Efficacy of Paxlovid in patients with acute kidney injury who developed COVID-19
et al., Journal of Infection, doi:10.1016/j.jinf.2022.10.002, Dec 2022
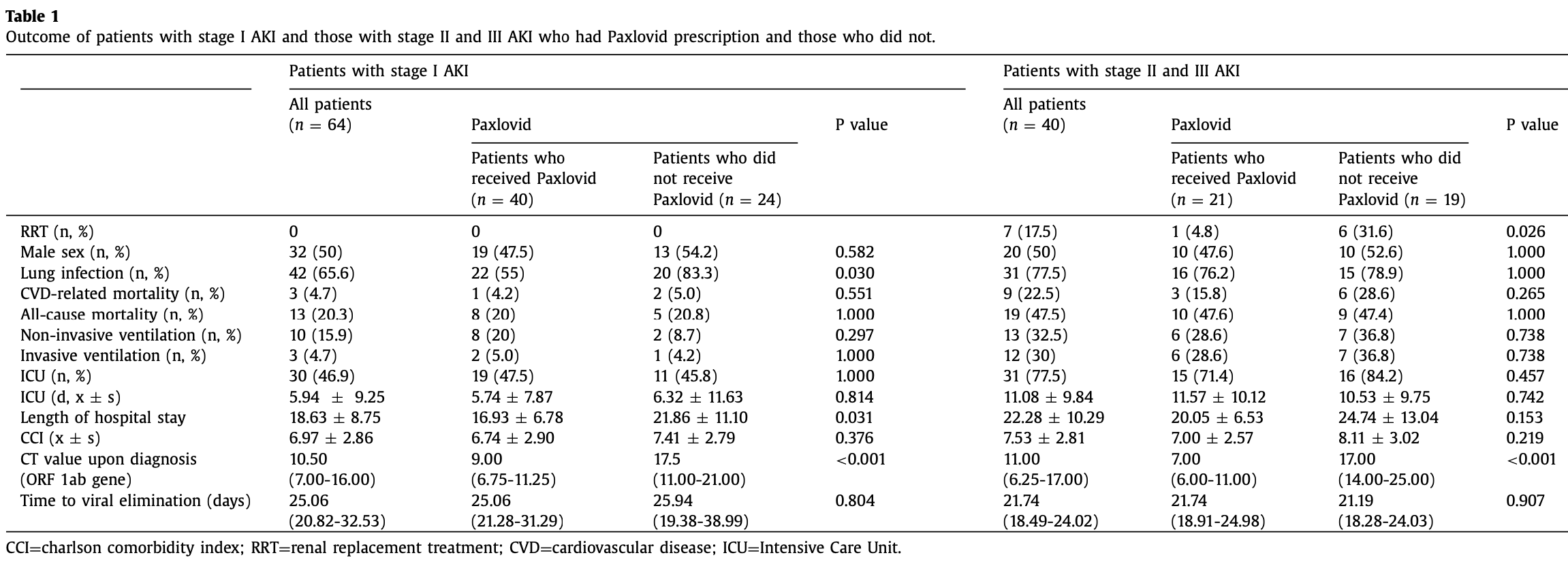
Retrospective 104 AKI patients with COVID-19 in China, 61 treated with paxlovid, showing faster viral clearance and shorter hospitalization with treatment, but no significant difference in morality or ICU admission.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
This may explain in part the very high mortality seen in this study.
|
risk of death, 9.4% lower, RR 0.91, p = 0.83, treatment 18 of 61 (29.5%), control 14 of 43 (32.6%), NNT 33.
|
|
risk of mechanical ventilation, 29.5% lower, RR 0.70, p = 0.58, treatment 8 of 61 (13.1%), control 8 of 43 (18.6%), NNT 18.
|
|
risk of ICU admission, 11.2% lower, RR 0.89, p = 0.55, treatment 34 of 61 (55.7%), control 27 of 43 (62.8%), NNT 14.
|
|
ICU time, 2.9% lower, relative time 0.97, p = 0.91, treatment 61, control 43.
|
|
hospitalization time, 20.0% lower, relative time 0.80, p = 0.01, treatment 61, control 43.
|
|
risk of no viral clearance, 47.1% lower, RR 0.53, p = 0.001, treatment 61, control 43.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Cai et al., 31 Dec 2022, retrospective, China, peer-reviewed, 5 authors, study period 7 April, 2022 - 21 June, 2022.
Contact: Chexj@126.com, shan_mou@shsmu.edu.cn.
Abstract: Since January 2020 Elsevier has created a COVID-19 resource centre with
free information in English and Mandarin on the novel coronavirus COVID19. The COVID-19 resource centre is hosted on Elsevier Connect, the
company's public news and information website.
Elsevier hereby grants permission to make all its COVID-19-related
research that is available on the COVID-19 resource centre - including this
research content - immediately available in PubMed Central and other
publicly funded repositories, such as the WHO COVID database with rights
for unrestricted research re-use and analyses in any form or by any means
with acknowledgement of the original source. These permissions are
granted for free by Elsevier for as long as the COVID-19 resource centre
remains active.
Journal of Infection 85 (2022) 702–769
Contents lists available at ScienceDirect
Journal of Infection
journal homepage: www.elsevier.com/locate/jinf
Letters to the Editor
Highly Pathogenic Avian Influenza outbreaks amongst
bird populations in Europe - a view from China
Dear Editor,
A recent letter in this journal described an avian influenza virus
of wild bird origin that is well adapted to a mammalian host, posing a potential threat to animal and human health.1 This is reminiscent of the recent warnings about highly pathogenic avian influenza (HPAI) in Europe. HPAI, caused by highly pathogenic avian
influenza virus (HPAIV), is a zoonotic disease that seriously endangers the poultry industry, human life, and public health. In October 2022, the European centre for Disease Prevention and Control (ECDC) issued an alert stating that the 2021–2022 HPAI epidemic season in Europe was the largest ever. The latest data (as
of September 9, 2022) showed 3573 HPAI cases in wild birds and
2467 outbreaks in poultry, with 48 million birds culled.2 The geographical extent of the outbreak was unprecedented, affecting 37
European countries from the Svalbard islands to southern Portugal
and eastern Portugal to Ukraine.3
HPAI data from Europe (Fig. 1) showed both explosive growth
and significant change in the epidemic strain over the past 3 years
(Table 1). In 2018, the main epidemic strain was H5N6, whereas
in 2019–2020, H5N8 was more prevalent. In 2021, the dominant
strain changed again, and between September 2021 and September 2022, most cases were H5N1 (96.78%, 5311/5489). New strains
of HPAIV constantly emerge through mutation, insertion, or recombination of the HA and NA genes.4
Long-distance migration of birds can spread HPAI. A study of
the H5N8 HPAI outbreaks in Europe and Japan in 2015 showed
Fig. 1. HPAI outbreaks in Europe, 2018–2022. According to ECDC data on HPAI
outbreaks in the past 5 years. The 2018 data include HPAI outbreaks reported
from November 16, 2017 to November 15, 2018; 2019 data include outbreaks from
November 16, 2018 to November 15, 2019; 2020 data include outbreaks from
November 16, 2019 to December 27, 2020; 2021 data include outbreaks from December 8, 2020 to December 8, 2021; and 2022 data include outbreaks from December 9, 2021 to September 9, 2022.
Table 1
HPAI outbreaks in Europe, 2018–2022. The table shows data for HPAI outbreaks
reported by ECDC in the past 5 years. Domestic birds include poultry and captive
birds. The main strain refers to the most prevalent HPAI strain reported during that
period. (https://www.ecdc.europa.eu/en/publications-data/surveillance report avian
influenza overview).
Number of HPAI infected birds
Time..
DOI record:
{
"DOI": "10.1016/j.jinf.2022.10.002",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2022.10.002",
"alternative-id": [
"S016344532200562X"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy of Paxlovid in patients with acute kidney injury who developed COVID-19"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2022.10.002"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The British Infection Association. Published by Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3728-2681",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cai",
"given": "Hong",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yan",
"given": "Jiayi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Jieying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Che",
"given": "Xiajing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mou",
"given": "Shan",
"sequence": "additional"
}
],
"container-title": "Journal of Infection",
"container-title-short": "Journal of Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"journalofinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
8
]
],
"date-time": "2022-10-08T05:54:50Z",
"timestamp": 1665208490000
},
"deposited": {
"date-parts": [
[
2023,
1,
17
]
],
"date-time": "2023-01-17T00:15:35Z",
"timestamp": 1673914535000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"81970574",
"82170685"
],
"doi-asserted-by": "publisher",
"name": "National Natural Science Foundation of China"
},
{
"DOI": "10.13039/501100004921",
"doi-asserted-by": "publisher",
"name": "Shanghai Jiao Tong University"
},
{
"DOI": "10.13039/501100008233",
"doi-asserted-by": "publisher",
"name": "School of Medicine, Shanghai Jiao Tong University"
},
{
"DOI": "10.13039/100017950",
"award": [
"18ZXY001",
"ZXYXZ-201904"
],
"doi-asserted-by": "publisher",
"name": "Shanghai Municipal Health Commission"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
8
]
],
"date-time": "2024-08-08T07:05:04Z",
"timestamp": 1723100704964
},
"is-referenced-by-count": 7,
"issue": "6",
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S016344532200562X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S016344532200562X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "702-769",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30374-3",
"article-title": "WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?",
"author": "Heymann",
"doi-asserted-by": "crossref",
"first-page": "542",
"issue": "10224",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2022.10.002_bib0001",
"volume": "395",
"year": "2020"
},
{
"article-title": "Efficacy and safety of Paxlovid for COVID-19: a meta-analysis",
"author": "Zheng",
"journal-title": "J Inf",
"key": "10.1016/j.jinf.2022.10.002_bib0002",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge",
"author": "Arbel",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2022.10.002_bib0003",
"year": "2022"
},
{
"DOI": "10.1681/ASN.2020050615",
"article-title": "Mount Sinai COVID Informatics Center (MSCIC). AKI in Hospitalized patients with COVID-19",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "151",
"issue": "1",
"journal-title": "J Am Soc Nephrol.",
"key": "10.1016/j.jinf.2022.10.002_bib0004",
"volume": "32",
"year": "2021"
}
],
"reference-count": 4,
"references-count": 4,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S016344532200562X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of Paxlovid in patients with acute kidney injury who developed COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "85"
}