom@chem.uoa.gr (corresponding author), nikitage@chem.uoa.gr, alexroutsi@chem.uoa.gr, errpets@chem.uoa.gr, nikosstav@chem.uoa.gr, nikolzoup@chem.uoa.gr, kmoschovou@chem.uoa.gr, sofki@chem.uoa.gr, up1068935@ac.upatras.gr, xrifre@chem.uoa.gr.
Quercetin: A Potential Polydynamic Drug
Molecules, doi:10.3390/molecules28248141
The study of natural products as potential drug leads has gained tremendous research interest. Quercetin is one of those natural products. It belongs to the family of flavonoids and, more specifically, flavonols. This review summarizes the beneficial pharmaceutical effects of quercetin, such as its anti-cancer, anti-inflammatory, and antimicrobial properties, which are some of the quercetin effects described in this review. Nevertheless, quercetin shows poor bioavailability and low solubility. For this reason, its encapsulation in macromolecules increases its bioavailability and therefore pharmaceutical efficiency. In this review, a brief description of the different forms of encapsulation of quercetin are described, and new ones are proposed. The beneficial effects of applying new pharmaceutical forms of nanotechnology are outlined.
References
Agrawal, Agrawal, Blunden, Quercetin: Antiviral Significance and Possible COVID-19 Integrative Considerations, Nat. Prod. Commun, doi:10.1177/1934578X20976293
Ahmedova, Paradowska, Wawer, 1H, 13C MAS NMR and DFT GIAO Study of Quercetin and Its Complex with Al(III) in Solid State, J. Inorg. Biochem, doi:10.1016/j.jinorgbio.2012.02.007
Ahrens, Thompson, Atm Metabolics Lllp. Composition for Treating Diabetes and Metabolic Disorders with Quercetin, Myrcetin and Chlorogenic Acid, EP
Alabri, Hussain, Mabood, Rehman, Ali et al., Fluorescence Spectroscopy-Partial Least Square Regression Method for the Quantification of Quercetin in Euphorbia Masirahensis, Measurement, doi:10.1016/j.measurement.2018.02.036
Alban, Monteiro, Diz, Miranda, Scheid et al., New Quercetin-Coated Titanate Nanotubes and Their Radiosensitization Effect on Human Bladder Cancer, Mater. Sci. Eng. C, doi:10.1016/j.msec.2020.110662
Ali, Sudi, Shi-Jing, Rozianoor, Hassan et al., Dual Anti-Malarial and GSK3 β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria, Pharmaceuticals, doi:10.3390/ph14030248
Amanzadeh, Esmaeili, Rahgozar, Nourbakhshnia, Application of Quercetin in Neurological Disorders: From Nutrition to Nanomedicine, Rev. Neurosci, doi:10.1515/revneuro-2018-0080
Ansari, Choudhury, Seidel, Rahman, Aziz et al., Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus, Life, doi:10.3390/life12081146
Anusuya, Gromiha, Quercetin Derivatives as Non-Nucleoside Inhibitors for Dengue Polymerase: Molecular Docking, Molecular Dynamics Simulation, and Binding Free Energy Calculation, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2016.1234416
Asfaram, Arabi, Ostovan, Sadeghi, Ghaedi, Simple and Selective Detection of Quercetin in Extracts of Plants and Food Samples by Dispersive-Micro-Solid Phase Extraction Based on Core-Shell Magnetic Molecularly Imprinted Polymers, New J. Chem
Bairwa, Kakwani, Tawari, Lalchandani, Ray et al., Novel Molecular Hybrids of Cinnamic Acids and Guanylhydrazones as Potential Antitubercular Agents, Bioorg. Med. Chem. Lett, doi:10.1016/j.bmcl.2010.01.031
Baskar, Garrick, Lalitha, Chamundeeswari, Gold Nanoparticle Mediated Delivery of Fungal Asparaginase against Cancer Cells, J. Drug Deliv. Sci. Technol, doi:10.1016/j.jddst.2018.02.007
Belščak-Cvitanović, Valinger, Benković, Tušek, Jurina et al., Integrated Approach for Bioactive Quality Evaluation of Medicinal Plant Extracts Using HPLC-DAD, Spectrophotometric, near Infrared Spectroscopy and Chemometric Techniques, Int. J. Food Prop, doi:10.1080/10942912.2017.1373122
Braga, Rosa, Dias, Synthesis and Characterization of Molecularly Imprinted Silica Mediated by Al for Solid Phase Extraction of Quercetin in Ginkgo biloba L. Anal, Methods, doi:10.1039/C4AY00471J
Brown, Treatment of Fragile X Syndrome with Ibudilast in Combination with Metformin, Cannbidiol, Sertraline or Quercetin, WO2021044158A
Burger, Granger, Scott, Skin Care Compositions Containing Naringenin and/or Quercetin and a Retinoid, US5665367A
Caira, Bourne, Samsodien, Smith, Inclusion Complexes of 2-Methoxyestradiol with Dimethylated and Permethylated β-Cyclodextrins: Models for Cyclodextrin-Steroid Interaction, Beilstein J. Org. Chem, doi:10.3762/bjoc.11.281
Canini, Alesiani, D'arcangelo, Tagliatesta, Gas Chromatography-Mass Spectrometry Analysis of Phenolic Compounds from Carica papaya L. Leaf, J. Food Compos. Anal, doi:10.1016/j.jfca.2007.03.009
Caro, Pourmadadi, Eshaghi, Rahmani, Shojaei et al., Nanomaterials Loaded with Quercetin as an Advanced Tool for Cancer Treatment, J. Drug Deliv. Sci. Technol, doi:10.1016/j.jddst.2022.103938
Castro, Barbiric, Molecular Modeling and Cyclodextrins: A Relationship Strengthened By Complexes, Curr. Org. Chem, doi:10.2174/138527206776818928
Cecchi, Ieri, Vignolini, Mulinacci, Romani, Characterization of Volatile and Flavonoid Composition of Different Cuts of Dried Onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GC×GC-TOF and HPLC-DAD, Molecules, doi:10.3390/molecules25020408
Chen, Chang, Gilson, Calculation of Cyclodextrin Binding Affinities: Energy, Entropy, and Implications for Drug Design, Biophys. J, doi:10.1529/biophysj.104.049494
Chen, Zhang, Ye, Determination of Rutin and Quercetin in Plants by Capillary Electrophoresis with Electrochemical Detection, Anal. Chim. Acta, doi:10.1016/S0003-2670(00)01099-0
Chen, Zhang, Zhu, Liu, Chen et al., Quercetin Inhibits TNF-α Induced HUVECs Apoptosis and Inflammation via Downregulating NF-KB and AP-1 Signaling Pathway in vitro, Medicine, doi:10.1097/MD.0000000000022241
Chodoeva, Quercetin-Based Composition for Treating Rhinosinusitis, US2021000787A
Chontzopoulou, Papaemmanouil, Chatziathanasiadou, Kolokouris, Kiriakidi et al., Molecular Investigation of Artificial and Natural Sweeteners as Potential Anti-Inflammatory Agents, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2021.1973565
Cornard, Merlin, Boudet, Vrielynck, Structural Study of Quercetin by Vibrational and Electronic Spectroscopies Combined with Semiempirical Calculations, Biospectroscopy, doi:10.1002/(SICI)1520-6343(1997)3:3%3C183::AID-BSPY2%3E3.0.CO;2-7
D'mello, Joshi, Shetgiri, Dasgupta, Darji, A Simple HPLC Method for Quantitation of Quercetin in Herbal Extracts, J. AOAC Int, doi:10.1093/jaoac/94.1.100
Da Silva, Maia, Lopes, De Albuquerque, Valle et al., Characterization and Antichagasic Evaluation of Thiosemicarbazones Prepared from Chalcones and Dibenzalacetones, J. Mol. Struct, doi:10.1016/j.molstruc.2021.130014
Daina, Michielin, Zoete, SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules, Sci. Rep, doi:10.1038/srep42717
Das, Saha, Mahata, China, Chatterjee et al., Quercetin and 5-Fu Loaded Chitosan Nanoparticles Trigger Cell-Cycle Arrest and Induce Apoptosis in HCT116 Cells via Modulation of the P53/P21 Axis, ACS Omega, doi:10.1021/acsomega.3c03933
De La Torre, Tomé, Silva, Cavaleiro, Synthesis of [60]Fullerene-Quercetin Dyads, Tetrahedron Lett, doi:10.1016/S0040-4039(02)00867-5
De, Bedos-Belval, Vanucci-Bacque, Baltas, Cinnamic Acid Derivatives in Tuberculosis, Malaria and Cardiovascular Diseases-A Review, Curr. Org. Chem, doi:10.2174/138527212799958020
Debnath, Jana, Jana, Quercetin Encapsulated Polymer Nanoparticle for Inhibiting Intracellular Polyglutamine Aggregation, ACS Appl. Bio Mater, doi:10.1021/acsabm.9b00518
Deng, Zito, Development and Validation of a Gas Chromatographic-Mass Spectrometric Method for Simultaneous Identification and Quantification of Marker Compounds Including Bilobalide, Ginkgolides and Flavonoids in Ginkgo biloba L. Extract and Pharmaceutical Preparatio, J. Chromatogr. A, doi:10.1016/S0021-9673(02)01921-0
Dhanya, Quercetin for Managing Type 2 Diabetes and Its Complications, an Insight into Multitarget Therapy, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.112560
Di Petrillo, Orrù, Fais, Fantini, Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review, Phyther. Res, doi:10.1002/ptr.7309
Diamantis, Ramesova, Chatzigiannis, Degano, Gerogianni et al., Exploring the Oxidation and Iron Binding Profile of a Cyclodextrin Encapsulated Quercetin Complex Unveiled a Controlled Complex Dissociation through a Chemical Stimulus, Biochim. Biophys. Acta-Gen. Subj, doi:10.1016/j.bbagen.2018.06.006
Down, Nair, Thurairaja, Bladder, Cancer, None, Surgery, doi:10.1016/j.mpsur.2016.08.001
Ekinci, Supercritical Fluid Extraction of Quercetin from Sumac (Rhus coriaria L.): Effects of Supercritical Extraction Parameters, Sep. Sci. Technol, doi:10.1080/01496395.2021.1893333
Erdogan, Turkekul, Dibirdik, Doganlar, Doganlar et al., Midkine Downregulation Increases the Efficacy of Quercetin on Prostate Cancer Stem Cell Survival and Migration through PI3K/AKT and MAPK/ERK Pathway, Biomed. Pharmacother, doi:10.1016/j.biopha.2018.08.061
Ezzat, Choucry, Kandil, Antioxidant, and Topical Anti-Inflammatory Activities of Bergia Ammannioides: A Wound-Healing Plant, Pharm. Biol, doi:10.3109/13880209.2015.1028079
Ferreira-Silva, Faria-Silva, Carvalheiro, Simões, Marinho et al., Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment, Pharmaceutics, doi:10.3390/pharmaceutics14010104
Fogaça, Feuser, Ricci-Júnior, Hermes De Araújo, Sayer et al., ZnO and Quercetin Encapsulated Nanoparticles for Sun Protection Obtained by Miniemulsion Polymerization Using Alternative Co-Stabilizers, Mater. Res. Express, doi:10.1088/2053-1591/ab6c8e
Formica, Regelson, Review of the Biology of Quercetin and Related Bioflavonoids, Food Chem. Toxicol, doi:10.1016/0278-6915(95)00077-1
Frison-Norrie, Sporns, Identification and Quantification of Flavonol Glycosides in Almond Seedcoats Using MALDI-TOF MS, J. Agric. Food Chem, doi:10.1021/jf0115894
Fábio, Rocha, Sales, Gleiciane, Galdino et al., Antifungal Effects of the Flavonoids Kaempferol and Quercetin: A Possible Alternative for the Control of Fungal Biofilms, Biofouling, doi:10.1080/08927014.2019.1604948
George, Parimelazhagan, Sajeesh, Saravanan, Antitumor and Wound Healing Properties of Rubus Niveus Thunb, Root. J. Environ. Pathol. Toxicol. Oncol, doi:10.1615/JEnvironPatholToxicolOncol.2014010949
Georgiou, Cheilari, Karta, Chontzopoulou, Plavec et al., Conformational Properties and Putative Bioactive Targets for Novel Thiosemicarbazone Derivatives, Molecules, doi:10.3390/molecules27144548
Georgiou, Chontzopoulou, Cheilari, Katsogiannou, Karta et al., Thiocarbohydrazone and Chalcone-Derived 3,4-Dihydropyrimidinethione as Lipid Peroxidation and Soybean Lipoxygenase Inhibitors, ACS Omega, doi:10.1021/acsomega.2c07625
Georgiou, Gouleni, Chontzopoulou, Skoufas, Gkionis et al., Structure Assignment, Conformational Properties and Discovery of Potential Targets of the Ugi Cinnamic Adduct NGI25, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2021.2017356
Georgiou, Katsogiannou, Skourtis, Iatrou, Tzeli et al., Conformational Properties of New Thiosemicarbazone and Thiocarbohydrazone Derivatives and Their Possible Targets, Molecules, doi:10.3390/molecules27082537
Ghosh, Sarmah, Patel, Mukerjee, Mishra et al., Nonlinear Molecular Dynamics of Quercetin in Gynocardia Odorata and Diospyros Malabarica Fruits: Its Mechanistic Role in Hepatoprotection, PLoS ONE, doi:10.1371/journal.pone.0263917
Ginex, Vazquez, Gilbert, Herrero, Luque, Lipophilicity in Drug Design: An Overview of Lipophilicity Descriptors in 3D-QSAR Studies, Future Med. Chem, doi:10.4155/fmc-2018-0435
Gross, Pfeiffer, Martini, Campbell, Slavin et al., The Quantitation of Metabolites of Quercetin Flavonols in Human Urine, Cancer Epidemiol. Biomark. Prev
Guan, Yang, Cai, Sun, Di et al., ADMET-Score-A Comprehensive Scoring Function for Evaluation of Chemical Drug-Likeness, Medchemcomm, doi:10.1039/C8MD00472B
Guss, Ziyatdinova, Zhupanova, Budnikov, Voltammetric Determination of Quercetin and Rutin on Their Simultaneous Presence on an Electrode Modified with Polythymolphthalein, J. Anal. Chem, doi:10.1134/S106193482004005X
Gutierrez, Gehlen, Time Resolved Fluorescence Spectroscopy of Quercetin and Morin Complexes with Al3+. Spectrochim, Acta Part A Mol. Biomol. Spectrosc, doi:10.1016/S1386-1425(01)00515-7
Gwonhwa, Whasun, Sunwoo, Pharmaceutical Composition for Preventing or Treating Endometriosis Comprising Quercetin Luteolin Delphinidin or Mixture Thereof, KR20210044409A
Haimhoffer, Rusznyák, Réti-Nagy, Vasvári, Váradi et al., Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers, Sci. Pharm, doi:10.3390/scipharm87040033
Hamed, Abdallah, Bedair, Mansour, Sample Preparation Methods for Determination of Quercetin and Quercetin Glycosides in Diverse Matrices, Microchem. J, doi:10.1016/j.microc.2023.109233
Han, Zeng, Jiang, Xing, Huang, Talanta MIL-101 (Cr) as Matrix for Sensitive Detection of Quercetin by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry, Talanta, doi:10.1016/j.talanta.2016.11.044
Hisaka, Sakai, Sato, Goto, Nomura et al., Quercetin Suppresses Proliferation of Liver Cancer Cell Lines In Vitro, Anticancer Res, doi:10.21873/anticanres.14469
Hosseini, Razavi, Banach, Hosseinzadeh, Quercetin and Metabolic Syndrome: A Review, Phyther. Res, doi:10.1002/ptr.7144
Huang, Czech, The GLUT4 Glucose Transporter, Cell Metab, doi:10.1016/j.cmet.2007.03.006
Huang, Feng, Tang, Li, Zhang et al., Development and Validation of a Fast SFC Method for the Analysis of Flavonoids in Plant Extracts, J. Pharm. Biomed. Anal, doi:10.1016/j.jpba.2017.03.012
Huang, Wang, Li, Xia, Xia, Nanostructured Lipid Carrier (NLC) as a Strategy for Encapsulation of Quercetin and Linseed Oil: Preparation and in Vitro Characterization Studies, J. Food Eng, doi:10.1016/j.jfoodeng.2017.07.002
Hussain, Rehman, Mabood, Al-Harrasi, Ali et al., Application of Fluorescence Spectroscopy Coupled with PLSR for the Estimation of Quercetin in Four Medicinal Plants, Chem. Data Collect, doi:10.1016/j.cdc.2019.100228
Ivanov, Ivanova, Roomi, Niedzwicki, Rath, Novel Composition and Method for the Treatment of Hypertension, US2004242504A
Jalili, Quercetin Supplementation to Treat Hypertenstion, US
Jeung-Hye, Composition Containing Quercetin and Vitamin D for Alleviation of Acnegenic Skin, WO
Jeung-Hye, Composition, Containing Quercetin, Genistein, and Alpha-Lipoic Acid, for Relieving Acne Skin, WO2020111757A
Joshi, Aggarwal, Hiprara, Jaggi, Singh et al., Novel Quercetin Derivatives as Anti-Cancer Agents, US
Jullian, Moyano, Yañez, Olea-Azar, Complexation of Quercetin with Three Kinds of Cyclodextrins: An Antioxidant Study, Spectrochim. Acta-Part A Mol. Biomol. Spectrosc, doi:10.1016/j.saa.2006.07.006
Jurasekova, Domingo, Garcia-Ramos, Sanchez-Cortes, Effect of PH on the Chemical Modification of Quercetin and Structurally Related Flavonoids Characterized by Optical (UV-Visible and Raman) Spectroscopy, Phys. Chem. Chem. Phys, doi:10.1039/C4CP00864B
Jurasekova, Torreggiani, Tamba, Sanchez-Cortes, Garcia-Ramos, Raman and Surface-Enhanced Raman Scattering (SERS) Investigation of the Quercetin Interaction with Metals: Evidence of Structural Changing Processes in Aqueous Solution and on Metal Nanoparticles, J. Mol. Struct, doi:10.1016/j.molstruc.2008.07.025
Karaboga, Perez-Neuno, Souchet, Decaudin, Muscle Atrophy Inhibitor Containing Quercetin Glycoside, CN106255500A
Kellici, Chatziathanasiadou, Diamantis, Chatzikonstantinou, Andreadelis et al., Mapping the Interactions and Bioactivity of Quercetin(2-Hydroxypropyl)-β-Cyclodextrin Complex, Int. J. Pharm, doi:10.1016/j.ijpharm.2016.07.008
Kellici, Chatziathanasiadou, Lee, Sayyad, Geromichalou et al., Rational Design and Structure-Activity Relationship Studies of Quercetin-Amino Acid Hybrids Targeting the Anti-Apoptotic Protein Bcl-XL, Org. Biomol. Chem, doi:10.1039/C7OB02045G
Kfoury, Landy, Ruellan, Auezova, Greige-Gerges et al., Determination of Formation Constants and Structural Characterization of Cyclodextrin Inclusion Complexes with Two Phenolic Isomers: Carvacrol and Thymol, Beilstein J. Org. Chem, doi:10.3762/bjoc.12.5
Khan, Khan, Asiri, Rub, Azum et al., A New Trend on Biosensor for Neurotransmitter Choline/Acetylcholine-An Overview, Appl. Biochem. Biotechnol, doi:10.1007/s12010-013-0099-0
Khan, Khan, Asiri, Rub, Rahman et al., In Vitro Studies of Carbon Fiber Microbiosensor for Dopamine Neurotransmitter Supported by Copper-Graphene Oxide Composite, Microchim. Acta, doi:10.1007/s00604-014-1202-0
Khan, Khan, Asiri, Toward Design and Measurement of Electrical Conductivity and Thermal Properties of Silver Nanoparticle Embedded Poly(o-anisidine) Molybdophosphate Nanocomposite and Its Application as Microbiosensor, Polym. Compos, doi:10.1002/pc.23981
Kicuntod, Khuntawee, Wolschann, Pongsawasdi, Chavasiri et al., Inclusion Complexation of Pinostrobin with Various Cyclodextrin Derivatives, J. Mol. Graph. Model, doi:10.1016/j.jmgm.2015.11.005
Kikiowo, Ahmad, Alade, Ijatuyi, Iwaloye et al., Molecular Dynamics Simulation and Pharmacokinetics Studies of Ombuin and Quercetin against Human Pancreatic α-Amylase, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2022.2155699
Kim, Park, Quercetin and Its Role in Biological Functions: An Updated Review, EXCLI J, doi:10.17179/excli2018-1538
Kokalj Ladan, Straus, Tavčar Benković, Kreft, FT-IR-Based Method for Rutin, Quercetin and Quercitrin Quantification in Different Buckwheat (Fagopyrum) Species, Sci. Rep, doi:10.1038/s41598-017-07665-z
Koontz, Marcy, O'keefe, Duncan, Cyclodextrin Inclusion Complex Formation and Solid-State Characterization of the Natural Antioxidants α-Tocopherol and Quercetin, J. Agric. Food Chem, doi:10.1021/jf802823q
Korotkova, Voronova, Dorozhko, Study of Antioxidant Properties of Flavonoids by Voltammetry, J. Solid State Electrochem, doi:10.1007/s10008-012-1707-6
Kratz, Teixeira, Ferronato, Teixeira, Koester et al., Characterization, and In Vitro Intestinal Permeability Evaluation of Thalidomide-Hydroxypropyl-β-Cyclodextrin Complexes, AAPS PharmSciTech, doi:10.1208/s12249-011-9739-2
Kroslakova, Pedrussio, Wolfram, Direct Coupling of HPTLC with MALDI-TOF MS for Qualitative Detection of Flavonoids on Phytochemical Fingerprints, Phytochem. Anal, doi:10.1002/pca.2621
Kruthiventi, Javed, Pharmaceutical Co-Crystals of Quercetin, US20120258170A
Kuebler, Medicament, Polifenoles, Useful to Treat or Prevent Malignantly Transformed Cells, e.g., Adeno-Carcinoma, Prostate Carcinoma and Breast Carcinoma, Comprises a Mixture of Quercetin and Myrecetin and/or Anisomycin and Rapamycin as Kinase Inhibitors. DE102006036307A1
Kumar, Malik, Tewary, A New Method for Determination of Myricetin and Quercetin Using Solid Phase Microextraction-High Performance Liquid Chromatography-Ultra Violet/Visible System in Grapes, Vegetables and Red Wine Samples, Anal. Chim. Acta, doi:10.1016/j.aca.2008.10.038
Kumar, Verma, Singh, Morphological and in vitro Antibacterial Efficacy of Quercetin Loaded Nanoparticles against Food-Borne Microorganisms, LWT-Food Sci. Technol, doi:10.1016/j.lwt.2015.11.004
Kumari, Yadav, Pakade, Singh, Yadav, Development of Biodegradable Nanoparticles for Delivery of Quercetin, Colloids Surf. B Biointerfaces, doi:10.1016/j.colsurfb.2010.06.002
Kurzawa, Determination of Quercetin and Rutin in Selected Herbs and Pharmaceutical Preparations, Anal. Lett, doi:10.1080/00032710903491070
Lan, Hou, Liu, Ding, Zhang et al., Synthesis and Evaluation of Novel Cinnamic Acid Derivatives Bearing N-Benzyl Pyridinium Moiety as Multifunctional Cholinesterase Inhibitors for Alzheimer's Disease, J. Enzym. Inhib. Med. Chem, doi:10.1080/14756366.2016.1256883
Larson, Symons, Jalili, Quercetin: A Treatment for Hypertension?-A Review of Efficacy and Mechanisms, Pharmaceuticals, doi:10.3390/ph3010237
Leonis, Vakali, Zoupanou, Georgiou, Diamantis et al., Computational and Spectroscopic Analysis of the Quercetin Encapsulation in (2HP-β-CD)2 and (2,6Me-β-CD)2 Complexes, J. Mol. Struct, doi:10.1016/j.molstruc.2023.136430
Li, Li, Yu, Liao, Wang, Fast Disintegrating Quercetin-Loaded Drug Delivery Systems Fabricated Using Coaxial Electrospinning, Int. J. Mol. Sci, doi:10.3390/ijms141121647
Li, Liu, Gao, The Application of Nanoparticles in Diagnosis and Theranostics of Gastric Cancer, Cancer Lett, doi:10.1016/j.canlet.2016.10.032
Li, Wang, Fei, Wu, Tao et al., A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules, ChemMed-Chem, doi:10.1002/cmdc.201600182
Li, Yao, Han, Yang, Chaudhry et al., Inflammation and Immunity, Nutrients, doi:10.3390/nu8030167
Li, Zhang, Su, Zhou, Wang, Nanoparticles Designed to Regulate Tumor Microenvironment for Cancer Therapy, Life Sci, doi:10.1016/j.lfs.2018.03.044
Li, Zhang, Yuan, Separation and Determination of Rutin and Quercetin in the Flowers of Sophora japonica L. by Capillary Electrophoresis with Electrochemical Detection, Chromatographia, doi:10.1007/BF02492150
Liao, Li, Zhao, Jiang, Yan et al., Synthesis and Biological Evaluation of Novel Carboline-Cinnamic Acid Hybrids as Multifunctional Agents for Treatment of Alzheimer's Disease, Bioorg. Chem, doi:10.1016/j.bioorg.2020.103844
Ligor, Kornyšova, Maruška, Buszewski, Determination of Flavonoids in Tea and Rooibos Extracts by TLC and HPLC, J. Planar Chromatogr.-Mod. TLC, doi:10.1556/JPC.21.2008.5.7
Lines, Joo, Jun, Young, Ju et al., Reducing Cholesterol Levels with Combined Use of Quercetin and Statin. WO2010027572A2, KR100553266B
Lines, Quercetin-Containing Compositions for Use in Treating Amyotrophic Lateral Sclerosis, WO2022243942A
Liossi, Ntountaniotis, Kellici, Chatziathanasiadou, Megariotis et al., Exploring the Interactions of Irbesartan and Irbesartan-2-Hydroxypropyl-β-Cyclodextrin Complex with Model Membranes, Biochim. Biophys. Acta-Biomembr, doi:10.1016/j.bbamem.2017.03.003
Liu, Dong, Chen, Zheng, Sun et al., Inclusion Complexes of Quercetin with Three β-Cyclodextrins Derivatives at Physiological PH: Spectroscopic Study and Antioxidant Activity, Spectrochim. Acta-Part A Mol. Biomol. Spectrosc, doi:10.1016/j.saa.2013.07.008
Lodhi, Jain, Jain, Pawar, Singhai, Effects of Flavonoids from Martynia Annua and Tephrosia Purpurea on Cutaneous Wound Healing, Avicenna J. Phytomed
Lokhande, Ballav, Yadav, Swamy, Basu, Probing Intermolecular Interactions and Binding Stability of Kaempferol, Quercetin Resveratrol Derivatives with PPAR-γ: Docking, Molecular Dynamics and MM/GBSA Approach to Reveal Potent PPAR-γ Agonist against Cancer, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1820380
Lopez Munoz, Espinosa Juarez, Jaramilo Morales, Pharmaceutical Composition of Haloperidol and Quercetin with Analgesic Effect In Neuropathic Pain, MX2017007166A
Lozano, Azarang, Wilaisakditipakorn, Hagerman, Fragile X Syndrome: A Review of Clinical Management, Intractable Rare Dis. Res, doi:10.5582/irdr.2016.01048
Ma, Li, Xie, Liu, Liu, Quercetin Protects Mouse Liver against CCl4-Induced Inflammation by the TLR2/4 and MAPK/NF-KB Pathway, Int. Immunopharmacol, doi:10.1016/j.intimp.2015.06.036
Magnuszewska, Krogulec, Analytica Chimica Acta Application of Hot Platinum Microelectrodes for Determination of Flavonoids in Flow Injection Analysis and Capillary Electrophoresis, Anal. Chim. Acta, doi:10.1016/j.aca.2013.05.031
Malkhasian, Howlin, Docking and DFT Studies on Ligand Binding to Quercetin 2,3-Dioxygenase, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2015.1123190
Mansour, Abdallah, Bedair, Hamed, Analytical Methods for the Determination of Quercetin and Quercetin Glycosides in Pharmaceuticals and Biological Samples, Crit. Rev. Anal. Chem, doi:10.1080/10408347.2023.2269421
Manta, Papakyriakopoulou, Chountoulesi, Diamantis, Spaneas et al., Preparation and Biophysical Characterization of Quercetin Inclusion Complexes with β-Cyclodextrin Derivatives to Be Formulated as Possible Nose-to-Brain Quercetin Delivery Systems, Mol. Pharm, doi:10.1021/acs.molpharmaceut.0c00672
Manta, Papakyriakopoulou, Nikolidaki, Balafas, Kostomitsopoulos et al., Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles, Pharmaceutics, doi:10.3390/pharmaceutics15082036
Maran, Gangadharan, Emerson, Molecular Dynamics Study of Quercetin Families and Its Derivative Compounds from Carica Papaya Leaf as Breast Cancer Inhibitors, Chem. Phys. Lett, doi:10.1016/j.cplett.2022.139470
Marchi, Feige, Horcajada, Compositions and Methods Using a Combination of Oleuropein and Quercetin for Use in Cartilage Degeneration, WO2022106410A
Maroto, Synergic Polyphenol Combination ES2391211B1, B
Mathew, Carradori, Guglielmi, Uddin, Kim, New Aspects of Monoamine Oxidase B Inhibitors: The Key Role of Halogens to Open the Golden Door, Curr. Med. Chem, doi:10.2174/0929867327666200121165931
Mehranfar, Bordbar, Parastar, A Combined Spectroscopic, Molecular Docking and Molecular Dynamic Simulation Study on the Interaction of Quercetin with β-Casein Nanoparticles, J. Photochem. Photobiol. B Biol, doi:10.1016/j.jphotobiol.2013.07.019
Memon, Solangi, Memon, Mallah, Memon et al., Simultaneous Determination of Quercetin, Rutin, Naringin, and Naringenin in Different Fruits by Capillary Zone Electrophoresis, Food Anal. Methods, doi:10.1007/s12161-016-0552-0
Mendoza, Burd, Quercetin as a Systemic Chemopreventative Agent: Structural and Functional Mechanisms, Mini-Rev. Med. Chem, doi:10.2174/13895575111091216
Mi-La, Min-Jung, Seon-Yeong, Sung-Hee, Eun-Ji et al., Composition for Preventing or Treating Immune Disease Comprising Metformin and Quercetin as Active Ingredients, KR20140132932A
Mohammed, Syeda, Wasan, Wasan, An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery, Pharmaceutics, doi:10.3390/pharmaceutics9040053
Molinelli, Weiss, Mizaikoff, Advanced Solid Phase Extraction Using Molecularly Imprinted Polymers for the Determination of Quercetin in Red Wine, J. Agric. Food Chem, doi:10.1021/jf011213q
Moon, Wang, Morris, Dietary Flavonoids: Effects on Xenobiotic and Carcinogen Metabolism, Toxicol. Vitr, doi:10.1016/j.tiv.2005.06.048
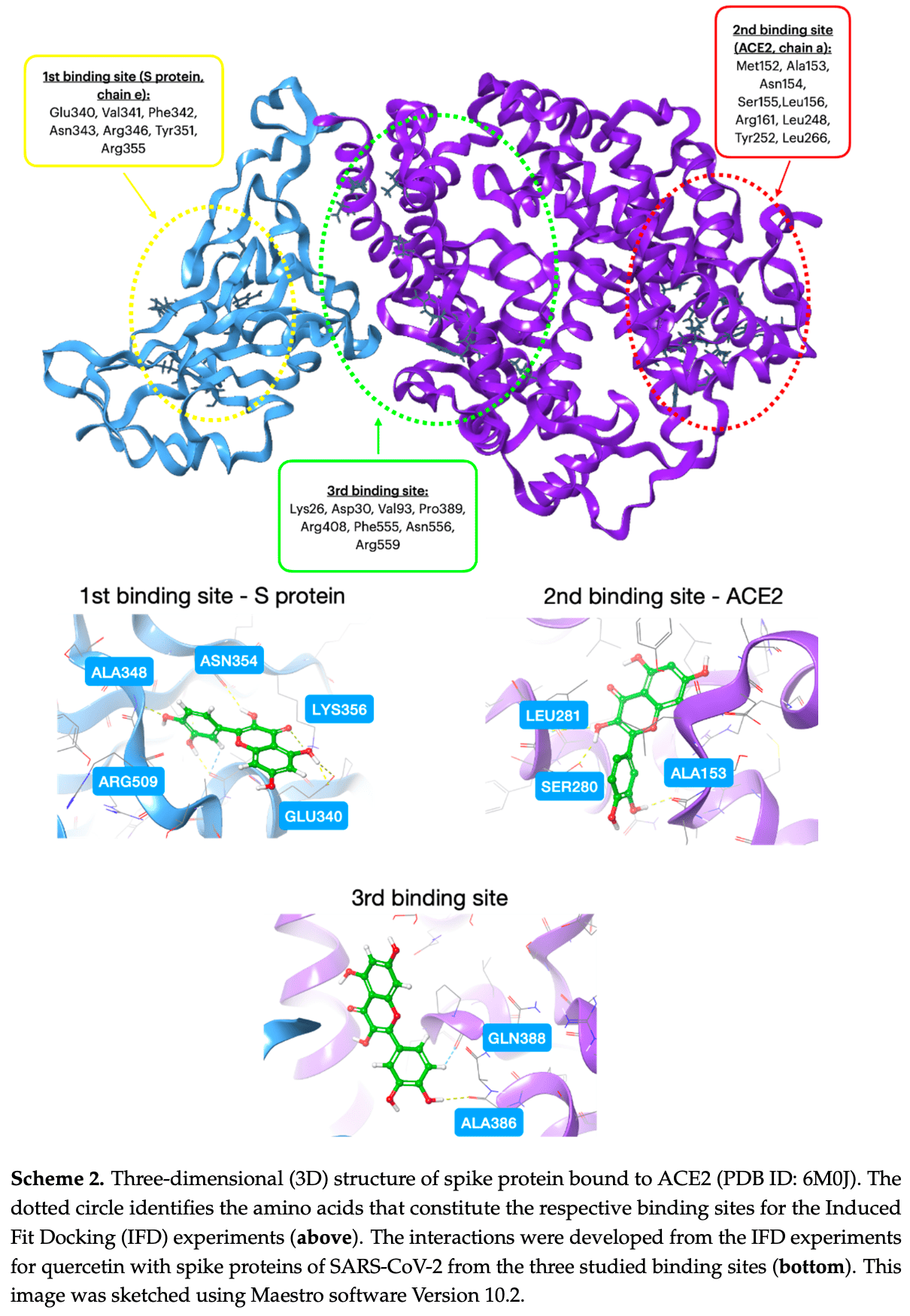
Moschovou, Antoniou, Chontzopoulou, Papavasileiou, Melagraki et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, Int. J. Mol. Sci, doi:10.3390/ijms242115894
Mosleh, Ghoreishi, Masoum, Khoobi, Determination of Quercetin in the Presence of Tannic Acid in Soft Drinks Based on Carbon Nanotubes Modified Electrode Using Chemometric Approaches, Sens. Actuators B Chem, doi:10.1016/j.snb.2018.05.172
Muller, Ernst, Schroder, Wilhelm, Wang, Synergistic Composition Comprising Quercetin and Polyphosphate for Treatment of Bone Disorders, WO
Nakamura, Fukuma, Notsu, Kono, Quercetin and HSC70 Coregulate the Anti-Inflammatory Action of the Ubiquitin-like Protein MNSFβ, Mol. Biol. Rep, doi:10.1007/s11033-021-06949-y
Nazir, Karim, Abdel-Halim, Khan, Wadood et al., Phytochemical Analysis, Molecular Docking and Antiamnesic Effects of Methanolic Extract of Silybum marianum (L.) Gaertn Seeds in Scopolamine Induced Memory Impairment in Mice, J. Ethnopharmacol, doi:10.1016/j.jep.2017.08.026
Nday, Halevas, Jackson, Salifoglou, Quercetin Encapsulation in Modified Silica Nanoparticles: Potential Use against Cu(II)-Induced Oxidative Stress in Neurodegeneration, J. Inorg. Biochem, doi:10.1016/j.jinorgbio.2015.01.001
Niazvand, Orazizadeh, Khorsandi, Abbaspour, Mansouri et al., Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells, Medicina, doi:10.3390/medicina55040114
Nickel, Hanssen, Sisic, Pfeiler, Summo et al., Immunoregulatory Effects of the Flavonol Quercetin in vitro and in vivo, Eur. J. Nutr, doi:10.1007/s00394-010-0125-8
Numata, Tanaka, Quantitative Analysis of Quercetin Using Raman Spectroscopy, Food Chem, doi:10.1016/j.foodchem.2010.11.059
Nunes, Vieira, Queiroz, Leal, Maia Morais et al., Use of Flavonoids and Cinnamates, the Main Photoprotectors with Natural Origin, Adv. Pharmacol. Sci, doi:10.1155/2018/5341487
Olszewska, Separation of Quercetin, Sexangularetin, Kaempferol and Isorhamnetin for Simultaneous HPLC Determination of Flavonoid Aglycones in Inflorescences, Leaves and Fruits of Three Sorbus Species, J. Pharm. Biomed. Anal, doi:10.1016/j.jpba.2008.06.004
Omirin, Omotuyi, Afokhume, Okoh, Boboye et al., Molecular Dynamics Simulations on Quercetin-3-(6-Malonylglucoside) From Morus Alba Shows Optimal Inhibition of Bcl-2 with Favorable Anti-Tumor Activities, bioRxiv, doi:10.1111/tpj.12882
Otsuka, Egawa, Kanzaki, Izumo, Rogi et al., Quercetin Glycosides Prevent Dexamethasone-Induced Muscle Atrophy in Mice, Biochem. Biophys. Rep, doi:10.1016/j.bbrep.2019.100618
Oz, Selcuk, Arik, Gungor, Targeted Agents in Ovarian Carcinoma, Med. Sci, doi:10.5455/medscience.2015.04.8351
Paczkowska, Lewandowska, Bednarski, Mizera, Podborska et al., Application of Spectroscopic Methods for Identification (FT-IR, Raman Spectroscopy) and Determination (UV, EPR) of Quercetin-3-O-Rutinoside. Experimental and DFT Based Approach, Spectrochim. Acta Part A Mol. Biomol. Spectrosc, doi:10.1016/j.saa.2014.12.050
Pallag, Bungau, Tit, Jurca, Sirbu et al., Comparative Study of Polyphenols, Flavonoids and Chlorophylls in Equisetum arvense L. Populations, Rev. Chim
Palli, Leonis, Zoupanou, Georgiou, Chountoulesi et al., Losartan Interactions with 2-Hydroxypropyl-β-CD, Molecules, doi:10.3390/molecules27082421
Panche, Diwan, Chandra, Flavonoids: An Overview, J. Nutr. Sci, doi:10.1017/jns.2016.41
Pastorino, Marchetti, Borghesi, Cornara, Ribulla et al., Biological Activities of the Legume Crops Melilotus Officinalis and Lespedeza Capitata for Skin Care and Pharmaceutical Applications, Ind. Crops Prod, doi:10.1016/j.indcrop.2016.11.047
Patel, Amin, Patwari, Shah, Validated High Performance Thin Layer Chromatography Method for Simultaneous Determination of Quercetin and Gallic Acid in Leea Indica, Rev. Bras. Farmacogn, doi:10.1016/j.bjp.2016.05.017
Pejic, Kuntic, Vujic, Micic, Direct Spectrophotometric Determination of Quercetin in the Presence of Ascorbic Acid, Il Farm, doi:10.1016/j.farmac.2003.07.013
Pham, Stempel, Shields, Spaulding, Kumar et al., Quercetin Enhances the Anti-Tumor Effects of BET Inhibitors by Suppressing HnRNPA1, Int. J. Mol. Sci, doi:10.3390/ijms20174293
Pham-Hoang, Winckler, Waché, Fluorescence Lifetime and UV-Vis Spectroscopy to Evaluate the Interactions between Quercetin and Its Yeast Microcapsule, Biotechnol. J, doi:10.1002/biot.201700389
Pires, Blundell, Ascher, Pkcsm, Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures, J. Med. Chem, doi:10.1021/acs.jmedchem.5b00104
Polera, Badolato, Perri, Carullo, Aiello, Quercetin and Its Natural Sources in Wound Healing Management, Curr. Med. Chem, doi:10.2174/0929867325666180713150626
Poór, Boda, Kunsági-Máté, Needs, Kroon et al., Fluorescence Spectroscopic Evaluation of the Interactions of Quercetin, Isorhamnetin, and Quercetin-3 ′ -Sulfate with Different Albumins, J. Lumin, doi:10.1016/j.jlumin.2017.10.024
Pralhad, Rajendrakumar, Study of Freeze-Dried Quercetin-Cyclodextrin Binary Systems by DSC, FT-IR, X-ray Diffraction and SEM Analysis, J. Pharm. Biomed. Anal, doi:10.1016/S0731-7085(03)00529-6
Prasongsidh, Skurray, Capillary Electrophoresis Analysis of Trans-and Cis-Resveratrol, Quercetin, Catechin and Gallic Acid in Wine, Food Chem, doi:10.1016/S0308-8146(97)00153-2
Pugazhendhi, Edison, Karuppusamy, Kathirvel, Inorganic Nanoparticles: A Potential Cancer Therapy for Human Welfare, Int. J. Pharm, doi:10.1016/j.ijpharm.2018.01.034
Rahimi, Bahar, Heydari, Amininasab, Determination of Quercetin Using a Molecularly Imprinted Polymer as Solid-Phase Microextraction Sorbent and High-Performance Liquid Chromatography, Microchem. J, doi:10.1016/j.microc.2019.05.032
Randhawa, Kumar, Jamwal, Kumar, Screening of Antidepressant Activity and Estimation of Quercetin from Coccinia Indica Using TLC Densitometry, Pharm. Biol, doi:10.3109/13880209.2015.1025289
Reddaiah, Reddy, Swamy, Electrochemical Determination of Quercetin at β-Cyclodextrin Modified Chemical Sensor: A Voltammetric Study, Anal. Bioanal. Electrochem
Reed, Stability of Drugs, Drug Candidates, and Metabolites in Blood and Plasma, Curr. Protoc. Pharmacol, doi:10.1002/cpph.16
Reeves, Doms, Human Immunodeficiency Virus Type 2, J. Gen. Virol, doi:10.1099/0022-1317-83-6-1253
Renjit, Sickle Cell Anemia Treatment
Ruwizhi, Aderibigbe, Cinnamic Acid Derivatives and Their Biological Efficacy, Int. J. Mol. Sci, doi:10.3390/ijms21165712
Sah, Gautam, Pokhrel, Ghani, Bhattarai, Quantification of the Quercetin Nanoemulsion Technique Using Various Parameters, Molecules, doi:10.3390/molecules28062540
Sahoo, Kakran, Shaal, Li, Müller et al., Preparation and Characterization of Quercetin Nanocrystals, J. Pharm. Sci, doi:10.1002/jps.22446
Saldanha, Vilegas, Dokkedal, Characterization of Flavonoids and Phenolic Acids in Myrcia Bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS Combined with NMR, Molecules, doi:10.3390/molecules18078402
Salehi, Machin, Monzote, Sharifi-Rad, Ezzat et al., Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health, ACS Omega, doi:10.1021/acsomega.0c01818
Sang-Chan, Park, Kim, Kim, Park et al., Composition for Preventing or Treating Liver Disease, Comprising Icaritin and Quercetin, WO2022065550A
Sangpheak, Kicuntod, Schuster, Rungrotmongkol, Wolschann et al., Physical Properties and Biological Activities of Hesperetin and Naringenin in Complex with Methylated P-Cyclodextrin, Beilstein J. Org. Chem, doi:10.3762/bjoc.11.297
Sasikumar, Ghosh, Dusthackeer, Antimycobacterial Potentials of Quercetin and Rutin against Mycobacterium Tuberculosis H37, Rv. 3 Biotech, doi:10.1007/s13205-018-1450-5
Savic, Nikolic, Savic, Nikolic, Stankovic, Development and Validation of a New RP-HPLC Method for Determination of Quercetin in Green Tea, J. Anal. Chem, doi:10.1134/S1061934813100080
Savic, Nikolic, Savic-Gajic, Nikolic, Radovanovic et al., Investigation of Properties and Structural Characterization of the Quercetin Inclusion Complex with (2-Hydroxypropyl)-β-Cyclodextrin, J. Incl. Phenom. Macrocycl. Chem, doi:10.1007/s10847-015-0500-4
Savic-Gajic, Savic, Nikolic, Modelling and Optimization of Quercetin Extraction and Biological Activity of Quercetin-Rich Red Onion Skin Extract from Southeastern Serbia, J. Food Nutr. Res
Scheltens, De Strooper, Kivipelto, Holstege, Chételat et al., Alzheimer's Disease, Lancet, doi:10.1016/S0140-6736(20)32205-4
Sengupta, Sengupta, The Interaction of Quercetin with Human Serum Albumin: A Fluorescence Spectroscopic Study, Biochem. Biophys. Res. Commun, doi:10.1016/S0006-291X(02)02667-0
Sharp, Hahn, The Evolution of HIV-1 and the Origin of AIDS, Philos. Trans. R. Soc. B Biol. Sci, doi:10.1098/rstb.2010.0031
Singh, Arif, Bajguz, Hayat, The Role of Quercetin in Plants, Plant Physiol. Biochem, doi:10.1016/j.plaphy.2021.05.023
Singh, Selvaraj, Knowar, Singh, Singh et al., Competitive Inhibition of Quercetin and Apigenin at the ATP Binding Site of D-Alanine-D-Alanine Ligase of Helicobacter Pylori-A Molecular Modeling Approach, Curr. Biotechnol, doi:10.2174/2211550107666180612100441
Smirnova, Egorova, Lantsova, Chechetkin, Toporkova et al., Recombinant Soybean Lipoxygenase 2 (GmLOX2) Acts Primarily as a ω 6 (S)-Lipoxygenase, Curr. Issues Mol. Biol, doi:10.3390/cimb45080396
Song, Li, Wang, Chen, Quercetin Molecularly Imprinted Polymers: Preparation, Recognition Characteristics and Properties as Sorbent for Solid-Phase Extraction, Talanta, doi:10.1016/j.talanta.2009.07.051
Song, Tae, Ki, Yong, Myung et al., Composition Containing Rutin and Quercetin for Preventing or Treating Elevated Blood Lipid Level-Related Diseases, WO
Sotgiu, Centis, D'ambrosio, Tadolini, Castiglia et al., Do We Need a New Fleming Époque: The Nightmare of Drug-Resistant Tuberculosis, Int. J. Mycobacteriol, doi:10.1016/j.ijmyco.2013.07.001
Stefova, Kulevanova, Stafilov, Assay of Flavonols and Quantification of Quercetin in Medicinal Plants by Hplc with Uv-Diode Array Detection, J. Liq. Chromatogr. Relat. Technol, doi:10.1081/JLC-100105140
Stojković, Zdravkovski, Supercritical Fluid Extraction of Quercetin and Rutin from Hyperici Herba Supercritical Fluid Extraction of Quercetin and Rutin from Hyperici Herba, J. Liq. Chromatogr. Relat. Technol, doi:10.1081/JLC-120023798
Sturza, Pavel, Ancus, Danciu, Dehelean et al., Quercetin Exerts an Inhibitory Effect on Cellular Bioenergetics of the B164A5 Murine Melanoma Cell Line, Mol. Cell. Biochem, doi:10.1007/s11010-018-3296-x
Sul, Ra, Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-KB in Lung Epithelial Cells, Molecules, doi:10.3390/molecules26226949
Suntornsuk, Kasemsook, Wongyai, Quantitative Analysis of Aglycone Quercetin in Mulberry Leaves (Morus alba L.) by Capillary Zone Electrophoresis, Electrophoresis, doi:10.1002/elps.200390159
Tamayo-Ramos, Martel, Barros, Bol, Atilhan et al., On the Behavior of Quercetin + Organic Solvent Solutions and Their Role for C60 Fullerene Solubilization, J. Mol. Liq, doi:10.1016/j.molliq.2021.117714
Tanaka, Inflammation and Regeneration Rheumatoid Arthritis, BioMed Cent
Tang, Diao, Shu, Li, Xiong, Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model, BioMed Res. Int, doi:10.1155/2019/7039802
Tasdemir, Kaiser, Brun, Yardley, Schmidt et al., Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies, Antimicrob. Agents Chemother, doi:10.1128/AAC.50.4.1352-1364.2006
Tebbi, Sickle Cell Disease: A Review, Hemato, doi:10.3390/hemato3020024
Tiboc-Schnell, Filip, Man, Decea, Moldovan et al., Quercetin Attenuates Naso-Sinusal Inflammation and Inflammatory Response in Lungs and Brain on an Experimental Model of Acute Rhinosinusitis in Rats, J. Physiol. Pharmacol, doi:10.26402/jpp.2020.4.03
Tiwari, Tiwari, Rai, Cyclodextrins in Delivery Systems: Applications, J. Pharm. Bioallied Sci, doi:10.4103/0975-7406.67003
Trimboli, Gatti, Naccari, Combination of Catechin and Quercetin for Pharmaceutical or Dietary Use, WO
Tsiailanis, Renziehausen, Kiriakidi, Vrettos, Markopoulos et al., Enhancement of Glioblastoma Multiforme Therapy through a Novel Quercetin-Losartan Hybrid, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2020.08.007
Ulusoy, Sanlier, A Minireview of Quercetin: From Its Metabolism to Possible Mechanisms of Its Biological Activities, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2019.1683810
Vacek, Papoušková, Vrba, Zatloukalová, Křen et al., LC-MS Metabolic Study on Quercetin and Taxifolin Galloyl Esters Using Human Hepatocytes as Toxicity and Biotransformation in vitro Cell Model, J. Pharm. Biomed. Anal, doi:10.1016/j.jpba.2013.07.045
Vakali, Papadourakis, Georgiou, Zoupanou, Diamantis et al., Comparative Interaction Studies of Quercetin with 2-Hydroxyl-Propyl-β-Cyclodextrin and 2,6-Methylated-β-Cyclodextrin, Molecules, doi:10.3390/molecules27175490
Valencia-Lazcano, Hassan, Pourmadadi, Shamsabadipour, Behzadmehr et al., 5-Fluorouracil Nano-Delivery Systems as a Cutting-Edge for Cancer Therapy, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2022.114995
Vasquez Garzon, Carrasco Torres, Andrade Jorge, Trujillo Ferrara, Trevino et al., Quercetin and Maleic Anhydride Derivatives for The Treatment of Hepatocellular Carcinoma, MX2018008239A
Veber, Johnson, Cheng, Smith, Ward et al., Molecular Properties That Influence the Oral Bioavailability of Drug Candidates, J. Med. Chem, doi:10.1021/jm020017n
Verma, Sharma, Sharma, Kaur Lamba, Lamba et al., Characterization and Solubility Study of Solid Dispersion of Quercetin by Solvent Evaporation Method, Mater. Today Proc, doi:10.1016/j.matpr.2017.06.334
Verma, Trehan, HPLC Analysis of Methanolic Extract of Herbs for Quercetin Content, J. Pharmacogn. Phytochem
Viana Nunes, Das, Pereira De Andrade, Filgueiras, De Carvalho Maia et al., PreADMET Analysis and Clinical Aspects of Dogs Treated with the Organotellurium Compound RF07: A Possible Control for Canine Visceral Leishmaniasis?, Environ. Toxicol. Pharmacol, doi:10.1016/j.etap.2020.103470
Wang, Sporns, MALDI-TOF MS Analysis of Food Flavonol Glycosides, J. Agric. Food Chem, doi:10.1021/jf991035p
Wang, Wang, Han, Ultrasensitive Determination of Epicatechin, Rutin, and Quercetin by Capillary Electrophoresis Chemiluminescence, Acta Chromatogr, doi:10.1556/AChrom.24.2012.4.13
Wang, Wang, Yao, Gu, Zhao et al., Pharmacological Activity of Quercetin: An Updated Review, Evid.-Based Complement. Altern. Med, doi:10.1155/2022/3997190
Ward, Mir, Kapur, Gales, Carriere et al., Quercetin Inhibits Prostate Cancer by Attenuating Cell Survival and Inhibiting Anti-Apoptotic Pathways, World J. Surg. Oncol, doi:10.1186/s12957-018-1400-z
Wei, Zhang, Tang, Ji, Yan et al., Protective Effects of Quercetin against Inflammation and Oxidative Stress in a Rabbit Model of Knee Osteoarthritis, Drug Dev. Res, doi:10.1002/ddr.21510
Wu, Li, Liu, Li, Feng et al., Quercetin Shows Anti-tumor Effect in Hepatocellular Carcinoma LM3 Cells by Abrogating JAK2/STAT3 Signaling Pathway, Cancer Med, doi:10.1002/cam4.2388
Wybranowski, Kruszewski, Optical Spectroscopy Study of the Interaction between Quercetin and Human Serum Albumin, Acta Phys. Pol. A, doi:10.12693/APhysPolA.125.A-57
Wüpper, Lüersen, Rimbach, Cyclodextrins, Natural Compounds, and Plant Bioactives-A Nutritional Perspective, Biomolecules, doi:10.3390/biom11030401
Xiao, Wang, Peng, Huang, Yang et al., Molecular Docking, Kinetics Study, and Structure-Activity Relationship Analysis of Quercetin and Its Analogous as Helicobacter Pylori Urease Inhibitors, J. Agric. Food Chem, doi:10.1021/jf303393n
Xu, Hu, Wang, Cui, Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application, Molecules, doi:10.3390/molecules24061123
Yang, Wang, Long, Li, Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine, Oxid. Med. Cell. Longev, doi:10.1155/2020/8825387
Yang, Wu, Du, Li, Chen et al., Spectroscopy Study on the Interaction of Quercetin with Collagen, J. Agric. Food Chem, doi:10.1021/jf803671s
Yilmaz, Karanastasis, Chatziathanasiadou, Oguz, Kougioumtzi et al., Inclusion of Quercetin in Gold Nanoparticles Decorated with Supramolecular Hosts Amplifies Its Tumor Targeting Properties, ACS Appl. Bio Mater, doi:10.1021/acsabm.8b00748
Yoon, Jung, Lee, Cho, Jang et al., Anxiolytic-like Effects of Sinapic Acid in Mice, Life Sci, doi:10.1016/j.lfs.2007.05.007
York, May, The Crystal and Molecular Structure of Quercetin: A Biologically Active and Naturally Occurring Flavonoid, Bioorg. Chem
Yuan, Zhu, Lu, Jiang, Zhu et al., Quercetin Alleviates Rheumatoid Arthritis by Inhibiting Neutrophil Inflammatory Activities, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2020.108454
Zahedipour, Kesharwani, Sahebkar, Mechanisms of Multidrug Resistance in Cancer, doi:10.1016/B978-0-323-85881-6.00002-6
Zhang, Chen, Ouyang, Lu, Quercetin in Animal Models of Alzheimer's Disease: A Systematic Review of Preclinical Studies, Int. J. Mol. Sci, doi:10.3390/ijms21020493
Zhang, Li, Antipruritic Composition Containing Astragalin and Quercetin, KR20120121684A
Zhang, Li, Wang, Li, Assessing the Anti-inflammatory Effects of Quercetin Using Network Pharmacology and in Vitro Experiments, Exp. Ther. Med, doi:10.3892/etm.2022.11230
Zhang, Yang, Li, Gao, Preparation, Physicochemical Characterization and in Vitro Digestibility on Solid Complex of Maize Starches with Quercetin, LWT-Food Sci. Technol, doi:10.1016/j.lwt.2010.09.001
Zhao, Funk, Lipoxygenase Pathways in Atherogenesis, Trends Cardiovasc. Med, doi:10.1016/j.tcm.2004.04.003
Zhao, Yang, Li, Luan, Luo, Quercetin Derivatives and Their Medical Usages, US
Zielinska, Wiczkowski, Piskula, Determination of the Relative Contribution of Quercetin and Its Glucosides to the Antioxidant Capacity of Onion by Cyclic Voltammetry and Spectrophotometric Methods, J. Agric. Food Chem, doi:10.1021/jf073521f
Zwicker, Furie, Flaumenhaft, Method for Treating Sickle Cell Disease Using Quercetin-Containing Compositions, WO
DOI record:
{
"DOI": "10.3390/molecules28248141",
"ISSN": [
"1420-3049"
],
"URL": "http://dx.doi.org/10.3390/molecules28248141",
"abstract": "<jats:p>The study of natural products as potential drug leads has gained tremendous research interest. Quercetin is one of those natural products. It belongs to the family of flavonoids and, more specifically, flavonols. This review summarizes the beneficial pharmaceutical effects of quercetin, such as its anti-cancer, anti-inflammatory, and antimicrobial properties, which are some of the quercetin effects described in this review. Nevertheless, quercetin shows poor bioavailability and low solubility. For this reason, its encapsulation in macromolecules increases its bioavailability and therefore pharmaceutical efficiency. In this review, a brief description of the different forms of encapsulation of quercetin are described, and new ones are proposed. The beneficial effects of applying new pharmaceutical forms of nanotechnology are outlined.</jats:p>",
"alternative-id": [
"molecules28248141"
],
"author": [
{
"affiliation": [
{
"name": "Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
}
],
"family": "Georgiou",
"given": "Nikitas",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Laboratory of Organic Chemistry and Biochemistry, Department of Chemistry, University of Patras, 26504 Patras, Greece"
}
],
"family": "Kakava",
"given": "Margarita Georgia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
},
{
"name": "Center of Excellence for Drug Design and Discovery, National and Kapodistrian University of Athens, 15771 Athens, Greece"
}
],
"family": "Routsi",
"given": "Efthymios Alexandros",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
}
],
"family": "Petsas",
"given": "Errikos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
}
],
"family": "Stavridis",
"given": "Nikolaos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Analytical Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
}
],
"family": "Freris",
"given": "Christoforos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
}
],
"family": "Zoupanou",
"given": "Nikoletta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
}
],
"family": "Moschovou",
"given": "Kalliopi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2509-3277",
"affiliation": [
{
"name": "Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
},
{
"name": "Departamento de Quimica Orgánica, Facultade de Quimica, Universidade de Vigo, 36310 Vigo, Spain"
}
],
"authenticated-orcid": false,
"family": "Kiriakidi",
"given": "Sofia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5309-992X",
"affiliation": [
{
"name": "Laboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15771 Athens, Greece"
}
],
"authenticated-orcid": false,
"family": "Mavromoustakos",
"given": "Thomas",
"sequence": "additional"
}
],
"container-title": "Molecules",
"container-title-short": "Molecules",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
12,
18
]
],
"date-time": "2023-12-18T16:28:07Z",
"timestamp": 1702916887000
},
"deposited": {
"date-parts": [
[
2023,
12,
19
]
],
"date-time": "2023-12-19T11:05:07Z",
"timestamp": 1702983907000
},
"indexed": {
"date-parts": [
[
2023,
12,
20
]
],
"date-time": "2023-12-20T00:36:08Z",
"timestamp": 1703032568536
},
"is-referenced-by-count": 0,
"issue": "24",
"issued": {
"date-parts": [
[
2023,
12,
17
]
]
},
"journal-issue": {
"issue": "24",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
17
]
],
"date-time": "2023-12-17T00:00:00Z",
"timestamp": 1702771200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1420-3049/28/24/8141/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "8141",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
12,
17
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
17
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"article-title": "Comparative Study of Polyphenols, Flavonoids and Chlorophylls in Equisetum arvense L. Populations",
"author": "Pallag",
"first-page": "530",
"journal-title": "Rev. Chim.",
"key": "ref_1",
"volume": "67",
"year": "2016"
},
{
"DOI": "10.1017/jns.2016.41",
"article-title": "Flavonoids: An Overview",
"author": "Panche",
"doi-asserted-by": "crossref",
"first-page": "e47",
"journal-title": "J. Nutr. Sci.",
"key": "ref_2",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.3390/nu8030167",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Li, Y., Yao, J., Han, C., Yang, J., Chaudhry, M.T., Wang, S., Liu, H., and Yin, Y. (2016). Quercetin, Inflammation and Immunity. Nutrients, 8."
},
{
"DOI": "10.1016/0278-6915(95)00077-1",
"article-title": "Review of the Biology of Quercetin and Related Bioflavonoids",
"author": "Formica",
"doi-asserted-by": "crossref",
"first-page": "1061",
"journal-title": "Food Chem. Toxicol.",
"key": "ref_4",
"volume": "33",
"year": "1995"
},
{
"DOI": "10.1155/2020/8825387",
"article-title": "Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "8825387",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "ref_5",
"volume": "2020",
"year": "2020"
},
{
"article-title": "Quercetin and Its Role in Biological Functions: An Updated Review",
"author": "Kim",
"first-page": "856",
"journal-title": "EXCLI J.",
"key": "ref_6",
"volume": "17",
"year": "2018"
},
{
"DOI": "10.1016/j.plaphy.2021.05.023",
"article-title": "The Role of Quercetin in Plants",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "10",
"journal-title": "Plant Physiol. Biochem.",
"key": "ref_7",
"volume": "166",
"year": "2021"
},
{
"DOI": "10.1515/revneuro-2018-0080",
"article-title": "Application of Quercetin in Neurological Disorders: From Nutrition to Nanomedicine",
"author": "Amanzadeh",
"doi-asserted-by": "crossref",
"first-page": "555",
"journal-title": "Rev. Neurosci.",
"key": "ref_8",
"volume": "30",
"year": "2019"
},
{
"article-title": "Quercetin as a Systemic Chemopreventative Agent: Structural and Functional Mechanisms",
"author": "Burd",
"first-page": "1216",
"journal-title": "Mini-Rev. Med. Chem.",
"key": "ref_9",
"volume": "11",
"year": "2012"
},
{
"DOI": "10.1038/srep42717",
"article-title": "SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules",
"author": "Daina",
"doi-asserted-by": "crossref",
"first-page": "42717",
"journal-title": "Sci. Rep.",
"key": "ref_10",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1021/acs.jmedchem.5b00104",
"article-title": "PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures",
"author": "Pires",
"doi-asserted-by": "crossref",
"first-page": "4066",
"journal-title": "J. Med. Chem.",
"key": "ref_11",
"volume": "58",
"year": "2015"
},
{
"DOI": "10.1016/j.etap.2020.103470",
"article-title": "PreADMET Analysis and Clinical Aspects of Dogs Treated with the Organotellurium Compound RF07: A Possible Control for Canine Visceral Leishmaniasis?",
"author": "Filgueiras",
"doi-asserted-by": "crossref",
"first-page": "103470",
"journal-title": "Environ. Toxicol. Pharmacol.",
"key": "ref_12",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1039/C8MD00472B",
"article-title": "ADMET-Score—A Comprehensive Scoring Function for Evaluation of Chemical Drug-Likeness",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "148",
"journal-title": "Medchemcomm",
"key": "ref_13",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1021/acsomega.2c07625",
"article-title": "Thiocarbohydrazone and Chalcone-Derived 3,4-Dihydropyrimidinethione as Lipid Peroxidation and Soybean Lipoxygenase Inhibitors",
"author": "Georgiou",
"doi-asserted-by": "crossref",
"first-page": "11966",
"journal-title": "ACS Omega",
"key": "ref_14",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1080/07391102.2021.2017356",
"article-title": "Structure Assignment, Conformational Properties and Discovery of Potential Targets of the Ugi Cinnamic Adduct NGI25",
"author": "Georgiou",
"doi-asserted-by": "crossref",
"first-page": "1253",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_15",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.3390/molecules27144548",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Georgiou, N., Cheilari, A., Karta, D., Chontzopoulou, E., Plavec, J., Tzeli, D., Vassiliou, S., and Mavromoustakos, T. (2022). Conformational Properties and Putative Bioactive Targets for Novel Thiosemicarbazone Derivatives. Molecules, 27."
},
{
"DOI": "10.3390/molecules27082537",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Georgiou, N., Katsogiannou, A., Skourtis, D., Iatrou, H., Tzeli, D., Vassiliou, S., Javornik, U., Plavec, J., and Mavromoustakos, T. (2022). Conformational Properties of New Thiosemicarbazone and Thiocarbohydrazone Derivatives and Their Possible Targets. Molecules, 27."
},
{
"DOI": "10.4155/fmc-2018-0435",
"article-title": "Lipophilicity in Drug Design: An Overview of Lipophilicity Descriptors in 3D-QSAR Studies",
"author": "Ginex",
"doi-asserted-by": "crossref",
"first-page": "1177",
"journal-title": "Future Med. Chem.",
"key": "ref_18",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1021/jm020017n",
"article-title": "Molecular Properties That Influence the Oral Bioavailability of Drug Candidates",
"author": "Veber",
"doi-asserted-by": "crossref",
"first-page": "2615",
"journal-title": "J. Med. Chem.",
"key": "ref_19",
"volume": "45",
"year": "2002"
},
{
"key": "ref_20",
"unstructured": "Li, G., Wang, Y., Fei, T., Wu, D., and Tao, L. Application of Quercetin or Quercetin Derivative for Relieving Smoke Harm."
},
{
"DOI": "10.1002/cmdc.201600182",
"article-title": "A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules",
"author": "Daina",
"doi-asserted-by": "crossref",
"first-page": "1117",
"journal-title": "ChemMedChem",
"key": "ref_21",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.2174/0929867327666200121165931",
"article-title": "New Aspects of Monoamine Oxidase B Inhibitors: The Key Role of Halogens to Open the Golden Door",
"author": "Mathew",
"doi-asserted-by": "crossref",
"first-page": "266",
"journal-title": "Curr. Med. Chem.",
"key": "ref_22",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1016/j.tcm.2004.04.003",
"article-title": "Lipoxygenase Pathways in Atherogenesis",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "191",
"journal-title": "Trends Cardiovasc. Med.",
"key": "ref_23",
"volume": "14",
"year": "2004"
},
{
"DOI": "10.1002/ptr.7144",
"article-title": "Quercetin and Metabolic Syndrome: A Review",
"author": "Hosseini",
"doi-asserted-by": "crossref",
"first-page": "5352",
"journal-title": "Phyther. Res.",
"key": "ref_24",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2007.05.007",
"article-title": "Anxiolytic-like Effects of Sinapic Acid in Mice",
"author": "Yoon",
"doi-asserted-by": "crossref",
"first-page": "234",
"journal-title": "Life Sci.",
"key": "ref_25",
"volume": "81",
"year": "2007"
},
{
"article-title": "Use of Flavonoids and Cinnamates, the Main Photoprotectors with Natural Origin",
"author": "Nunes",
"first-page": "5341487",
"journal-title": "Adv. Pharmacol. Sci.",
"key": "ref_26",
"volume": "2018",
"year": "2018"
},
{
"DOI": "10.1088/2053-1591/ab6c8e",
"article-title": "ZnO and Quercetin Encapsulated Nanoparticles for Sun Protection Obtained by Miniemulsion Polymerization Using Alternative Co-Stabilizers",
"author": "Feuser",
"doi-asserted-by": "crossref",
"first-page": "015096",
"journal-title": "Mater. Res. Express",
"key": "ref_27",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1099/0022-1317-83-6-1253",
"article-title": "Human Immunodeficiency Virus Type 2",
"author": "Reeves",
"doi-asserted-by": "crossref",
"first-page": "1253",
"journal-title": "J. Gen. Virol.",
"key": "ref_28",
"volume": "83",
"year": "2002"
},
{
"DOI": "10.1098/rstb.2010.0031",
"article-title": "The Evolution of HIV-1 and the Origin of AIDS",
"author": "Sharp",
"doi-asserted-by": "crossref",
"first-page": "2487",
"journal-title": "Philos. Trans. R. Soc. B Biol. Sci.",
"key": "ref_29",
"volume": "365",
"year": "2010"
},
{
"DOI": "10.1002/ptr.7309",
"article-title": "Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review",
"author": "Fais",
"doi-asserted-by": "crossref",
"first-page": "266",
"journal-title": "Phyther. Res.",
"key": "ref_30",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1155/2022/3997190",
"article-title": "Pharmacological Activity of Quercetin: An Updated Review",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "3997190",
"journal-title": "Evid.-Based Complement. Altern. Med.",
"key": "ref_31",
"volume": "2022",
"year": "2022"
},
{
"article-title": "Quercetin: Antiviral Significance and Possible COVID-19 Integrative Considerations",
"author": "Agrawal",
"first-page": "1934578X20976293",
"journal-title": "Nat. Prod. Commun.",
"key": "ref_32",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.3390/ijms242115894",
"doi-asserted-by": "crossref",
"key": "ref_33",
"unstructured": "Moschovou, K., Antoniou, M., Chontzopoulou, E., Papavasileiou, K.D., Melagraki, G., Afantitis, A., and Mavromoustakos, T. (2023). Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.5455/medscience.2015.04.8351",
"article-title": "Targeted Agents in Ovarian Carcinoma",
"author": "Oz",
"doi-asserted-by": "crossref",
"first-page": "547",
"journal-title": "Med. Sci.",
"key": "ref_34",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.1016/j.biopha.2018.08.061",
"article-title": "Midkine Downregulation Increases the Efficacy of Quercetin on Prostate Cancer Stem Cell Survival and Migration through PI3K/AKT and MAPK/ERK Pathway",
"author": "Erdogan",
"doi-asserted-by": "crossref",
"first-page": "793",
"journal-title": "Biomed. Pharmacother.",
"key": "ref_35",
"volume": "107",
"year": "2018"
},
{
"DOI": "10.1186/s12957-018-1400-z",
"article-title": "Quercetin Inhibits Prostate Cancer by Attenuating Cell Survival and Inhibiting Anti-Apoptotic Pathways",
"author": "Ward",
"doi-asserted-by": "crossref",
"first-page": "108",
"journal-title": "World J. Surg. Oncol.",
"key": "ref_36",
"volume": "16",
"year": "2018"
},
{
"DOI": "10.21873/anticanres.14469",
"article-title": "Quercetin Suppresses Proliferation of Liver Cancer Cell Lines In Vitro",
"author": "Hisaka",
"doi-asserted-by": "crossref",
"first-page": "4695",
"journal-title": "Anticancer Res.",
"key": "ref_37",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.3390/medicina55040114",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Niazvand, F., Orazizadeh, M., Khorsandi, L., Abbaspour, M., Mansouri, E., and Khodadadi, A. (2019). Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells. Medicina (B. Aires), 55."
},
{
"DOI": "10.3390/ijms20174293",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Pham, T.N.D., Stempel, S., Shields, M.A., Spaulding, C., Kumar, K., Bentrem, D.J., Matsangou, M., and Munshi, H.G. (2019). Quercetin Enhances the Anti-Tumor Effects of BET Inhibitors by Suppressing HnRNPA1. Int. J. Mol. Sci., 20."
},
{
"DOI": "10.1007/s11010-018-3296-x",
"article-title": "Quercetin Exerts an Inhibitory Effect on Cellular Bioenergetics of the B164A5 Murine Melanoma Cell Line",
"author": "Sturza",
"doi-asserted-by": "crossref",
"first-page": "103",
"journal-title": "Mol. Cell. Biochem.",
"key": "ref_40",
"volume": "447",
"year": "2018"
},
{
"DOI": "10.1002/cam4.2388",
"article-title": "Quercetin Shows Anti-tumor Effect in Hepatocellular Carcinoma LM3 Cells by Abrogating JAK2/STAT3 Signaling Pathway",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "4806",
"journal-title": "Cancer Med.",
"key": "ref_41",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1016/j.jddst.2022.103938",
"article-title": "Nanomaterials Loaded with Quercetin as an Advanced Tool for Cancer Treatment",
"author": "Caro",
"doi-asserted-by": "crossref",
"first-page": "103938",
"journal-title": "J. Drug Deliv. Sci. Technol.",
"key": "ref_42",
"volume": "78",
"year": "2022"
},
{
"article-title": "Inflammation and Regeneration Rheumatoid Arthritis",
"author": "Tanaka",
"first-page": "1",
"journal-title": "BioMed Cent.",
"key": "ref_43",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1016/j.jnutbio.2020.108454",
"doi-asserted-by": "crossref",
"key": "ref_44",
"unstructured": "Yuan, K., Zhu, Q., Lu, Q., Jiang, H., Zhu, M., Li, X., Huang, G., and Xu, A. (2020). Quercetin Alleviates Rheumatoid Arthritis by Inhibiting Neutrophil Inflammatory Activities. J. Nutr. Biochem., 84."
},
{
"DOI": "10.1080/07391102.2021.1973565",
"article-title": "Molecular Investigation of Artificial and Natural Sweeteners as Potential Anti-Inflammatory Agents",
"author": "Chontzopoulou",
"doi-asserted-by": "crossref",
"first-page": "12608",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_45",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.3390/cimb45080396",
"article-title": "Recombinant Soybean Lipoxygenase 2 (GmLOX2) Acts Primarily as a ω 6 (S)-Lipoxygenase",
"author": "Smirnova",
"doi-asserted-by": "crossref",
"first-page": "6283",
"journal-title": "Curr. Issues Mol. Biol.",
"key": "ref_46",
"volume": "2",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(20)32205-4",
"article-title": "Alzheimer’s Disease",
"author": "Scheltens",
"doi-asserted-by": "crossref",
"first-page": "1577",
"journal-title": "Lancet",
"key": "ref_47",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.3390/ijms21165712",
"doi-asserted-by": "crossref",
"key": "ref_48",
"unstructured": "Ruwizhi, N., and Aderibigbe, B.A. (2020). Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci., 21."
},
{
"DOI": "10.1016/j.bioorg.2020.103844",
"doi-asserted-by": "crossref",
"key": "ref_49",
"unstructured": "Liao, Q., Li, Q., Zhao, Y., Jiang, P., Yan, Y., Sun, H., Liu, W., Feng, F., and Qu, W. (2020). Design, Synthesis and Biological Evaluation of Novel Carboline-Cinnamic Acid Hybrids as Multifunctional Agents for Treatment of Alzheimer’s Disease. Bioorg. Chem., 99."
},
{
"DOI": "10.1080/14756366.2016.1256883",
"article-title": "Design, Synthesis and Evaluation of Novel Cinnamic Acid Derivatives Bearing N-Benzyl Pyridinium Moiety as Multifunctional Cholinesterase Inhibitors for Alzheimer’s Disease",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "776",
"journal-title": "J. Enzym. Inhib. Med. Chem.",
"key": "ref_50",
"volume": "32",
"year": "2017"
},
{
"DOI": "10.1021/acsomega.0c01818",
"article-title": "Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health",
"author": "Salehi",
"doi-asserted-by": "crossref",
"first-page": "11849",
"journal-title": "ACS Omega",
"key": "ref_51",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.3390/molecules24061123",
"doi-asserted-by": "crossref",
"key": "ref_52",
"unstructured": "Xu, D., Hu, M.-J., Wang, Y.-Q., and Cui, Y.-L. (2019). Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules, 24."
},
{
"DOI": "10.1016/j.freeradbiomed.2020.08.007",
"article-title": "Enhancement of Glioblastoma Multiforme Therapy through a Novel Quercetin-Losartan Hybrid",
"author": "Tsiailanis",
"doi-asserted-by": "crossref",
"first-page": "391",
"journal-title": "Free Radic. Biol. Med.",
"key": "ref_53",
"volume": "160",
"year": "2020"
},
{
"DOI": "10.1080/10408398.2019.1683810",
"article-title": "A Minireview of Quercetin: From Its Metabolism to Possible Mechanisms of Its Biological Activities",
"author": "Ulusoy",
"doi-asserted-by": "crossref",
"first-page": "3290",
"journal-title": "Crit. Rev. Food Sci. Nutr.",
"key": "ref_54",
"volume": "60",
"year": "2020"
},
{
"key": "ref_55",
"unstructured": "Maroto, J.Á.M. (2013). Synergic Polyphenol Combination ES2391211B1. (ES2391211B1)."
},
{
"DOI": "10.2174/138527212799958020",
"article-title": "Cinnamic Acid Derivatives in Tuberculosis, Malaria and Cardiovascular Diseases—A Review",
"author": "De",
"doi-asserted-by": "crossref",
"first-page": "747",
"journal-title": "Curr. Org. Chem.",
"key": "ref_56",
"volume": "16",
"year": "2012"
},
{
"key": "ref_57",
"unstructured": "Ivanov, V., Ivanova, S., Roomi, W., Niedzwicki, A., and Rath, M. (2003). Novel Composition and Method for the Treatment of Hypertension. (US2004242504A1)."
},
{
"key": "ref_58",
"unstructured": "Jalili, T. (2004). Quercetin Supplementation to Treat Hypertenstion. (US2004258674A1)."
},
{
"DOI": "10.3390/ph3010237",
"article-title": "Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms",
"author": "Larson",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "Pharmaceuticals",
"key": "ref_59",
"volume": "3",
"year": "2010"
},
{
"DOI": "10.1615/JEnvironPatholToxicolOncol.2014010949",
"article-title": "Antitumor and Wound Healing Properties of Rubus Niveus Thunb. Root",
"author": "George",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "J. Environ. Pathol. Toxicol. Oncol.",
"key": "ref_60",
"volume": "33",
"year": "2014"
},
{
"DOI": "10.2174/0929867325666180713150626",
"article-title": "Quercetin and Its Natural Sources in Wound Healing Management",
"author": "Polera",
"doi-asserted-by": "crossref",
"first-page": "5825",
"journal-title": "Curr. Med. Chem.",
"key": "ref_61",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.3109/13880209.2015.1028079",
"article-title": "Antibacterial, Antioxidant, and Topical Anti-Inflammatory Activities of Bergia Ammannioides: A Wound-Healing Plant",
"author": "Ezzat",
"doi-asserted-by": "crossref",
"first-page": "215",
"journal-title": "Pharm. Biol.",
"key": "ref_62",
"volume": "54",
"year": "2016"
},
{
"DOI": "10.1016/j.indcrop.2016.11.047",
"article-title": "Biological Activities of the Legume Crops Melilotus Officinalis and Lespedeza Capitata for Skin Care and Pharmaceutical Applications",
"author": "Pastorino",
"doi-asserted-by": "crossref",
"first-page": "158",
"journal-title": "Ind. Crops Prod.",
"key": "ref_63",
"volume": "96",
"year": "2017"
},
{
"article-title": "Effects of Flavonoids from Martynia Annua and Tephrosia Purpurea on Cutaneous Wound Healing",
"author": "Lodhi",
"first-page": "578",
"journal-title": "Avicenna J. Phytomed.",
"key": "ref_64",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1155/2019/7039802",
"doi-asserted-by": "crossref",
"key": "ref_65",
"unstructured": "Tang, J., Diao, P., Shu, X., Li, L., and Xiong, L. (2019). Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed Res. Int., 2019."
},
{
"DOI": "10.1002/ddr.21510",
"article-title": "Protective Effects of Quercetin against Inflammation and Oxidative Stress in a Rabbit Model of Knee Osteoarthritis",
"author": "Wei",
"doi-asserted-by": "crossref",
"first-page": "360",
"journal-title": "Drug Dev. Res.",
"key": "ref_66",
"volume": "80",
"year": "2019"
},
{
"DOI": "10.3390/molecules26226949",
"doi-asserted-by": "crossref",
"key": "ref_67",
"unstructured": "Sul, O.-J., and Ra, S.W. (2021). Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-KB in Lung Epithelial Cells. Molecules, 26."
},
{
"DOI": "10.1007/s11033-021-06949-y",
"article-title": "Quercetin and HSC70 Coregulate the Anti-Inflammatory Action of the Ubiquitin-like Protein MNSFβ",
"author": "Nakamura",
"doi-asserted-by": "crossref",
"first-page": "1213",
"journal-title": "Mol. Biol. Rep.",
"key": "ref_68",
"volume": "49",
"year": "2022"
},
{
"DOI": "10.1097/MD.0000000000022241",
"article-title": "Quercetin Inhibits TNF-α Induced HUVECs Apoptosis and Inflammation via Downregulating NF-KB and AP-1 Signaling Pathway in vitro",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "e22241",
"journal-title": "Medicine",
"key": "ref_69",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2015.06.036",
"article-title": "Quercetin Protects Mouse Liver against CCl4-Induced Inflammation by the TLR2/4 and MAPK/NF-ΚB Pathway",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_70",
"volume": "28",
"year": "2015"
},
{
"DOI": "10.1016/j.bmcl.2010.01.031",
"article-title": "Novel Molecular Hybrids of Cinnamic Acids and Guanylhydrazones as Potential Antitubercular Agents",
"author": "Bairwa",
"doi-asserted-by": "crossref",
"first-page": "1623",
"journal-title": "Bioorg. Med. Chem. Lett.",
"key": "ref_71",
"volume": "20",
"year": "2010"
},
{
"DOI": "10.1016/j.ijmyco.2013.07.001",
"article-title": "Do We Need a New Fleming Époque: The Nightmare of Drug-Resistant Tuberculosis",
"author": "Sotgiu",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "Int. J. Mycobacteriol.",
"key": "ref_72",
"volume": "2",
"year": "2013"
},
{
"DOI": "10.1007/s13205-018-1450-5",
"article-title": "Antimycobacterial Potentials of Quercetin and Rutin against Mycobacterium Tuberculosis H37Rv",
"author": "Sasikumar",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "3 Biotech",
"key": "ref_73",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1016/j.cmet.2007.03.006",
"article-title": "The GLUT4 Glucose Transporter",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "Cell Metab.",
"key": "ref_74",
"volume": "5",
"year": "2007"
},
{
"DOI": "10.1016/j.biopha.2021.112560",
"doi-asserted-by": "crossref",
"key": "ref_75",
"unstructured": "Dhanya, R. (2022). Quercetin for Managing Type 2 Diabetes and Its Complications, an Insight into Multitarget Therapy. Biomed. Pharmacother., 146."
},
{
"key": "ref_76",
"unstructured": "Ahrens, M.J., Thompson, D.L., and Atm Metabolics Lllp (2008). Composition for Treating Diabetes and Metabolic Disorders with Quercetin, Myrcetin and Chlorogenic Acid. (EP2129371B1)."
},
{
"key": "ref_77",
"unstructured": "Kruthiventi, A., and Javed, I. (2009). Pharmaceutical Co-Crystals of Quercetin. (US20120258170A1)."
},
{
"DOI": "10.3390/life12081146",
"doi-asserted-by": "crossref",
"key": "ref_78",
"unstructured": "Ansari, P., Choudhury, S.T., Seidel, V., Rahman, A.B., Aziz, M.A., Richi, A.E., Rahman, A., Jafrin, U.H., Hannan, J.M.A., and Abdel-Wahab, Y.H.A. (2022). Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life, 12."
},
{
"DOI": "10.3390/ph14030248",
"doi-asserted-by": "crossref",
"key": "ref_79",
"unstructured": "Ali, A.H., Sudi, S., Shi-Jing, N., Rozianoor, W., Hassan, M., Basir, R., Agustar, H.K., Embi, N., Sidek, H.M., and Latip, J. (2021). Dual Anti-Malarial and GSK3 β-Mediated Cytokine-Modulating Activities of Quercetin Are Requisite of Its Potential as a Plant-Derived Therapeutic in Malaria. Pharmaceuticals, 14."
},
{
"DOI": "10.1016/j.molstruc.2021.130014",
"article-title": "Synthesis, Characterization and Antichagasic Evaluation of Thiosemicarbazones Prepared from Chalcones and Dibenzalacetones",
"author": "Maia",
"doi-asserted-by": "crossref",
"first-page": "130014",
"journal-title": "J. Mol. Struct.",
"key": "ref_80",
"volume": "1232",
"year": "2021"
},
{
"DOI": "10.1128/AAC.50.4.1352-1364.2006",
"article-title": "Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies",
"author": "Tasdemir",
"doi-asserted-by": "crossref",
"first-page": "1352",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_81",
"volume": "50",
"year": "2006"
},
{
"DOI": "10.1080/08927014.2019.1604948",
"article-title": "Antifungal Effects of the Flavonoids Kaempferol and Quercetin: A Possible Alternative for the Control of Fungal Biofilms",
"author": "Rocha",
"doi-asserted-by": "crossref",
"first-page": "320",
"journal-title": "Biofouling",
"key": "ref_82",
"volume": "35",
"year": "2019"
},
{
"DOI": "10.3390/hemato3020024",
"article-title": "Sickle Cell Disease: A Review",
"author": "Tebbi",
"doi-asserted-by": "crossref",
"first-page": "341",
"journal-title": "Hemato",
"key": "ref_83",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.5582/irdr.2016.01048",
"article-title": "Fragile X Syndrome: A Review of Clinical Management",
"author": "Lozano",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "Intractable Rare Dis. Res.",
"key": "ref_84",
"volume": "5",
"year": "2016"
},
{
"key": "ref_85",
"unstructured": "Chodoeva, R. (2021). Quercetin-Based Composition for Treating Rhinosinusitis. (US2021000787A1)."
},
{
"article-title": "Quercetin Attenuates Naso-Sinusal Inflammation and Inflammatory Response in Lungs and Brain on an Experimental Model of Acute Rhinosinusitis in Rats",
"author": "Filip",
"first-page": "479",
"journal-title": "J. Physiol. Pharmacol.",
"key": "ref_86",
"volume": "71",
"year": "2020"
},
{
"key": "ref_87",
"unstructured": "Zahedipour, F., Kesharwani, P., and Sahebkar, A. (2022). Aptamers Engineered Nanocarriers for Cancer Therapy, Elsevier."
},
{
"key": "ref_88",
"unstructured": "Joshi, N.S., Aggarwal, P., Hiprara, V.K., Jaggi, M., Singh, A., Awasthi, A., and Verma, R. (2008). Novel Quercetin Derivatives as Anti-Cancer Agents. (US2011034413A1)."
},
{
"DOI": "10.1016/j.bbrep.2019.100618",
"doi-asserted-by": "crossref",
"key": "ref_89",
"unstructured": "Otsuka, Y., Egawa, K., Kanzaki, N., Izumo, T., Rogi, T., and Shibata, H. (2019). Quercetin Glycosides Prevent Dexamethasone-Induced Muscle Atrophy in Mice. Biochem. Biophys. Rep., 18."
},
{
"key": "ref_90",
"unstructured": "Karaboga, A.S., Perez-Neuno, V.I., Souchet, M., and Decaudin, D. (2015). Muscle Atrophy Inhibitor Containing Quercetin Glycoside. (CN106255500A)."
},
{
"DOI": "10.1016/j.molstruc.2023.136430",
"article-title": "Computational and Spectroscopic Analysis of the Quercetin Encapsulation in (2HP-β-CD)2 and (2,6Me-β-CD)2 Complexes",
"author": "Leonis",
"doi-asserted-by": "crossref",
"first-page": "136430",
"journal-title": "J. Mol. Struct.",
"key": "ref_91",
"volume": "1294",
"year": "2023"
},
{
"DOI": "10.3390/molecules27175490",
"doi-asserted-by": "crossref",
"key": "ref_92",
"unstructured": "Vakali, V., Papadourakis, M., Georgiou, N., Zoupanou, N., Diamantis, D.A., Javornik, U., Papakyriakopoulou, P., Plavec, J., Valsami, G., and Tzakos, A.G. (2022). Comparative Interaction Studies of Quercetin with 2-Hydroxyl-Propyl-β-Cyclodextrin and 2,6-Methylated-β-Cyclodextrin. Molecules, 27."
},
{
"DOI": "10.3390/pharmaceutics15082036",
"doi-asserted-by": "crossref",
"key": "ref_93",
"unstructured": "Manta, K., Papakyriakopoulou, P., Nikolidaki, A., Balafas, E., Kostomitsopoulos, N., Banella, S., Colombo, G., and Valsami, G. (2023). Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles. Pharmaceutics, 15."
},
{
"DOI": "10.1007/s10847-015-0500-4",
"article-title": "Investigation of Properties and Structural Characterization of the Quercetin Inclusion Complex with (2-Hydroxypropyl)-β-Cyclodextrin",
"author": "Savic",
"doi-asserted-by": "crossref",
"first-page": "383",
"journal-title": "J. Incl. Phenom. Macrocycl. Chem.",
"key": "ref_94",
"volume": "82",
"year": "2015"
},
{
"DOI": "10.1021/acs.molpharmaceut.0c00672",
"article-title": "Preparation and Biophysical Characterization of Quercetin Inclusion Complexes with β-Cyclodextrin Derivatives to Be Formulated as Possible Nose-to-Brain Quercetin Delivery Systems",
"author": "Manta",
"doi-asserted-by": "crossref",
"first-page": "4241",
"journal-title": "Mol. Pharm.",
"key": "ref_95",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.3390/molecules27082421",
"doi-asserted-by": "crossref",
"key": "ref_96",
"unstructured": "Palli, V., Leonis, G., Zoupanou, N., Georgiou, N., Chountoulesi, M., Naziris, N., Tzeli, D., Demetzos, C., Valsami, G., and Marousis, K.D. (2022). Losartan Interactions with 2-Hydroxypropyl-β-CD. Molecules, 27."
},
{
"DOI": "10.3762/bjoc.12.5",
"article-title": "Determination of Formation Constants and Structural Characterization of Cyclodextrin Inclusion Complexes with Two Phenolic Isomers: Carvacrol and Thymol",
"author": "Kfoury",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Beilstein J. Org. Chem.",
"key": "ref_97",
"volume": "12",
"year": "2016"
},
{
"DOI": "10.3762/bjoc.11.281",
"article-title": "Inclusion Complexes of 2-Methoxyestradiol with Dimethylated and Permethylated β-Cyclodextrins: Models for Cyclodextrin-Steroid Interaction",
"author": "Caira",
"doi-asserted-by": "crossref",
"first-page": "2616",
"journal-title": "Beilstein J. Org. Chem.",
"key": "ref_98",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.3390/scipharm87040033",
"doi-asserted-by": "crossref",
"key": "ref_99",
"unstructured": "Haimhoffer, Á., Rusznyák, Á., Réti-Nagy, K., Vasvári, G., Váradi, J., Vecsernyés, M., Bácskay, I., Fehér, P., Ujhelyi, Z., and Fenyvesi, F. (2019). Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm., 87."
},
{
"DOI": "10.4103/0975-7406.67003",
"doi-asserted-by": "crossref",
"key": "ref_100",
"unstructured": "Tiwari, G., Tiwari, R., and Rai, A. (2010). Cyclodextrins in Delivery Systems: Applications. J. Pharm. Bioallied Sci., 2."
},
{
"DOI": "10.3390/biom11030401",
"doi-asserted-by": "crossref",
"key": "ref_101",
"unstructured": "Wüpper, S., Lüersen, K., and Rimbach, G. (2021). Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules, 11."
},
{
"DOI": "10.1529/biophysj.104.049494",
"article-title": "Calculation of Cyclodextrin Binding Affinities: Energy, Entropy, and Implications for Drug Design",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "3035",
"journal-title": "Biophys. J.",
"key": "ref_102",
"volume": "87",
"year": "2004"
},
{
"DOI": "10.1016/j.bbamem.2017.03.003",
"article-title": "Exploring the Interactions of Irbesartan and Irbesartan–2-Hydroxypropyl-β-Cyclodextrin Complex with Model Membranes",
"author": "Liossi",
"doi-asserted-by": "crossref",
"first-page": "1089",
"journal-title": "Biochim. Biophys. Acta-Biomembr.",
"key": "ref_103",
"volume": "1859",
"year": "2017"
},
{
"DOI": "10.1016/j.bbagen.2018.06.006",
"article-title": "Exploring the Oxidation and Iron Binding Profile of a Cyclodextrin Encapsulated Quercetin Complex Unveiled a Controlled Complex Dissociation through a Chemical Stimulus",
"author": "Diamantis",
"doi-asserted-by": "crossref",
"first-page": "1913",
"journal-title": "Biochim. Biophys. Acta-Gen. Subj.",
"key": "ref_104",
"volume": "1862",
"year": "2018"
},
{
"DOI": "10.2174/138527206776818928",
"article-title": "Molecular Modeling and Cyclodextrins: A Relationship Strengthened By Complexes",
"author": "Castro",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Curr. Org. Chem.",
"key": "ref_105",
"volume": "10",
"year": "2006"
},
{
"DOI": "10.1016/j.ijpharm.2016.07.008",
"article-title": "Mapping the Interactions and Bioactivity of Quercetin(2-Hydroxypropyl)-β-Cyclodextrin Complex",
"author": "Kellici",
"doi-asserted-by": "crossref",
"first-page": "303",
"journal-title": "Int. J. Pharm.",
"key": "ref_106",
"volume": "511",
"year": "2016"
},
{
"DOI": "10.1039/C7OB02045G",
"article-title": "Rational Design and Structure-Activity Relationship Studies of Quercetin-Amino Acid Hybrids Targeting the Anti-Apoptotic Protein Bcl-XL",
"author": "Kellici",
"doi-asserted-by": "crossref",
"first-page": "7956",
"journal-title": "Org. Biomol. Chem.",
"key": "ref_107",
"volume": "15",
"year": "2017"
},
{
"DOI": "10.1021/acsabm.9b00518",
"article-title": "Quercetin Encapsulated Polymer Nanoparticle for Inhibiting Intracellular Polyglutamine Aggregation",
"author": "Debnath",
"doi-asserted-by": "crossref",
"first-page": "5298",
"journal-title": "ACS Appl. Bio Mater.",
"key": "ref_108",
"volume": "2",
"year": "2019"
},
{
"DOI": "10.1016/j.saa.2006.07.006",
"article-title": "Complexation of Quercetin with Three Kinds of Cyclodextrins: An Antioxidant Study",
"author": "Jullian",
"doi-asserted-by": "crossref",
"first-page": "230",
"journal-title": "Spectrochim. Acta-Part A Mol. Biomol. Spectrosc.",
"key": "ref_109",
"volume": "67",
"year": "2007"
},
{
"DOI": "10.1016/j.saa.2013.07.008",
"article-title": "Inclusion Complexes of Quercetin with Three β-Cyclodextrins Derivatives at Physiological PH: Spectroscopic Study and Antioxidant Activity",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "854",
"journal-title": "Spectrochim. Acta-Part A Mol. Biomol. Spectrosc.",
"key": "ref_110",
"volume": "115",
"year": "2013"
},
{
"DOI": "10.3762/bjoc.11.297",
"article-title": "Physical Properties and Biological Activities of Hesperetin and Naringenin in Complex with Methylated P-Cyclodextrin",
"author": "Sangpheak",
"doi-asserted-by": "crossref",
"first-page": "2763",
"journal-title": "Beilstein J. Org. Chem.",
"key": "ref_111",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1016/j.jmgm.2015.11.005",
"article-title": "Inclusion Complexation of Pinostrobin with Various Cyclodextrin Derivatives",
"author": "Kicuntod",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "J. Mol. Graph. Model.",
"key": "ref_112",
"volume": "63",
"year": "2016"
},
{
"DOI": "10.1016/j.colsurfb.2010.06.002",
"article-title": "Development of Biodegradable Nanoparticles for Delivery of Quercetin",
"author": "Kumari",
"doi-asserted-by": "crossref",
"first-page": "184",
"journal-title": "Colloids Surf. B Biointerfaces",
"key": "ref_113",
"volume": "80",
"year": "2010"
},
{
"key": "ref_114",
"unstructured": "Zwicker, J.I., Furie, B., and Flaumenhaft, R. (2023). Method for Treating Sickle Cell Disease Using Quercetin-Containing Compositions. (WO 2023/288044 A1)."
},
{
"key": "ref_115",
"unstructured": "Renjit, S. (2005). Sickle Cell Anemia Treatment. (US 2006/0115459 A1)."
},
{
"key": "ref_116",
"unstructured": "Trimboli, D., Gatti, V., and Naccari, G.C. (2001). Combination of Catechin and Quercetin for Pharmaceutical or Dietary Use. (WO 02/34262 A1)."
},
{
"key": "ref_117",
"unstructured": "Zhang, J., and Li, B. (2021). New Application of Quercetin and Kaempferol. (CN113018293A)."
},
{
"key": "ref_118",
"unstructured": "(2011). Antipruritic Composition Containing Astragalin and Quercetin. (KR20120121684A)."
},
{
"key": "ref_119",
"unstructured": "Lines, T. (2009). Reducing Cholesterol Levels with Combined Use of Quercetin and Statin. (WO2010027572A2)."
},
{
"key": "ref_120",
"unstructured": "Won, L.K., Joo, L.H., Jun, L.S., Young, C.J., Ju, K.N., Hoon, K.J., and Hyuk, L.J. (2006). Composition for Inhibiting Liver Cancer Containing Doxorubicin and Quercetin. (KR100553266B1)."
},
{
"key": "ref_121",
"unstructured": "Marchi, D., Feige, J., and Horcajada, M. (2021). Compositions and Methods Using a Combination of Oleuropein and Quercetin for Use in Cartilage Degeneration. (WO2022106410A1)."
},
{
"key": "ref_122",
"unstructured": "Brown, D. (2020). Treatment of Fragile X Syndrome with Ibudilast in Combination with Metformin, Cannbidiol, Sertraline or Quercetin. (WO2021044158A1)."
},
{
"key": "ref_123",
"unstructured": "Lines, T. (2022). Quercetin-Containing Compositions for Use in Treating Amyotrophic Lateral Sclerosis. (WO2022243942A1)."
},
{
"key": "ref_124",
"unstructured": "Song, H.B., Tae, S.J., Ki, H.B., Yong, B.P., Myung, S.C., Sik, M.S., Yong, K.K., Lee, E.S., Byung, H.H., and Yang, K.C. (1999). Composition Containing Rutin and Quercetin for Preventing or Treating Elevated Blood Lipid Level-Related Diseases. (WO0015237A1)."
},
{
"key": "ref_125",
"unstructured": "Muller, W., Ernst, L.G., Schroder, H.-C., Wilhelm, F., and Wang, X. (2015). Synergistic Composition Comprising Quercetin and Polyphosphate for Treatment of Bone Disorders. (WO2015132304A1)."
},
{
"key": "ref_126",
"unstructured": "Sang-Chan, K.I.M., Park, S.M., Kim, J.K., Kim, E.O., PARK, C.A., Sung-Hui, B.Y.U.N., and Sook-Jahr, P.A.R.K. (2020). Composition for Preventing or Treating Liver Disease, Comprising Icaritin and Quercetin. (WO2022065550A1)."
},
{
"key": "ref_127",
"unstructured": "Jeung-Hye, P. (2018). Composition Containing Quercetin and Vitamin D for Alleviation of Acnegenic Skin. (WO2018230824A1)."
},
{
"key": "ref_128",
"unstructured": "Burger, A.R., Granger, S.P., and Scott, I.R. (1996). Skin Care Compositions Containing Naringenin and/or Quercetin and a Retinoid. (US5665367A)."
},
{
"key": "ref_129",
"unstructured": "Jeung-Hye, P. (2019). Composition, Containing Quercetin, Genistein, and Alpha-Lipoic Acid, for Relieving Acne Skin. (WO2020111757A1)."
},
{
"key": "ref_130",
"unstructured": "Vasquez Garzon, V.R., Carrasco Torres, G., Andrade Jorge, E., Trujillo Ferrara, J.G., and Trevino Villa, S. (2018). Quercetin and Maleic Anhydride Derivatives for The Treatment of Hepatocellular Carcinoma. (MX2018008239A)."
},
{
"key": "ref_131",
"unstructured": "Lopez Munoz, F.J., Espinosa Juarez, J.V., and Jaramilo Morales, O.A. (2017). Pharmaceutical Composition of Haloperidol and Quercetin with Analgesic Effect In Neuropathic Pain. (MX2017007166A)."
},
{
"key": "ref_132",
"unstructured": "Mi-La, C., Min-Jung, P., Seon-Yeong, L., Sung-Hee, L., Eun-Ji, Y., and Hye-Jin, S. (2013). Composition for Preventing or Treating Immune Disease Comprising Metformin and Quercetin as Active Ingredients. (KR20140132932A)."
},
{
"key": "ref_133",
"unstructured": "Gwonhwa, S., Whasun, L., and Sunwoo, P. (2019). Pharmaceutical Composition for Preventing or Treating Endometriosis Comprising Quercetin Luteolin Delphinidin or Mixture Thereof. (KR20210044409A)."
},
{
"key": "ref_134",
"unstructured": "Kuebler, U. (2006). Medicament, Useful to Treat or Prevent Malignantly Transformed Cells, e.g., Adeno-Carcinoma, Prostate Carcinoma and Breast Carcinoma, Comprises a Mixture of Quercetin and Myrecetin and/or Anisomycin and Rapamycin as Kinase Inhibitors. (DE102006036307A1)."
},
{
"key": "ref_135",
"unstructured": "(2012). Polifenoles, C, Combinación Sinérgica de Polifenoles. (WO2012150370A1)."
},
{
"DOI": "10.1016/j.jinorgbio.2015.01.001",
"article-title": "Quercetin Encapsulation in Modified Silica Nanoparticles: Potential Use against Cu(II)-Induced Oxidative Stress in Neurodegeneration",
"author": "Nday",
"doi-asserted-by": "crossref",
"first-page": "51",
"journal-title": "J. Inorg. Biochem.",
"key": "ref_136",
"volume": "145",
"year": "2015"
},
{
"key": "ref_137",
"unstructured": "Zhao, Y., Yang, M., Li, Y., Luan, X., and Luo, Z. (2003). Quercetin Derivatives and Their Medical Usages. (US2004132671A1)."
},
{
"DOI": "10.3390/pharmaceutics14010104",
"doi-asserted-by": "crossref",
"key": "ref_138",
"unstructured": "Ferreira-Silva, M., Faria-Silva, C., Carvalheiro, M.C., Simões, S., Marinho, H.S., Marcelino, P., Campos, M.C., Metselaar, J.M., Fernandes, E., and Baptista, P.V. (2022). Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics, 14."
},
{
"DOI": "10.1016/j.molliq.2021.117714",
"article-title": "On the Behavior of Quercetin + Organic Solvent Solutions and Their Role for C60 Fullerene Solubilization",
"author": "Martel",
"doi-asserted-by": "crossref",
"first-page": "117714",
"journal-title": "J. Mol. Liq.",
"key": "ref_139",
"volume": "345",
"year": "2022"
},
{
"DOI": "10.1016/S0040-4039(02)00867-5",
"article-title": "Synthesis of [60]Fullerene–Quercetin Dyads",
"author": "Silva",
"doi-asserted-by": "crossref",
"first-page": "4617",
"journal-title": "Tetrahedron Lett.",
"key": "ref_140",
"volume": "43",
"year": "2002"
},
{
"DOI": "10.1021/acsomega.3c03933",
"article-title": "Quercetin and 5-Fu Loaded Chitosan Nanoparticles Trigger Cell-Cycle Arrest and Induce Apoptosis in HCT116 Cells via Modulation of the P53/P21 Axis",
"author": "Das",
"doi-asserted-by": "crossref",
"first-page": "36893",
"journal-title": "ACS Omega",
"key": "ref_141",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.3390/pharmaceutics9040053",
"doi-asserted-by": "crossref",
"key": "ref_142",
"unstructured": "Mohammed, M.A., Syeda, J.T.M., Wasan, K.M., and Wasan, E.K. (2017). An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics, 9."
},
{
"DOI": "10.1016/j.ejmech.2022.114995",
"article-title": "5-Fluorouracil Nano-Delivery Systems as a Cutting-Edge for Cancer Therapy",
"author": "Hassan",
"doi-asserted-by": "crossref",
"first-page": "114995",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_143",
"volume": "246",
"year": "2023"
},
{
"DOI": "10.1016/j.tiv.2005.06.048",
"article-title": "Dietary Flavonoids: Effects on Xenobiotic and Carcinogen Metabolism",
"author": "Moon",
"doi-asserted-by": "crossref",
"first-page": "187",
"journal-title": "Toxicol. Vitr.",
"key": "ref_144",
"volume": "20",
"year": "2006"
},
{
"DOI": "10.1016/j.canlet.2016.10.032",
"article-title": "The Application of Nanoparticles in Diagnosis and Theranostics of Gastric Cancer",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "Cancer Lett.",
"key": "ref_145",
"volume": "386",
"year": "2017"
},
{
"DOI": "10.1016/j.ijpharm.2018.01.034",
"article-title": "Inorganic Nanoparticles: A Potential Cancer Therapy for Human Welfare",
"author": "Pugazhendhi",
"doi-asserted-by": "crossref",
"first-page": "104",
"journal-title": "Int. J. Pharm.",
"key": "ref_146",
"volume": "539",
"year": "2018"
},
{
"DOI": "10.1007/s00604-014-1202-0",
"article-title": "In Vitro Studies of Carbon Fiber Microbiosensor for Dopamine Neurotransmitter Supported by Copper-Graphene Oxide Composite",
"author": "Khan",
"doi-asserted-by": "crossref",
"first-page": "1049",
"journal-title": "Microchim. Acta",
"key": "ref_147",
"volume": "181",
"year": "2014"
},
{
"DOI": "10.1208/s12249-011-9739-2",
"article-title": "Preparation, Characterization, and In Vitro Intestinal Permeability Evaluation of Thalidomide–Hydroxypropyl-β-Cyclodextrin Complexes",
"author": "Kratz",
"doi-asserted-by": "crossref",
"first-page": "118",
"journal-title": "AAPS PharmSciTech",
"key": "ref_148",
"volume": "13",
"year": "2012"
},
{
"DOI": "10.1002/pc.23981",
"article-title": "Toward Design and Measurement of Electrical Conductivity and Thermal Properties of Silver Nanoparticle Embedded Poly(o-anisidine) Molybdophosphate Nanocomposite and Its Application as Microbiosensor",
"author": "Khan",
"doi-asserted-by": "crossref",
"first-page": "E237",
"journal-title": "Polym. Compos.",
"key": "ref_149",
"volume": "38",
"year": "2017"
},
{
"DOI": "10.1007/s12010-013-0099-0",
"article-title": "A New Trend on Biosensor for Neurotransmitter Choline/Acetylcholine—An Overview",
"author": "Khan",
"doi-asserted-by": "crossref",
"first-page": "1927",
"journal-title": "Appl. Biochem. Biotechnol.",
"key": "ref_150",
"volume": "169",
"year": "2013"
},
{
"DOI": "10.1016/j.lfs.2018.03.044",
"article-title": "Nanoparticles Designed to Regulate Tumor Microenvironment for Cancer Therapy",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "37",
"journal-title": "Life Sci.",
"key": "ref_151",
"volume": "201",
"year": "2018"
},
{
"DOI": "10.1016/j.jddst.2018.02.007",
"article-title": "Gold Nanoparticle Mediated Delivery of Fungal Asparaginase against Cancer Cells",
"author": "Baskar",
"doi-asserted-by": "crossref",
"first-page": "498",
"journal-title": "J. Drug Deliv. Sci. Technol.",
"key": "ref_152",
"volume": "44",
"year": "2018"
},
{
"DOI": "10.1016/j.msec.2020.110662",
"article-title": "New Quercetin-Coated Titanate Nanotubes and Their Radiosensitization Effect on Human Bladder Cancer",
"author": "Alban",
"doi-asserted-by": "crossref",
"first-page": "110662",
"journal-title": "Mater. Sci. Eng. C",
"key": "ref_153",
"volume": "110",
"year": "2020"
},
{
"article-title": "Bladder Cancer",
"author": "Down",
"first-page": "532",
"journal-title": "Surgery",
"key": "ref_154",
"volume": "34",
"year": "2016"
},
{
"DOI": "10.1021/acsabm.8b00748",
"article-title": "Inclusion of Quercetin in Gold Nanoparticles Decorated with Supramolecular Hosts Amplifies Its Tumor Targeting Properties",
"author": "Yilmaz",
"doi-asserted-by": "crossref",
"first-page": "2715",
"journal-title": "ACS Appl. Bio Mater.",
"key": "ref_155",
"volume": "2",
"year": "2019"
},
{
"DOI": "10.1080/10408347.2023.2269421",
"doi-asserted-by": "crossref",
"key": "ref_156",
"unstructured": "Mansour, F.R., Abdallah, I.A., Bedair, A., and Hamed, M. (2023). Analytical Methods for the Determination of Quercetin and Quercetin Glycosides in Pharmaceuticals and Biological Samples. Crit. Rev. Anal. Chem., 1–26."
},
{
"DOI": "10.1016/j.jinorgbio.2012.02.007",
"article-title": "1H, 13C MAS NMR and DFT GIAO Study of Quercetin and Its Complex with Al(III) in Solid State",
"author": "Ahmedova",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "J. Inorg. Biochem.",
"key": "ref_157",
"volume": "110",
"year": "2012"
},
{
"DOI": "10.1016/j.jphotobiol.2013.07.019",
"article-title": "A Combined Spectroscopic, Molecular Docking and Molecular Dynamic Simulation Study on the Interaction of Quercetin with β-Casein Nanoparticles",
"author": "Mehranfar",
"doi-asserted-by": "crossref",
"first-page": "100",
"journal-title": "J. Photochem. Photobiol. B Biol.",
"key": "ref_158",
"volume": "127",
"year": "2013"
},
{
"DOI": "10.1016/j.jep.2017.08.026",
"article-title": "Phytochemical Analysis, Molecular Docking and Antiamnesic Effects of Methanolic Extract of Silybum marianum (L.) Gaertn Seeds in Scopolamine Induced Memory Impairment in Mice",
"author": "Nazir",
"doi-asserted-by": "crossref",
"first-page": "198",
"journal-title": "J. Ethnopharmacol.",
"key": "ref_159",
"volume": "210",
"year": "2018"
},
{
"DOI": "10.1080/07391102.2015.1123190",
"article-title": "Docking and DFT Studies on Ligand Binding to Quercetin 2,3-Dioxygenase",
"author": "Malkhasian",
"doi-asserted-by": "crossref",
"first-page": "2453",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_160",
"volume": "34",
"year": "2016"
},
{
"DOI": "10.1021/jf303393n",
"article-title": "Molecular Docking, Kinetics Study, and Structure–Activity Relationship Analysis of Quercetin and Its Analogous as Helicobacter Pylori Urease Inhibitors",
"author": "Xiao",
"doi-asserted-by": "crossref",
"first-page": "10572",
"journal-title": "J. Agric. Food Chem.",
"key": "ref_161",
"volume": "60",
"year": "2012"
},
{
"DOI": "10.2174/2211550107666180612100441",
"article-title": "Competitive Inhibition of Quercetin and Apigenin at the ATP Binding Site of D-Alanine-D-Alanine Ligase of Helicobacter Pylori—A Molecular Modeling Approach",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "340",
"journal-title": "Curr. Biotechnol.",
"key": "ref_162",
"volume": "7",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0263917",
"doi-asserted-by": "crossref",
"key": "ref_163",
"unstructured": "Ghosh, A., Sarmah, P., Patel, H., Mukerjee, N., Mishra, R., Alkahtani, S., Varma, R.S., and Baishya, D. (2022). Nonlinear Molecular Dynamics of Quercetin in Gynocardia Odorata and Diospyros Malabarica Fruits: Its Mechanistic Role in Hepatoprotection. PLoS ONE, 17."
},
{
"DOI": "10.1080/07391102.2022.2155699",
"article-title": "Molecular Dynamics Simulation and Pharmacokinetics Studies of Ombuin and Quercetin against Human Pancreatic α-Amylase",
"author": "Kikiowo",
"doi-asserted-by": "crossref",
"first-page": "10388",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_164",
"volume": "41",
"year": "2023"
},
{
"DOI": "10.1016/j.cplett.2022.139470",
"article-title": "Molecular Dynamics Study of Quercetin Families and Its Derivative Compounds from Carica Papaya Leaf as Breast Cancer Inhibitors",
"author": "Maran",
"doi-asserted-by": "crossref",
"first-page": "139470",
"journal-title": "Chem. Phys. Lett.",
"key": "ref_165",
"volume": "793",
"year": "2022"
},
{
"key": "ref_166",
"unstructured": "Omirin, E.S., Omotuyi, O., Afokhume, O.G., Okoh, E.F., Boboye, S.O., Olugbogi, E.A., Adelegan, O.O., Ibitoye, B.O., Aderiye, M.A., and Agosile, O.O. (2015). Molecular Dynamics Simulations on Quercetin-3-(6-Malonylglucoside) From Morus Alba Shows Optimal Inhibition of Bcl-2 with Favorable Anti-Tumor Activities. bioRxiv, 1–15."
},
{
"DOI": "10.1080/07391102.2016.1234416",
"article-title": "Quercetin Derivatives as Non-Nucleoside Inhibitors for Dengue Polymerase: Molecular Docking, Molecular Dynamics Simulation, and Binding Free Energy Calculation",
"author": "Anusuya",
"doi-asserted-by": "crossref",
"first-page": "2895",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_167",
"volume": "35",
"year": "2017"
},
{
"DOI": "10.1080/07391102.2020.1820380",
"article-title": "Probing Intermolecular Interactions and Binding Stability of Kaempferol, Quercetin and Resveratrol Derivatives with PPAR-γ: Docking, Molecular Dynamics and MM/GBSA Approach to Reveal Potent PPAR-γ Agonist against Cancer",
"author": "Lokhande",
"doi-asserted-by": "crossref",
"first-page": "971",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_168",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.3390/ijms141121647",
"article-title": "Fast Disintegrating Quercetin-Loaded Drug Delivery Systems Fabricated Using Coaxial Electrospinning",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "21647",
"journal-title": "Int. J. Mol. Sci.",
"key": "ref_169",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1002/jps.22446",
"article-title": "Preparation and Characterization of Quercetin Nanocrystals",
"author": "Sahoo",
"doi-asserted-by": "crossref",
"first-page": "2379",
"journal-title": "J. Pharm. Sci.",
"key": "ref_170",
"volume": "100",
"year": "2011"
},
{
"DOI": "10.1016/j.lwt.2010.09.001",
"article-title": "Preparation, Physicochemical Characterization and in Vitro Digestibility on Solid Complex of Maize Starches with Quercetin",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "787",
"journal-title": "LWT-Food Sci. Technol.",
"key": "ref_171",
"volume": "44",
"year": "2011"
},
{
"DOI": "10.1021/jf802823q",
"article-title": "Cyclodextrin Inclusion Complex Formation and Solid-State Characterization of the Natural Antioxidants α-Tocopherol and Quercetin",
"author": "Koontz",
"doi-asserted-by": "crossref",
"first-page": "1162",
"journal-title": "J. Agric. Food Chem.",
"key": "ref_172",
"volume": "57",
"year": "2009"
},
{
"DOI": "10.1016/S0006-291X(02)02667-0",
"article-title": "The Interaction of Quercetin with Human Serum Albumin: A Fluorescence Spectroscopic Study",
"author": "Sengupta",
"doi-asserted-by": "crossref",
"first-page": "400",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "ref_173",
"volume": "299",
"year": "2002"
},
{
"DOI": "10.1016/S1386-1425(01)00515-7",
"article-title": "Time Resolved Fluorescence Spectroscopy of Quercetin and Morin Complexes with Al3+",
"author": "Gutierrez",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Spectrochim. Acta Part A Mol. Biomol. Spectrosc.",
"key": "ref_174",
"volume": "58",
"year": "2002"
},
{
"DOI": "10.1016/j.jlumin.2017.10.024",
"article-title": "Fluorescence Spectroscopic Evaluation of the Interactions of Quercetin, Isorhamnetin, and Quercetin-3′-Sulfate with Different Albumins",
"author": "Boda",
"doi-asserted-by": "crossref",
"first-page": "156",
"journal-title": "J. Lumin.",
"key": "ref_175",
"volume": "194",
"year": "2018"
},
{
"DOI": "10.1016/j.measurement.2018.02.036",
"article-title": "Fluorescence Spectroscopy-Partial Least Square Regression Method for the Quantification of Quercetin in Euphorbia Masirahensis",
"author": "Alabri",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Measurement",
"key": "ref_176",
"volume": "121",
"year": "2018"
},
{
"DOI": "10.1021/jf803671s",
"article-title": "Spectroscopy Study on the Interaction of Quercetin with Collagen",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "3431",
"journal-title": "J. Agric. Food Chem.",
"key": "ref_177",
"volume": "57",
"year": "2009"
},
{
"DOI": "10.12693/APhysPolA.125.A-57",
"article-title": "Optical Spectroscopy Study of the Interaction between Quercetin and Human Serum Albumin",
"author": "Wybranowski",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "Acta Phys. Pol. A",
"key": "ref_178",
"volume": "125",
"year": "2014"
},
{
"DOI": "10.1002/biot.201700389",
"doi-asserted-by": "crossref",
"key": "ref_179",
"unstructured": "Pham-Hoang, B., Winckler, P., and Waché, Y. (2018). Fluorescence Lifetime and UV-Vis Spectroscopy to Evaluate the Interactions between Quercetin and Its Yeast Microcapsule. Biotechnol. J., 13."
},
{
"DOI": "10.1016/j.cdc.2019.100228",
"article-title": "Application of Fluorescence Spectroscopy Coupled with PLSR for the Estimation of Quercetin in Four Medicinal Plants",
"author": "Hussain",
"doi-asserted-by": "crossref",
"first-page": "100228",
"journal-title": "Chem. Data Collect.",
"key": "ref_180",
"volume": "21",
"year": "2019"
},
{
"DOI": "10.1016/j.matpr.2017.06.334",
"article-title": "Development, Characterization and Solubility Study of Solid Dispersion of Quercetin by Solvent Evaporation Method",
"author": "Verma",
"doi-asserted-by": "crossref",
"first-page": "10128",
"journal-title": "Mater. Today Proc.",
"key": "ref_181",
"volume": "4",
"year": "2017"
},
{
"article-title": "HPLC Analysis of Methanolic Extract of Herbs for Quercetin Content",
"author": "Verma",
"first-page": "159",
"journal-title": "J. Pharmacogn. Phytochem.",
"key": "ref_182",
"volume": "2",
"year": "2013"
},
{
"DOI": "10.1093/jaoac/94.1.100",
"article-title": "A Simple HPLC Method for Quantitation of Quercetin in Herbal Extracts",
"author": "Joshi",
"doi-asserted-by": "crossref",
"first-page": "100",
"journal-title": "J. AOAC Int.",
"key": "ref_183",
"volume": "94",
"year": "2011"
},
{
"DOI": "10.1016/j.microc.2019.05.032",
"article-title": "Determination of Quercetin Using a Molecularly Imprinted Polymer as Solid-Phase Microextraction Sorbent and High-Performance Liquid Chromatography",
"author": "Rahimi",
"doi-asserted-by": "crossref",
"first-page": "433",
"journal-title": "Microchem. J.",
"key": "ref_184",
"volume": "148",
"year": "2019"
},
{
"DOI": "10.1081/JLC-100105140",
"article-title": "Assay of Flavonols and Quantification of Quercetin in Medicinal Plants by Hplc with Uv-Diode Array Detection",
"author": "Stefova",
"doi-asserted-by": "crossref",
"first-page": "2283",
"journal-title": "J. Liq. Chromatogr. Relat. Technol.",
"key": "ref_185",
"volume": "24",
"year": "2001"
},
{
"DOI": "10.1016/j.jpba.2008.06.004",
"article-title": "Separation of Quercetin, Sexangularetin, Kaempferol and Isorhamnetin for Simultaneous HPLC Determination of Flavonoid Aglycones in Inflorescences, Leaves and Fruits of Three Sorbus Species",
"author": "Olszewska",
"doi-asserted-by": "crossref",
"first-page": "629",
"journal-title": "J. Pharm. Biomed. Anal.",
"key": "ref_186",
"volume": "48",
"year": "2008"
},
{
"DOI": "10.1016/j.jfca.2007.03.009",
"article-title": "Gas Chromatography–Mass Spectrometry Analysis of Phenolic Compounds from Carica papaya L. Leaf",
"author": "Canini",
"doi-asserted-by": "crossref",
"first-page": "584",
"journal-title": "J. Food Compos. Anal.",
"key": "ref_187",
"volume": "20",
"year": "2007"
},
{
"DOI": "10.1016/S0021-9673(02)01921-0",
"article-title": "Development and Validation of a Gas Chromatographic–Mass Spectrometric Method for Simultaneous Identification and Quantification of Marker Compounds Including Bilobalide, Ginkgolides and Flavonoids in Ginkgo biloba L. Extract and Pharmaceutical Preparatio",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "121",
"journal-title": "J. Chromatogr. A",
"key": "ref_188",
"volume": "986",
"year": "2003"
},
{
"article-title": "The Quantitation of Metabolites of Quercetin Flavonols in Human Urine",
"author": "Gross",
"first-page": "711",
"journal-title": "Cancer Epidemiol. Biomark. Prev.",
"key": "ref_189",
"volume": "5",
"year": "1996"
},
{
"DOI": "10.1016/j.farmac.2003.07.013",
"article-title": "Direct Spectrophotometric Determination of Quercetin in the Presence of Ascorbic Acid",
"author": "Pejic",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Il Farm.",
"key": "ref_190",
"volume": "59",
"year": "2004"
},
{
"DOI": "10.1080/10942912.2017.1373122",
"article-title": "Integrated Approach for Bioactive Quality Evaluation of Medicinal Plant Extracts Using HPLC-DAD, Spectrophotometric, near Infrared Spectroscopy and Chemometric Techniques",
"author": "Valinger",
"doi-asserted-by": "crossref",
"first-page": "S2463",
"journal-title": "Int. J. Food Prop.",
"key": "ref_191",
"volume": "20",
"year": "2017"
},
{
"DOI": "10.1080/00032710903491070",
"article-title": "Determination of Quercetin and Rutin in Selected Herbs and Pharmaceutical Preparations",
"author": "Kurzawa",
"doi-asserted-by": "crossref",
"first-page": "993",
"journal-title": "Anal. Lett.",
"key": "ref_192",
"volume": "43",
"year": "2010"
},
{
"DOI": "10.1556/JPC.21.2008.5.7",
"article-title": "Determination of Flavonoids in Tea and Rooibos Extracts by TLC and HPLC",
"author": "Ligor",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "J. Planar Chromatogr.—Mod. TLC",
"key": "ref_193",
"volume": "21",
"year": "2008"
},
{
"DOI": "10.3109/13880209.2015.1025289",
"article-title": "Screening of Antidepressant Activity and Estimation of Quercetin from Coccinia Indica Using TLC Densitometry",
"author": "Randhawa",
"doi-asserted-by": "crossref",
"first-page": "1867",
"journal-title": "Pharm. Biol.",
"key": "ref_194",
"volume": "53",
"year": "2015"
},
{
"article-title": "Stability of Drugs, Drug Candidates, and Metabolites in Blood and Plasma",
"author": "Reed",
"first-page": "7.6.1",
"journal-title": "Curr. Protoc. Pharmacol.",
"key": "ref_195",
"volume": "2016",
"year": "2016"
},
{
"DOI": "10.1016/j.bjp.2016.05.017",
"article-title": "Validated High Performance Thin Layer Chromatography Method for Simultaneous Determination of Quercetin and Gallic Acid in Leea Indica",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "50",
"journal-title": "Rev. Bras. Farmacogn.",
"key": "ref_196",
"volume": "27",
"year": "2017"
},
{
"DOI": "10.1016/S0003-2670(00)01099-0",
"article-title": "Determination of Rutin and Quercetin in Plants by Capillary Electrophoresis with Electrochemical Detection",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Anal. Chim. Acta",
"key": "ref_197",
"volume": "423",
"year": "2000"
},
{
"DOI": "10.1007/BF02492150",
"article-title": "Separation and Determination of Rutin and Quercetin in the Flowers of Sophora japonica L. by Capillary Electrophoresis with Electrochemical Detection",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "243",
"journal-title": "Chromatographia",
"key": "ref_198",
"volume": "55",
"year": "2002"
},
{
"DOI": "10.1002/elps.200390159",
"article-title": "Quantitative Analysis of Aglycone Quercetin in Mulberry Leaves (Morus alba L.) by Capillary Zone Electrophoresis",
"author": "Suntornsuk",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "Electrophoresis",
"key": "ref_199",
"volume": "24",
"year": "2003"
},
{
"DOI": "10.1556/AChrom.24.2012.4.13",
"article-title": "Ultrasensitive Determination of Epicatechin, Rutin, and Quercetin by Capillary Electrophoresis Chemiluminescence",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "679",
"journal-title": "Acta Chromatogr.",
"key": "ref_200",
"volume": "24",
"year": "2012"
},
{
"DOI": "10.1007/s12161-016-0552-0",
"article-title": "Simultaneous Determination of Quercetin, Rutin, Naringin, and Naringenin in Different Fruits by Capillary Zone Electrophoresis",
"author": "Memon",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Food Anal. Methods",
"key": "ref_201",
"volume": "10",
"year": "2017"
},
{
"DOI": "10.1021/jf073521f",
"article-title": "Determination of the Relative Contribution of Quercetin and Its Glucosides to the Antioxidant Capacity of Onion by Cyclic Voltammetry and Spectrophotometric Methods",
"author": "Zielinska",
"doi-asserted-by": "crossref",
"first-page": "3524",
"journal-title": "J. Agric. Food Chem.",
"key": "ref_202",
"volume": "56",
"year": "2008"
},
{
"key": "ref_203",
"unstructured": "Reddaiah, K., Reddy, T.M., and Swamy, K. (2012). Electrochemical Determination of Quercetin at β–Cyclodextrin Modified Chemical Sensor: A Voltammetric Study. Anal. Bioanal. Electrochem., 4."
},
{
"DOI": "10.1134/S106193482004005X",
"article-title": "Voltammetric Determination of Quercetin and Rutin on Their Simultaneous Presence on an Electrode Modified with Polythymolphthalein",
"author": "Guss",
"doi-asserted-by": "crossref",
"first-page": "526",
"journal-title": "J. Anal. Chem.",
"key": "ref_204",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1007/s10008-012-1707-6",
"article-title": "Study of Antioxidant Properties of Flavonoids by Voltammetry",
"author": "Korotkova",
"doi-asserted-by": "crossref",
"first-page": "2435",
"journal-title": "J. Solid State Electrochem.",
"key": "ref_205",
"volume": "16",
"year": "2012"
},
{
"DOI": "10.1016/j.foodchem.2010.11.059",
"article-title": "Quantitative Analysis of Quercetin Using Raman Spectroscopy",
"author": "Numata",
"doi-asserted-by": "crossref",
"first-page": "751",
"journal-title": "Food Chem.",
"key": "ref_206",
"volume": "126",
"year": "2011"
},
{
"DOI": "10.1039/C4CP00864B",
"article-title": "Effect of PH on the Chemical Modification of Quercetin and Structurally Related Flavonoids Characterized by Optical (UV-Visible and Raman) Spectroscopy",
"author": "Jurasekova",
"doi-asserted-by": "crossref",
"first-page": "12802",
"journal-title": "Phys. Chem. Chem. Phys.",
"key": "ref_207",
"volume": "16",
"year": "2014"
},
{
"DOI": "10.1016/j.saa.2014.12.050",
"article-title": "Application of Spectroscopic Methods for Identification (FT-IR, Raman Spectroscopy) and Determination (UV, EPR) of Quercetin-3-O-Rutinoside. Experimental and DFT Based Approach",
"author": "Paczkowska",
"doi-asserted-by": "crossref",
"first-page": "132",
"journal-title": "Spectrochim. Acta Part A Mol. Biomol. Spectrosc.",
"key": "ref_208",
"volume": "140",
"year": "2015"
},
{
"DOI": "10.1016/j.molstruc.2008.07.025",
"article-title": "Raman and Surface-Enhanced Raman Scattering (SERS) Investigation of the Quercetin Interaction with Metals: Evidence of Structural Changing Processes in Aqueous Solution and on Metal Nanoparticles",
"author": "Jurasekova",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "J. Mol. Struct.",
"key": "ref_209",
"volume": "918",
"year": "2009"
},
{
"DOI": "10.1002/(SICI)1520-6343(1997)3:3<183::AID-BSPY2>3.0.CO;2-7",
"article-title": "Structural Study of Quercetin by Vibrational and Electronic Spectroscopies Combined with Semiempirical Calculations",
"author": "Cornard",
"doi-asserted-by": "crossref",
"first-page": "183",
"journal-title": "Biospectroscopy",
"key": "ref_210",
"volume": "3",
"year": "1997"
},
{
"DOI": "10.1134/S1061934813100080",
"article-title": "Development and Validation of a New RP-HPLC Method for Determination of Quercetin in Green Tea",
"author": "Savic",
"doi-asserted-by": "crossref",
"first-page": "906",
"journal-title": "J. Anal. Chem.",
"key": "ref_211",
"volume": "68",
"year": "2013"
},
{
"DOI": "10.3390/molecules28062540",
"doi-asserted-by": "crossref",
"key": "ref_212",
"unstructured": "Sah, M.K., Gautam, B., Pokhrel, K.P., Ghani, L., and Bhattarai, A. (2023). Quantification of the Quercetin Nanoemulsion Technique Using Various Parameters. Molecules, 28."
},
{
"DOI": "10.1016/j.lwt.2015.11.004",
"article-title": "Morphological and in vitro Antibacterial Efficacy of Quercetin Loaded Nanoparticles against Food-Borne Microorganisms",
"author": "Verma",
"doi-asserted-by": "crossref",
"first-page": "638",
"journal-title": "LWT-Food Sci. Technol.",
"key": "ref_213",
"volume": "66",
"year": "2016"
},
{
"DOI": "10.1016/j.snb.2018.05.172",
"article-title": "Determination of Quercetin in the Presence of Tannic Acid in Soft Drinks Based on Carbon Nanotubes Modified Electrode Using Chemometric Approaches",
"author": "Mosleh",
"doi-asserted-by": "crossref",
"first-page": "605",
"journal-title": "Sens. Actuators B Chem.",
"key": "ref_214",
"volume": "272",
"year": "2018"
},
{
"article-title": "Modelling and Optimization of Quercetin Extraction and Biological Activity of Quercetin-Rich Red Onion Skin Extract from Southeastern Serbia",
"author": "Savic",
"first-page": "15",
"journal-title": "J. Food Nutr. Res.",
"key": "ref_215",
"volume": "57",
"year": "2018"
},
{
"DOI": "10.1016/j.jfoodeng.2017.07.002",
"article-title": "Nanostructured Lipid Carrier (NLC) as a Strategy for Encapsulation of Quercetin and Linseed Oil: Preparation and in Vitro Characterization Studies",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Food Eng.",
"key": "ref_216",
"volume": "215",
"year": "2017"
},
{
"DOI": "10.1016/j.jpba.2013.07.045",
"article-title": "LC–MS Metabolic Study on Quercetin and Taxifolin Galloyl Esters Using Human Hepatocytes as Toxicity and Biotransformation in vitro Cell Model",
"author": "Vacek",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "J. Pharm. Biomed. Anal.",
"key": "ref_217",
"volume": "86",
"year": "2013"
},
{
"DOI": "10.1038/s41598-017-07665-z",
"article-title": "FT-IR-Based Method for Rutin, Quercetin and Quercitrin Quantification in Different Buckwheat (Fagopyrum) Species",
"author": "Straus",
"doi-asserted-by": "crossref",
"first-page": "7226",
"journal-title": "Sci. Rep.",
"key": "ref_218",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1016/S0731-7085(03)00529-6",
"article-title": "Study of Freeze-Dried Quercetin–Cyclodextrin Binary Systems by DSC, FT-IR, X-ray Diffraction and SEM Analysis",
"author": "Pralhad",
"doi-asserted-by": "crossref",
"first-page": "333",
"journal-title": "J. Pharm. Biomed. Anal.",
"key": "ref_219",
"volume": "34",
"year": "2004"
},
{
"DOI": "10.1016/S0308-8146(97)00153-2",
"article-title": "Capillary Electrophoresis Analysis of Trans- and Cis-Resveratrol, Quercetin, Catechin and Gallic Acid in Wine",
"author": "Prasongsidh",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Food Chem.",
"key": "ref_220",
"volume": "62",
"year": "1998"
},
{
"DOI": "10.3892/etm.2022.11230",
"article-title": "Assessing the Anti-inflammatory Effects of Quercetin Using Network Pharmacology and in Vitro Experiments",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "301",
"journal-title": "Exp. Ther. Med.",
"key": "ref_221",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3390/ijms21020493",
"doi-asserted-by": "crossref",
"key": "ref_222",
"unstructured": "Zhang, X.-W., Chen, J.-Y., Ouyang, D., and Lu, J.-H. (2020). Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci., 21."
},
{
"DOI": "10.1007/s00394-010-0125-8",
"article-title": "Immunoregulatory Effects of the Flavonol Quercetin in vitro and in vivo",
"author": "Nickel",
"doi-asserted-by": "crossref",
"first-page": "163",
"journal-title": "Eur. J. Nutr.",
"key": "ref_223",
"volume": "50",
"year": "2011"
},
{
"DOI": "10.1080/01496395.2021.1893333",
"article-title": "Supercritical Fluid Extraction of Quercetin from Sumac (Rhus coriaria L.): Effects of Supercritical Extraction Parameters",
"author": "Ekinci",
"doi-asserted-by": "crossref",
"first-page": "256",
"journal-title": "Sep. Sci. Technol.",
"key": "ref_224",
"volume": "57",
"year": "2022"
},
{
"DOI": "10.1016/j.jpba.2017.03.012",
"article-title": "Development and Validation of a Fast SFC Method for the Analysis of Flavonoids in Plant Extracts",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "384",
"journal-title": "J. Pharm. Biomed. Anal.",
"key": "ref_225",
"volume": "140",
"year": "2017"
},
{
"DOI": "10.1016/j.aca.2013.05.031",
"article-title": "Analytica Chimica Acta Application of Hot Platinum Microelectrodes for Determination of Flavonoids in Flow Injection Analysis and Capillary Electrophoresis",
"author": "Magnuszewska",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Anal. Chim. Acta",
"key": "ref_226",
"volume": "786",
"year": "2013"
},
{
"DOI": "10.3390/molecules18078402",
"article-title": "Characterization of Flavonoids and Phenolic Acids in Myrcia Bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS Combined with NMR",
"author": "Saldanha",
"doi-asserted-by": "crossref",
"first-page": "8402",
"journal-title": "Molecules",
"key": "ref_227",
"volume": "18",
"year": "2013"
},
{
"DOI": "10.1016/j.aca.2008.10.038",
"article-title": "A New Method for Determination of Myricetin and Quercetin Using Solid Phase Microextraction–High Performance Liquid Chromatography–Ultra Violet/Visible System in Grapes, Vegetables and Red Wine Samples",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "Anal. Chim. Acta",
"key": "ref_228",
"volume": "631",
"year": "2009"
},
{
"DOI": "10.3390/molecules25020408",
"doi-asserted-by": "crossref",
"key": "ref_229",
"unstructured": "Cecchi, L., Ieri, F., Vignolini, P., Mulinacci, N., and Romani, A. (2020). Characterization of Volatile and Flavonoid Composition of Different Cuts of Dried Onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GC×GC-TOF and HPLC-DAD. Molecules, 25."
},
{
"article-title": "The Crystal and Molecular Structure of Quercetin: A Biologically Active and Naturally Occurring Flavonoid",
"author": "York",
"first-page": "55",
"journal-title": "Bioorg. Chem.",
"key": "ref_230",
"volume": "69",
"year": "1986"
},
{
"DOI": "10.1016/j.talanta.2016.11.044",
"article-title": "Talanta MIL-101 (Cr) as Matrix for Sensitive Detection of Quercetin by Matrix- Assisted Laser Desorption/Ionization Mass Spectrometry",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Talanta",
"key": "ref_231",
"volume": "164",
"year": "2017"
},
{
"DOI": "10.1021/jf991035p",
"article-title": "MALDI-TOF MS Analysis of Food Flavonol Glycosides",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1657",
"journal-title": "J. Agric. Food Chem.",
"key": "ref_232",
"volume": "48",
"year": "2000"
},
{
"DOI": "10.1021/jf0115894",
"article-title": "Identification and Quantification of Flavonol Glycosides in Almond Seedcoats Using MALDI-TOF MS",
"author": "Sporns",
"doi-asserted-by": "crossref",
"first-page": "2782",
"journal-title": "J. Agric. Food Chem.",
"key": "ref_233",
"volume": "50",
"year": "2002"
},
{
"DOI": "10.1002/pca.2621",
"article-title": "Direct Coupling of HPTLC with MALDI-TOF MS for Qualitative Detection of Flavonoids on Phytochemical Fingerprints",
"author": "Kroslakova",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "Phytochem. Anal.",
"key": "ref_234",
"volume": "27",
"year": "2016"
},
{
"article-title": "Supercritical Fluid Extraction of Quercetin and Rutin from Hyperici Herba Supercritical Fluid Extraction of Quercetin and Rutin from Hyperici Herba",
"author": "Zdravkovski",
"first-page": "2517",
"journal-title": "J. Liq. Chromatogr. Relat. Technol.",
"key": "ref_235",
"volume": "26",
"year": "2007"
},
{
"DOI": "10.1016/j.microc.2023.109233",
"article-title": "Sample Preparation Methods for Determination of Quercetin and Quercetin Glycosides in Diverse Matrices",
"author": "Hamed",
"doi-asserted-by": "crossref",
"first-page": "109233",
"journal-title": "Microchem. J.",
"key": "ref_236",
"volume": "194",
"year": "2023"
},
{
"DOI": "10.1021/jf011213q",
"article-title": "Advanced Solid Phase Extraction Using Molecularly Imprinted Polymers for the Determination of Quercetin in Red Wine",
"author": "Molinelli",
"doi-asserted-by": "crossref",
"first-page": "1804",
"journal-title": "J. Agric. Food Chem.",
"key": "ref_237",
"volume": "50",
"year": "2002"
},
{
"DOI": "10.1016/j.talanta.2009.07.051",
"article-title": "Quercetin Molecularly Imprinted Polymers: Preparation, Recognition Characteristics and Properties as Sorbent for Solid-Phase Extraction",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "694",
"journal-title": "Talanta",
"key": "ref_238",
"volume": "80",
"year": "2009"
},
{
"DOI": "10.1039/C4AY00471J",
"article-title": "Synthesis and Characterization of Molecularly Imprinted Silica Mediated by Al for Solid Phase Extraction of Quercetin in Ginkgo biloba L",
"author": "Braga",
"doi-asserted-by": "crossref",
"first-page": "4029",
"journal-title": "Anal. Methods",
"key": "ref_239",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.1039/C8NJ03349H",
"article-title": "Simple and Selective Detection of Quercetin in Extracts of Plants and Food Samples by Dispersive-Micro-Solid Phase Extraction Based on Core–Shell Magnetic Molecularly Imprinted Polymers",
"author": "Asfaram",
"doi-asserted-by": "crossref",
"first-page": "16144",
"journal-title": "New J. Chem.",
"key": "ref_240",
"volume": "42",
"year": "2018"
}
],
"reference-count": 240,
"references-count": 240,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1420-3049/28/24/8141"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Chemistry (miscellaneous)",
"Analytical Chemistry",
"Organic Chemistry",
"Physical and Theoretical Chemistry",
"Molecular Medicine",
"Drug Discovery",
"Pharmaceutical Science"
],
"subtitle": [],
"title": "Quercetin: A Potential Polydynamic Drug",
"type": "journal-article",
"volume": "28"
}