Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir
et al., Viruses, doi:10.3390/v14040670, Mar 2022
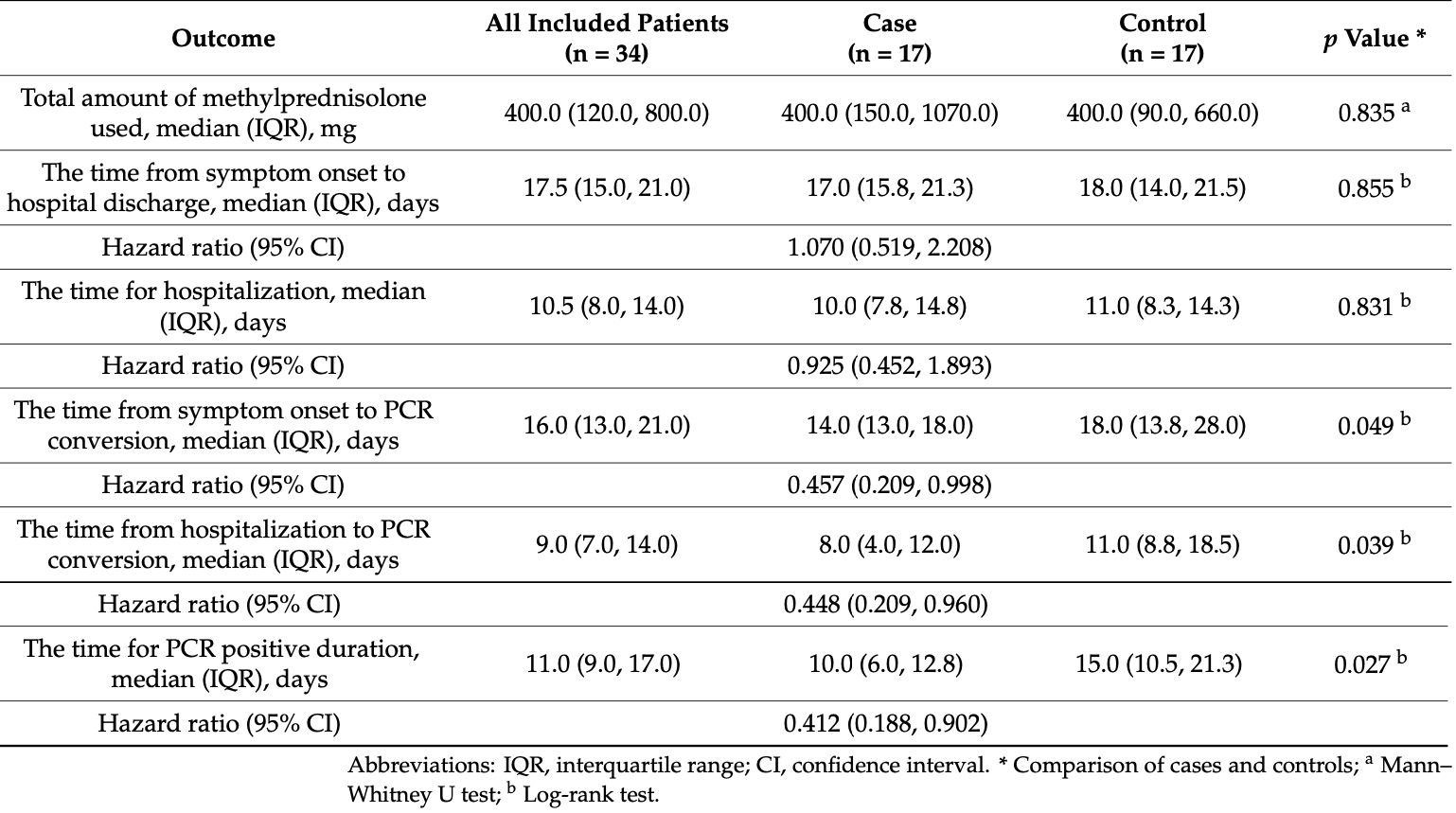
Retrospective 17 COVID+ patients treated with favipiravir and 17 matched controls in Japan, showing faster viral clearance with treatment. Favipiravir 3600mg day one, 1600mg per day for up to 14 days.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
hospitalization time, 7.5% lower, HR 0.93, p = 0.84, treatment 17, control 17.
|
|
viral clearance time, 55.2% lower, HR 0.45, p = 0.04, treatment 17, control 17.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Shinada et al., 24 Mar 2022, retrospective, Japan, peer-reviewed, 11 authors, study period 28 May, 2020 - 26 September, 2020, average treatment delay 8.9 days.
Contact: takashisato1220@gmail.com (corresponding author), kanakoshinada7@gmail.com, mshinopy@gmail.com, shin.ohta0915@gmail.com, miwapicco@outlook.jp, shinkai050169@gmail.com, m-mine@marianna-u.ac.jp, sayamrym@niid.go.jp, yuadachi@niid.go.jp, matt@niid.go.jp, ytakahas@niid.go.jp.
Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir
Viruses, doi:10.3390/v14040670
The effect of treatment with favipiravir, an antiviral purine nucleoside analog, for coronavirus disease 2019 (COVID-19) on the production and duration of neutralizing antibodies for SARS-CoV-2 was explored. There were 17 age-, gender-, and body mass index-matched pairs of favipiravir treated versus control selected from a total of 99 patients recovered from moderate COVID-19. These subjects participated in the longitudinal (>6 months) analysis of (i) SARS-CoV-2 spike protein's receptor-binding domain IgG, (ii) virus neutralization assay using authentic virus, and (iii) neutralization potency against original (WT) SARS-CoV-2 and cross-neutralization against B.1.351 (beta) variant carrying triple mutations of K417N, E484K, and N501Y. The results demonstrate that the use of favipiravir: (1) significantly accelerated the elimination of SARS-CoV-2 in the case vs. control groups (p = 0.027), (2) preserved the generation and persistence of neutralizing antibodies in the host, and (3) did not interfere the maturation of neutralizing potency of anti-SARS-CoV-2 and neutralizing breadth against SARS-CoV-2 variants. In conclusion, treatment of COVID-19 with favipiravir accelerates viral clearance and does not interfere the generation or maturation of neutralizing potency against both WT SARS-CoV-2 and its variants.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Tokyo Shinagawa Hospital (approval number 20-A-08 and approved on 20 May 2020). Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
Alqahtani, Oyelade, Aldhahir, Alghamdi, Almehmadi et al., Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis, PLoS ONE, doi:10.1371/journal.pone.0233147
Altay, Mohammadi, Lam, Turkez, Boren et al., Current Status of COVID-19 Therapies and Drug Repositioning Applications, iScience, doi:10.1016/j.isci.2020.101303
Bosnjak, Stein, Willenzon, Cordes, Puppe et al., Low serum neutralizing anti-SARS-CoV-2 S antibody levels in mildly affected COVID-19 convalescent patients revealed by two different detection methods, Cell. Mol. Immunol, doi:10.1038/s41423-020-00573-9
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering, doi:10.1016/j.eng.2020.03.007
Clinicaltrials, Gov, Favipiravir for Patients With Mild to Moderate Disease from Novel Coronavirus (COVID-19
Doi, Hibino, Hase, Yamamoto, Kasamatsu et al., Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrob. Agents Chemother, doi:10.1128/AAC.01897-20
Edalatifard, Akhtari, Salehi, Naderi, Jamshidi et al., Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial, Eur. Respir. J, doi:10.1183/13993003.02808-2020
Fujifilm, Fujifilm Announces the Start of a New Phase III Clinical Trial of Anti-Influenza Drug Avigan®Tablet in Japan, Targeting COVID-19 Patients
Garcia-Beltran, Lam, Astudillo, Yang, Miller et al., COVID-19-neutralizing antibodies predict disease severity and survival, Cell, doi:10.1016/j.cell.2020.12.015
Gluck, Grobecker, Tydykov, Salzberger, Gluck et al., SARS-CoV-2-directed antibodies persist for more than six months in a cohort with mild to moderate COVID-19, Infection, doi:10.1007/s15010-021-01598-6
Goto, Go, Miyakawa, Yamaoka, Ohtake et al., Sustained Neutralizing Antibodies 6 Months Following Infection in 376 Japanese COVID-19 Survivors, Front. Microbiol, doi:10.3389/fmicb.2021.661187
Gottlieb, Nirula, Chen, Boscia, Heller et al., Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.0202
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials, Sci. Rep
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in Hospitalized Patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Jeronimo, Farias, Val, Sampaio, Alexandre et al., Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease, Viruses
Lau, Tsang, Hui, Kwan, Chan et al., Neutralizing antibody titres in SARS-CoV-2 infections, Nat. Commun, doi:10.1038/s41467-020-20247-4
Marois, Cloutier, Garneau, Lesur, Richter, The administration of oseltamivir results in reduced effector and memory CD8+ T cell responses to influenza and affects protective immunity, FASEB J, doi:10.1096/fj.14-260687
Metcovid, A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial, Clin. Infect. Dis
Moriyama, Adachi, Sato, Tonouchi, Sun et al., Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants, Immunity, doi:10.1016/j.immuni.2021.06.015
Ong, Chiew, Ang, Mak, Cui et al., Clinical and virological features of SARS-CoV-2 variants of concern: A retrospective cohort study comparing B.1.1.7, Clin. Infect. Dis, doi:10.1093/cid/ciab721
Ranjbar, Moghadami, Mirahmadizadeh, Fallahi, Khaloo et al., Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: A triple-blinded randomized controlled trial, BMC Infect. Dis, doi:10.1186/s12879-021-06045-3
Salton, Confalonieri, Meduri, Santus, Harari et al., Prolonged Low-Dose Methylprednisolone in Patients With Severe COVID-19 Pneumonia, Open Forum Infect. Dis
Shinahara, Takahashi, Sawabuchi, Arai, Hirotsu et al., Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: A retrospective analysis, PLoS ONE, doi:10.1371/journal.pone.0070060
Shinkai, Tsushima, Tanaka, Hagiwara, Tarumoto et al., Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial, Infect. Dis. Ther, doi:10.1007/s40121-021-00517-4
Takahashi, Kataoka, Fujii, Chida, Mizuno et al., Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus, Microbes Infect, doi:10.1016/j.micinf.2010.04.013
Tegally, Wilkinson, Giovanetti, Iranzadeh, Fonseca et al., Detection of a SARS-CoV-2 variant of concern in South Africa, Nature, doi:10.1038/s41586-021-03402-9
Torjesen, COVID-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear, BMJ, doi:10.1136/bmj.n2943
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.11.142
Volz, Mishra, Chand, Barrett, Johnson et al., Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England, Nature, doi:10.1038/s41586-021-03470-x
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2035002
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors associated with COVID-19-related death using OpenSAFELY, Nature, doi:10.1038/s41586-020-2521-4
Wu, Liang, Chen, Wang, Fang et al., SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19, Nat. Commun, doi:10.1038/s41467-021-22034-1
Yu, Tostanoski, Peter, Mercado, Mcmahan et al., DNA vaccine protection against SARS-CoV-2 in rhesus macaques, Science, doi:10.1126/science.abc6284
DOI record:
{
"DOI": "10.3390/v14040670",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v14040670",
"abstract": "<jats:p>The effect of treatment with favipiravir, an antiviral purine nucleoside analog, for coronavirus disease 2019 (COVID-19) on the production and duration of neutralizing antibodies for SARS-CoV-2 was explored. There were 17 age-, gender-, and body mass index-matched pairs of favipiravir treated versus control selected from a total of 99 patients recovered from moderate COVID-19. These subjects participated in the longitudinal (>6 months) analysis of (i) SARS-CoV-2 spike protein’s receptor-binding domain IgG, (ii) virus neutralization assay using authentic virus, and (iii) neutralization potency against original (WT) SARS-CoV-2 and cross-neutralization against B.1.351 (beta) variant carrying triple mutations of K417N, E484K, and N501Y. The results demonstrate that the use of favipiravir: (1) significantly accelerated the elimination of SARS-CoV-2 in the case vs. control groups (p = 0.027), (2) preserved the generation and persistence of neutralizing antibodies in the host, and (3) did not interfere the maturation of neutralizing potency of anti-SARS-CoV-2 and neutralizing breadth against SARS-CoV-2 variants. In conclusion, treatment of COVID-19 with favipiravir accelerates viral clearance and does not interfere the generation or maturation of neutralizing potency against both WT SARS-CoV-2 and its variants.</jats:p>",
"alternative-id": [
"v14040670"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0697-6659",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shinada",
"given": "Kanako",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-2676-3781",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sato",
"given": "Takashi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2057-9198",
"affiliation": [],
"authenticated-orcid": false,
"family": "Moriyama",
"given": "Saya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adachi",
"given": "Yu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0288-5637",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shinoda",
"given": "Masahiro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0922-8656",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ota",
"given": "Shinichiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morikawa",
"given": "Miwa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mineshita",
"given": "Masamichi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1760-3484",
"affiliation": [],
"authenticated-orcid": false,
"family": "Matsumura",
"given": "Takayuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takahashi",
"given": "Yoshimasa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shinkai",
"given": "Masaharu",
"sequence": "additional"
}
],
"container-title": [
"Viruses"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
25
]
],
"date-time": "2022-03-25T03:31:43Z",
"timestamp": 1648179103000
},
"deposited": {
"date-parts": [
[
2022,
3,
25
]
],
"date-time": "2022-03-25T05:20:34Z",
"timestamp": 1648185634000
},
"funder": [
{
"DOI": "10.13039/100009619",
"award": [
"JP19fk0108104, JP20fk0108104"
],
"doi-asserted-by": "publisher",
"name": "Japan Agency for Medical Research and Development"
}
],
"indexed": {
"date-parts": [
[
2022,
3,
31
]
],
"date-time": "2022-03-31T22:57:45Z",
"timestamp": 1648767465618
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1999-4915"
}
],
"issue": "4",
"issued": {
"date-parts": [
[
2022,
3,
24
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
24
]
],
"date-time": "2022-03-24T00:00:00Z",
"timestamp": 1648080000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/14/4/670/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "670",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
3,
24
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
24
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "COVID-19 Weekly Epidemiological Updatehttps://covid19.who.int/"
},
{
"DOI": "10.1016/j.isci.2020.101303",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1128/AAC.01897-20",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"key": "ref5"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"key": "ref7"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"key": "ref9",
"unstructured": "Fujifilm Announces the Start of a New Phase III Clinical Trial of Anti-Influenza Drug Avigan® Tablet in Japan, Targeting COVID-19 Patientshttps://www.fujifilm.com/jp/en/news/hq/6478"
},
{
"key": "ref10",
"unstructured": "Phase III Clinical Trial of Favipiravir in Early-Onset COVID-19 Patients with Severe Risk Factors-Placebo-Controlled, Stratified Randomized, Multicenter, Double-Blind Studyhttps://jrct.niph.go.jp/en-latest-detail/jRCT2041210004"
},
{
"DOI": "10.1096/fj.14-260687",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1371/journal.pone.0070060",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.micinf.2010.04.013",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1126/science.abc6284",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1001/jama.2021.0202",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1038/s41423-020-00573-9",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1038/s41467-021-22034-1",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/j.cell.2020.12.015",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.immuni.2021.06.015",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1093/cid/ciab721",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1038/s41586-021-03402-9",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1136/bmj.n2943",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1038/s41586-021-03470-x",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"key": "ref25",
"unstructured": "Clinical Management of Patients with COVID-19: A Guide for Front-Line Healthcare Workers (Version 2.1)https://www.mhlw.go.jp/content/000646531.pdf"
},
{
"key": "ref26",
"unstructured": "Multicenter, Adaptive, Randomized, Placebo-Controlled, Comparative Study to Evaluate the Efficacy and Safety of Favipiravir in Patients with COVID-19 Non-Severe Pneumoniahttps://www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-205238"
},
{
"key": "ref27"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1183/13993003.02808-2020",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1093/ofid/ofaa421",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1371/journal.pone.0233147",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1186/s12879-021-06045-3",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1093/cid/ciaa1177",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1007/s15010-021-01598-6",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1038/s41467-020-20247-4",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.3389/fmicb.2021.661187",
"doi-asserted-by": "publisher",
"key": "ref38"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/14/4/670"
}
},
"score": 1,
"short-container-title": [
"Viruses"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": [
"Longitudinal Analysis of Neutralizing Potency against SARS-CoV-2 in the Recovered Patients after Treatment with or without Favipiravir"
],
"type": "journal-article",
"volume": "14"
}