Oral antivirals for COVID-19 among patients with cancer
et al., Research Square, doi:10.21203/rs.3.rs-3876022/v1, Jan 2024
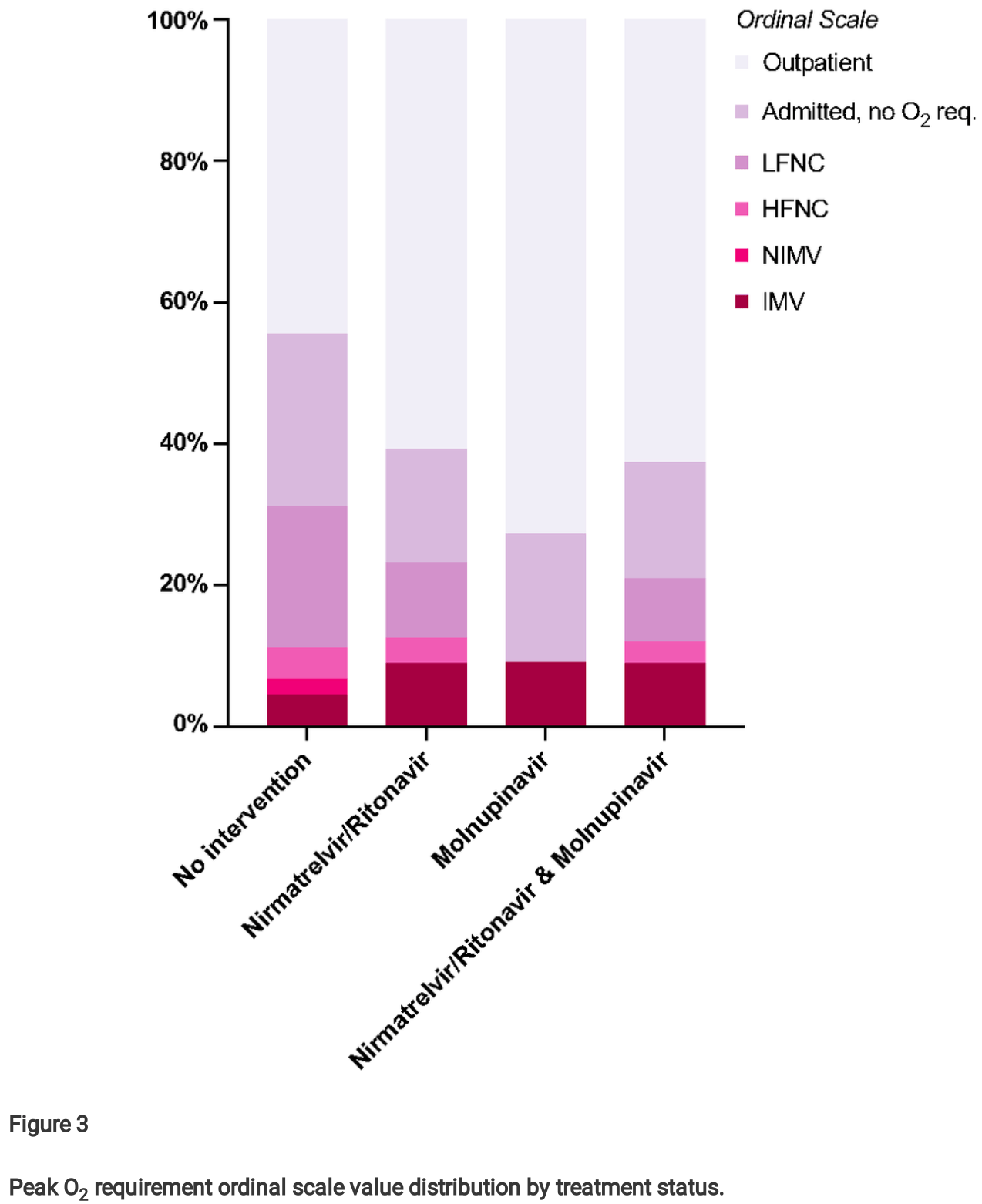
Retrospective 67 cancer outpatients treated with nirmatrelvir/ritonavir or molnupiravir, compared to 56 untreated concurrent controls, reporting lower mortality with treatment. However, Figure 3 shows the opposite results for invasive mechanical ventilation, ~2 times higher for the treatment groups versus the control group. The discrepancy suggests that the groups are not comparable in some key aspect such as treatment availability or decisions, baseline conditions, and/or prevalence of non-COVID-19 related death. ECOG ≥2 was much more common for controls vs. treated patients. One possibility is that controls had more advanced existing disease and were more likely to choose not to receive life-sustaining treatments. The higher use of remdesivir for controls may also indicate greater severity and/or more delayed treatment based on prescribing guidelines, and may also contribute to increased mortality due to side effects within a more vulnerable population. Remdesivir shows increased mortality with longer followup1.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity2-16. Multiple analyses have identified variants potentially created by molnupiravir17-21. Studies show significantly increased risk of acute kidney injury22, cardiovascular toxocity23, and neurological symptoms22. Treatment may increase viral rebound24,25.
Study covers molnupiravir and paxlovid.
2.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
3.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
4.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
5.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
6.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
7.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
8.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
9.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
10.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
11.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
12.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
13.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
14.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
15.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
16.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
17.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
18.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
19.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
20.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
22.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
23.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Guermazi et al., 24 Jan 2024, retrospective, preprint, 5 authors, study period 1 April, 2020 - 1 August, 2023.
Contact: dorra_guermazi@brown.edu.
Oral antivirals for COVID-19 among patients with cancer
doi:10.21203/rs.3.rs-3876022/v1
Purpose: Immunocompromised individuals, such as those diagnosed with cancer, are at a signi cantly higher risk for severe illness and mortality when infected with SARS-CoV-2 (COVID-19) than the general population. Two oral antiviral treatments are approved for COVID-19: Paxlovid® (nirmatrelvir/ritonavir) and Lagevrio® (molnupiravir). There is a paucity of data regarding the bene t from these antivirals among immunocompromised patients with cancer, and recent studies have questioned their e cacy among vaccinated patients, even those with risk factors for severe COVID-19. Methods: We evaluated the e cacy and safety of nirmatrelvir/ritonavir and molnupiravir in preventing severe illness and death using our database of 457 patients with cancer and COVID-19 from Brown University-a liated hospitals. 67 patients received nirmatrelvir/ritonavir or molnupiravir and were compared to 56 concurrent controls who received no antiviral treatment despite being eligible to receive it. Results: Administration of nirmatrelvir/ritonavir or molnupiravir was associated with improved survival and lower 90-day all-cause and COVID-19-attributed mortality (p<0.05) and with lower peak O2 requirements (ordinal odds ratio [OR] 1.52, 95% con dence interval [CI] 0.92-2.56). Conclusion: Acknowledging the small size of our sample as a limitation, we concluded that early antiviral treatment might be bene cial to immunocompromised individuals, particularly those with cancer, when infected with SARS-CoV-2. Larger-scale, well-strati ed studies are needed in this patient population.
Declarations Competing Interests DF has received research support from Viracor, Astellas and Merck, and consultant fee from Viracor. All other authors have nothing to disclose.
Ethics approval: The study was approved by the Lifespan Institutional Review Board (IRB). The study was conducted in accordance with the declaration of Helsinki.
Consent to publish: All authors agreed to the publication of the manuscript.
Consent to participate: The study was approved by the Lifespan Institutional Review Board (IRB) with a waiver of informed consent given its retrospective design and de-identi ed data.
Supplementary Files This is a list of supplementary les associated with this preprint. Click to download. SupplementaryMaterial.pdf
References
Anwar, Nguyen, Nagasaka, Ou, Chan, Overview of Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Targeted Therapy and Supportive Care for Lung Cancer, JTO Clin Res Rep, doi:10.1016/j.jtocrr.2022.100452
Arayici, Effects of SARS-CoV-2 infections in patients with cancer on mortality, ICU admission and incidence: a systematic review with meta-analysis involving 709,908 participants and 31,732 cancer patients, J Cancer Res Clin Oncol, doi:10.1007/s00432-022-04191-y
Arvanitis, Lerner, Vieira, Almaghlouth, Farmakiotis, Outpatient anti-spike monoclonal antibody administration is associated with decreased morbidity and mortality among patients with cancer and COVID-19, Clin Exp Med, doi:10.1007/s10238-023-01019-y
Bernal, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Butler, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platformadaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Choueiri, Breakthrough SARS-CoV-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: a retrospective observational study from the COVID-19 and Cancer Consortium, Lancet Reg Health Am, doi:10.1016/j.lana.2023.100445
Dodd, Freidlin, Korn, Platform Trials -Beware the Noncomparable Control Group, N Engl J Med, doi:10.1056/NEJMc2102446
Dormuth, Kim, Fisher, Piszczek, Kuo, Nirmatrelvir-Ritonavir and COVID-19 Mortality and Hospitalization Among Patients With Vulnerability to COVID-19 Complications, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.36678
Dryden-Peterson, Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System: A Population-Based Cohort Study, Ann Intern Med, doi:10.7326/m22-2141
Elkrief, Learning through a Pandemic: The Current State of Knowledge on COVID-19 and Cancer, Cancer Discov, doi:10.1158/2159-8290.Cd-21-1368
Eng, Disposition of Nirmatrelvir, an Orally Bioavailable Inhibitor of SARS-CoV-2 3C-Like Protease, across Animals and Humans, Drug Metab Dispos, doi:10.1124/dmd.121.000801
Farley, New Drug Application (NDA) 217188: PAXLOVID (nirmatrelvir tablets; ritonavir tablets), copackaged, FDA
Farmakiotis, COVID-19 Treatments for Nonhospitalized Patients, JAMA, doi:10.1001/jama.2022.6167
Fendler, Functional immune responses against SARS-CoV-2 variants of concern after fourth COVID-19 vaccine dose or infection in patients with blood cancer, Cell Rep Med, doi:10.1016/j.xcrm.2022.100781
Gentry, Nguyen, Thind, Kurdgelashvili, Williams, Characteristics and outcomes of US Veterans with immunocompromised conditions at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents, Clin Infect Dis, doi:10.1093/cid/ciad504
Gleeson, Kidney Transplant Recipients and Omicron: Outcomes, effect of vaccines and the e cacy and safety of novel treatments, medRxiv, doi:10.1101/2022.05.03.22274524
Grivas, Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium, Ann Oncol, doi:10.1016/j.annonc.2021.02.024
Guermazi, Arvanitis, Farmakiotis, Molnupiravir e cacy among immunocompromised patients with COVID-19: no proof of concept, Infection, doi:10.1007/s15010-023-02027-6
Hammond, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2118542
Johnson, Molnupiravir for the treatment of COVID-19 in immunocompromised participants: e cacy, safety, and virology results from the phase 3 randomized, placebo-controlled MOVe-OUT trial, Infection, doi:10.1007/s15010-022-01959-9
Kneidinger, Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study, Infection, doi:10.1007/s15010-022-01914-8
Lin, Chemotherapy Treatment Modi cations During the COVID-19 Outbreak at a Community Cancer Center in New York City, JCO Glob Oncol, doi:10.1200/go.20.00309
Liu, E cacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study, Lancet Reg Health West Pac, doi:10.1016/j.lanwpc.2023.100694
Malin, Weibel, Gruell, Kreuzberger, Stegemann et al., E cacy and safety of molnupiravir for the treatment of SARS-CoV-2 infection: a systematic review and meta-analysis, J Antimicrob Chemother, doi:10.1093/jac/dkad132
Paraskevis, Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in high-risk patients, J Infect Dis, doi:10.1093/infdis/jiad324
Radcliffe, Palacios, Azar, Cohen, Malinis, Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge, Am J Transplant, doi:10.1111/ajt.17098
Rusnak, PAXLOVID (nirmatrelvir / ritonavir): Main Protease Inhibitor of SARS-CoV-2 Corona Virus
Schmidt, COVID-19 vaccination and breakthrough infections in patients with cancer, Ann Oncol, doi:10.1016/j.annonc.2021.12.006
Sun, Lin, Wang, Gao, Ye, Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection, Lancet Infect Dis, doi:10.1016/s1473-3099(22)00430-3
Vaishampayan, Parchment, Jasti, Hussain, Taxanes: an overview of the pharmacokinetics and pharmacodynamics, Urology, doi:10.1016/s0090-4295(99)00451-3
Yao, Ding, Burchell, Wolf, Friedberg, Detoxication of vinca alkaloids by human P450 CYP3A4-mediated metabolism: implications for the development of drug resistance, J Pharmacol Exp Ther
Zhang, Yang, Zhou, Wei, Ma, Network Pharmacology and Bioinformatics Analysis Identi es Potential Therapeutic Targets of Paxlovid Against LUAD/COVID-19, Front Endocrinol, doi:10.3389/fendo.2022.935906
DOI record:
{
"DOI": "10.21203/rs.3.rs-3876022/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-3876022/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Purpose:\nImmunocompromised individuals, such as those diagnosed with cancer, are at a significantly higher risk for severe illness and mortality when infected with SARS-CoV-2 (COVID-19) than the general population. Two oral antiviral treatments are approved for COVID-19: Paxlovid® (nirmatrelvir/ritonavir) and Lagevrio® (molnupiravir). There is a paucity of data regarding the benefit from these antivirals among immunocompromised patients with cancer, and recent studies have questioned their efficacy among vaccinated patients, even those with risk factors for severe COVID-19.\nMethods:\nWe evaluated the efficacy and safety of nirmatrelvir/ritonavir and molnupiravir in preventing severe illness and death using our database of 457 patients with cancer and COVID-19 from Brown University-affiliated hospitals. 67 patients received nirmatrelvir/ritonavir or molnupiravir and were compared to 56 concurrent controls who received no antiviral treatment despite being eligible to receive it.\nResults:\nAdministration of nirmatrelvir/ritonavir or molnupiravir was associated with improved survival and lower 90-day all-cause and COVID-19-attributed mortality (p<0.05) and with lower peak O2 requirements (ordinal odds ratio [OR] 1.52, 95% confidence interval [CI] 0.92-2.56).\nConclusion:\nAcknowledging the small size of our sample as a limitation, we concluded that early antiviral treatment might be beneficial to immunocompromised individuals, particularly those with cancer, when infected with SARS-CoV-2. Larger-scale, well-stratified studies are needed in this patient population.</jats:p>",
"accepted": {
"date-parts": [
[
2024,
1,
18
]
]
},
"author": [
{
"affiliation": [
{
"name": "Brown University"
}
],
"family": "Guermazi",
"given": "Dorra",
"sequence": "first"
},
{
"affiliation": [
{
"name": "The Warren Alpert Medical School of Brown University"
}
],
"family": "Arvanitis",
"given": "Panos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Warren Alpert Medical School of Brown University"
}
],
"family": "Vieira",
"given": "Kendra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rhode Island Hospital"
}
],
"family": "Warner",
"given": "Jeremy L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Warren Alpert Medical School of Brown University"
}
],
"family": "Farmakiotis",
"given": "Dimitrios",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
1,
24
]
],
"date-time": "2024-01-24T18:57:57Z",
"timestamp": 1706122677000
},
"deposited": {
"date-parts": [
[
2024,
1,
24
]
],
"date-time": "2024-01-24T18:57:59Z",
"timestamp": 1706122679000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2024,
1,
25
]
],
"date-time": "2024-01-25T00:30:16Z",
"timestamp": 1706142616549
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
1,
24
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
24
]
],
"date-time": "2024-01-24T00:00:00Z",
"timestamp": 1706054400000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-3876022/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-3876022/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2024,
1,
24
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2024,
1,
24
]
]
},
"publisher": "Research Square Platform LLC",
"reference": [
{
"DOI": "10.1007/s00432-022-04191-y",
"article-title": "\"Effects of SARS-CoV-2 infections in patients with cancer on mortality, ICU admission and incidence: a systematic review with meta-analysis involving 709,908 participants and 31,732 cancer patients,\" (in eng)",
"author": "Arayici ME",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "J Cancer Res Clin Oncol",
"key": "ref1",
"unstructured": "M. E. Arayici et al., \"Effects of SARS-CoV-2 infections in patients with cancer on mortality, ICU admission and incidence: a systematic review with meta-analysis involving 709,908 participants and 31,732 cancer patients,\" (in eng), J Cancer Res Clin Oncol, pp. 1–14, Jul 13 2022, doi: 10.1007/s00432-022-04191-y.",
"year": "2022"
},
{
"DOI": "10.1016/j.xcrm.2022.100781",
"article-title": "\"Functional immune responses against SARS-CoV-2 variants of concern after fourth COVID-19 vaccine dose or infection in patients with blood cancer,\" (in eng)",
"author": "Fendler A",
"doi-asserted-by": "publisher",
"first-page": "100781",
"issue": "10",
"journal-title": "Cell Rep Med",
"key": "ref2",
"unstructured": "A. Fendler et al., \"Functional immune responses against SARS-CoV-2 variants of concern after fourth COVID-19 vaccine dose or infection in patients with blood cancer,\" (in eng), Cell Rep Med, vol. 3, no. 10, p. 100781, Oct 18 2022, doi: 10.1016/j.xcrm.2022.100781.",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1158/2159-8290.Cd-21-1368",
"article-title": "\"Learning through a Pandemic: The Current State of Knowledge on COVID-19 and Cancer,\" (in eng)",
"author": "Elkrief A",
"doi-asserted-by": "publisher",
"first-page": "303",
"issue": "2",
"journal-title": "Cancer Discov",
"key": "ref3",
"unstructured": "A. Elkrief et al., \"Learning through a Pandemic: The Current State of Knowledge on COVID-19 and Cancer,\" (in eng), Cancer Discov, vol. 12, no. 2, pp. 303–330, Feb 2022, doi: 10.1158/2159-8290.Cd-21-1368.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "\"Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19,\"",
"author": "Hammond J",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "New England Journal of Medicine",
"key": "ref4",
"unstructured": "J. Hammond et al., \"Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19,\" New England Journal of Medicine, vol. 386, no. 15, pp. 1397–1408, 2022, doi: 10.1056/NEJMoa2118542.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "\"Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients,\" (in eng)",
"author": "Jayk Bernal A",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "ref5",
"unstructured": "A. Jayk Bernal et al., \"Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients,\" (in eng), N Engl J Med, vol. 386, no. 6, pp. 509–520, Feb 10 2022, doi: 10.1056/NEJMoa2116044.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.7326/m22-2141",
"author": "Dryden-Peterson S",
"doi-asserted-by": "publisher",
"key": "ref6",
"unstructured": "S. Dryden-Peterson et al., \"Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System: A Population-Based Cohort Study,\" (in eng), Ann Intern Med, vol. 176, no. 1, pp. 77–84, Jan 2023, doi: 10.7326/m22-2141.",
"year": "2023"
},
{
"DOI": "10.1093/infdis/jiad324",
"article-title": "\"Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in high-risk patients,\" (in eng)",
"author": "Paraskevis D",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Dis, Aug",
"key": "ref7",
"unstructured": "D. Paraskevis et al., \"Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in high-risk patients,\" (in eng), J Infect Dis, Aug 11 2023, doi: 10.1093/infdis/jiad324.",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1007/s15010-022-01959-9",
"author": "Johnson MG",
"doi-asserted-by": "publisher",
"key": "ref8",
"unstructured": "M. G. Johnson et al., \"Molnupiravir for the treatment of COVID-19 in immunocompromised participants: efficacy, safety, and virology results from the phase 3 randomized, placebo-controlled MOVe-OUT trial,\" Infection, vol. 51, no. 5, pp. 1273–1284, 2023/10/01 2023, doi: 10.1007/s15010-022-01959-9.",
"year": "2023"
},
{
"DOI": "10.1111/ajt.17098",
"article-title": "Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge,\" (in eng)",
"author": "Radcliffe C",
"doi-asserted-by": "publisher",
"first-page": "2458",
"issue": "10",
"journal-title": "Am J Transplant",
"key": "ref9",
"unstructured": "C. Radcliffe, C. F. Palacios, M. M. Azar, E. Cohen, and M. Malinis, \"Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge,\" (in eng), Am J Transplant, vol. 22, no. 10, pp. 2458–2463, Oct 2022, doi: 10.1111/ajt.17098.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1101/2022.05.03.22274524",
"author": "Gleeson S",
"doi-asserted-by": "publisher",
"key": "ref10",
"unstructured": "S. Gleeson et al., \"Kidney Transplant Recipients and Omicron: Outcomes, effect of vaccines and the efficacy and safety of novel treatments,\" medRxiv, p. 2022.05.03.22274524, 2022, doi: 10.1101/2022.05.03.22274524.",
"year": "2022"
},
{
"DOI": "10.1007/s15010-023-02027-6",
"author": "Guermazi D",
"doi-asserted-by": "publisher",
"key": "ref11",
"unstructured": "D. Guermazi, P. Arvanitis, and D. Farmakiotis, \"Molnupiravir efficacy among immunocompromised patients with COVID-19: no proof of concept,\" (in eng), Infection, vol. 51, no. 5, pp. 1593–1595, Oct 2023, doi: 10.1007/s15010-023-02027-6.",
"year": "2023"
},
{
"DOI": "10.3389/fendo.2022.935906",
"article-title": "Network Pharmacology and Bioinformatics Analysis Identifies Potential Therapeutic Targets of Paxlovid Against LUAD/COVID-19,\" (in eng)",
"author": "Zhang W",
"doi-asserted-by": "publisher",
"first-page": "935906",
"journal-title": "Front Endocrinol (Lausanne)",
"key": "ref12",
"unstructured": "W. Zhang, Z. Yang, F. Zhou, Y. Wei, and X. Ma, \"Network Pharmacology and Bioinformatics Analysis Identifies Potential Therapeutic Targets of Paxlovid Against LUAD/COVID-19,\" (in eng), Front Endocrinol (Lausanne), vol. 13, p. 935906, 2022, doi: 10.3389/fendo.2022.935906.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.jtocrr.2022.100452",
"article-title": "Overview of Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Targeted Therapy and Supportive Care for Lung Cancer,\" (in eng)",
"author": "Anwar K",
"doi-asserted-by": "publisher",
"first-page": "100452",
"issue": "2",
"journal-title": "JTO Clin Res Rep",
"key": "ref13",
"unstructured": "K. Anwar, L. Nguyen, M. Nagasaka, S. I. Ou, and A. Chan, \"Overview of Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Targeted Therapy and Supportive Care for Lung Cancer,\" (in eng), JTO Clin Res Rep, vol. 4, no. 2, p. 100452, Feb 2023, doi: 10.1016/j.jtocrr.2022.100452.",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1001/jama.2022.6167",
"author": "Farmakiotis D",
"doi-asserted-by": "publisher",
"key": "ref14",
"unstructured": "D. Farmakiotis, \"COVID-19 Treatments for Nonhospitalized Patients,\" JAMA, vol. 327, no. 22, pp. 2247–2247, 2022, doi: 10.1001/jama.2022.6167.",
"year": "2022"
},
{
"DOI": "10.1016/j.lanwpc.2023.100694",
"author": "Liu J",
"doi-asserted-by": "publisher",
"key": "ref15",
"unstructured": "J. Liu et al., \"Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study,\" (in eng), Lancet Reg Health West Pac, vol. 33, p. 100694, Apr 2023, doi: 10.1016/j.lanwpc.2023.100694.",
"year": "2023"
},
{
"DOI": "10.1093/jac/dkad132",
"article-title": "Efficacy and safety of molnupiravir for the treatment of SARS-CoV-2 infection: a systematic review and meta-analysis,\" (in eng)",
"author": "Malin JJ",
"doi-asserted-by": "publisher",
"first-page": "1586",
"issue": "7",
"journal-title": "J Antimicrob Chemother",
"key": "ref16",
"unstructured": "J. J. Malin, S. Weibel, H. Gruell, N. Kreuzberger, M. Stegemann, and N. Skoetz, \"Efficacy and safety of molnupiravir for the treatment of SARS-CoV-2 infection: a systematic review and meta-analysis,\" (in eng), J Antimicrob Chemother, vol. 78, no. 7, pp. 1586–1598, Jul 5 2023, doi: 10.1093/jac/dkad132.",
"volume": "78",
"year": "2023"
},
{
"author": "Rusnak J",
"key": "ref17",
"unstructured": "J. Rusnak, \"PAXLOVID (nirmatrelvir / ritonavir): Main Protease Inhibitor of SARS-CoV-2 Corona Virus,\" A. D. A. Committee, Ed., ed, March 16, 2023, p. https://www.fda.gov/media/166238/download.",
"year": "2023"
},
{
"author": "Farley J",
"key": "ref18",
"unstructured": "J. Farley, \"New Drug Application (NDA) 217188: PAXLOVID (nirmatrelvir tablets; ritonavir tablets), co-packaged \", ed. FDA, March 16, 2023, p. https://www.fda.gov/media/166237/download."
},
{
"DOI": "10.1007/s10238-023-01019-y",
"article-title": "Outpatient anti-spike monoclonal antibody administration is associated with decreased morbidity and mortality among patients with cancer and COVID-19,\" (in eng)",
"author": "Arvanitis P",
"doi-asserted-by": "publisher",
"first-page": "2739",
"issue": "6",
"journal-title": "Clin Exp Med",
"key": "ref19",
"unstructured": "P. Arvanitis, A. H. Lerner, K. Vieira, N. Almaghlouth, and D. Farmakiotis, \"Outpatient anti-spike monoclonal antibody administration is associated with decreased morbidity and mortality among patients with cancer and COVID-19,\" (in eng), Clin Exp Med, vol. 23, no. 6, pp. 2739–2748, Oct 2023, doi: 10.1007/s10238-023-01019-y.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/s1473-3099(22)00430-3",
"author": "Sun F",
"doi-asserted-by": "publisher",
"key": "ref20",
"unstructured": "F. Sun, Y. Lin, X. Wang, Y. Gao, and S. Ye, \"Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection,\" (in eng), Lancet Infect Dis, vol. 22, no. 9, p. 1279, Sep 2022, doi: 10.1016/s1473-3099(22)00430-3.",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2023.36678",
"article-title": "\"Nirmatrelvir-Ritonavir and COVID-19 Mortality and Hospitalization Among Patients With Vulnerability to COVID-19 Complications,\" (in eng)",
"author": "Dormuth CR",
"doi-asserted-by": "publisher",
"first-page": "e2336678",
"issue": "10",
"journal-title": "JAMA Netw Open",
"key": "ref21",
"unstructured": "C. R. Dormuth, J. D. Kim, A. Fisher, J. Piszczek, and I. F. Kuo, \"Nirmatrelvir-Ritonavir and COVID-19 Mortality and Hospitalization Among Patients With Vulnerability to COVID-19 Complications,\" (in eng), JAMA Netw Open, vol. 6, no. 10, p. e2336678, Oct 2 2023, doi: 10.1001/jamanetworkopen.2023.36678.",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1016/j.annonc.2021.02.024",
"article-title": "\"Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium,\"",
"author": "Grivas P",
"doi-asserted-by": "publisher",
"first-page": "787",
"issue": "6",
"journal-title": "Ann Oncol",
"key": "ref22",
"unstructured": "P. Grivas et al., \"Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium,\" Ann Oncol, vol. 32, no. 6, pp. 787–800, Jun 2021, doi: 10.1016/j.annonc.2021.02.024.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/j.lana.2023.100445",
"article-title": "\"Breakthrough SARS-CoV-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: a retrospective observational study from the COVID-19 and Cancer Consortium,\"",
"author": "Choueiri TK",
"doi-asserted-by": "publisher",
"first-page": "100445",
"journal-title": "Lancet Reg Health Am",
"key": "ref23",
"unstructured": "T. K. Choueiri et al., \"Breakthrough SARS-CoV-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: a retrospective observational study from the COVID-19 and Cancer Consortium,\" Lancet Reg Health Am, vol. 19, p. 100445, Mar 2023, doi: 10.1016/j.lana.2023.100445.",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1016/j.annonc.2021.12.006",
"article-title": "\"COVID-19 vaccination and breakthrough infections in patients with cancer,\"",
"author": "Schmidt AL",
"doi-asserted-by": "publisher",
"first-page": "340",
"issue": "3",
"journal-title": "Ann Oncol",
"key": "ref24",
"unstructured": "A. L. Schmidt et al., \"COVID-19 vaccination and breakthrough infections in patients with cancer,\" Ann Oncol, vol. 33, no. 3, pp. 340–346, Mar 2022, doi: 10.1016/j.annonc.2021.12.006.",
"volume": "33",
"year": "2022"
},
{
"DOI": "10.1124/dmd.121.000801",
"author": "Eng H",
"doi-asserted-by": "publisher",
"key": "ref25",
"unstructured": "H. Eng et al., \"Disposition of Nirmatrelvir, an Orally Bioavailable Inhibitor of SARS-CoV-2 3C-Like Protease, across Animals and Humans,\" (in eng), Drug Metab Dispos, vol. 50, no. 5, pp. 576–590, May 2022, doi: 10.1124/dmd.121.000801.",
"year": "2022"
},
{
"DOI": "10.1016/s0090-4295(99)00451-3",
"author": "Vaishampayan U",
"doi-asserted-by": "publisher",
"key": "ref26",
"unstructured": "U. Vaishampayan, R. E. Parchment, B. R. Jasti, and M. Hussain, \"Taxanes: an overview of the pharmacokinetics and pharmacodynamics,\" (in eng), Urology, vol. 54, no. 6A Suppl, pp. 22 – 9, Dec 1999, doi: 10.1016/s0090-4295(99)00451-3."
},
{
"author": "Yao D",
"key": "ref27",
"unstructured": "D. Yao, S. Ding, B. Burchell, C. R. Wolf, and T. Friedberg, \"Detoxication of vinca alkaloids by human P450 CYP3A4-mediated metabolism: implications for the development of drug resistance,\" (in eng), J Pharmacol Exp Ther, vol. 294, no. 1, pp. 387 – 95, Jul 2000.",
"year": "2000"
},
{
"DOI": "10.1200/go.20.00309",
"author": "Lin DD",
"doi-asserted-by": "publisher",
"key": "ref28",
"unstructured": "D. D. Lin et al., \"Chemotherapy Treatment Modifications During the COVID-19 Outbreak at a Community Cancer Center in New York City,\" (in eng), JCO Glob Oncol, vol. 6, pp. 1298–1305, Aug 2020, doi: 10.1200/go.20.00309.",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"author": "Butler CC",
"doi-asserted-by": "publisher",
"key": "ref29",
"unstructured": "C. C. Butler et al., \"Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial,\" Lancet, vol. 401, no. 10373, pp. 281–293, Jan 28 2023, doi: 10.1016/S0140-6736(22)02597-1."
},
{
"DOI": "10.1093/cid/ciad504",
"article-title": "Characteristics and outcomes of US Veterans with immunocompromised conditions at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents,\"",
"author": "Gentry CA",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis, Aug",
"key": "ref30",
"unstructured": "C. A. Gentry, P. N. Nguyen, S. K. Thind, G. Kurdgelashvili, and R. J. Williams, \"Characteristics and outcomes of US Veterans with immunocompromised conditions at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents,\" Clin Infect Dis, Aug 24 2023, doi: 10.1093/cid/ciad504.",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1007/s15010-022-01914-8",
"article-title": "\"Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study,\" (in eng), Infection",
"author": "Kneidinger N",
"doi-asserted-by": "publisher",
"first-page": "749",
"issue": "3",
"key": "ref31",
"unstructured": "N. Kneidinger et al., \"Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study,\" (in eng), Infection, vol. 51, no. 3, pp. 749–757, Jun 2023, doi: 10.1007/s15010-022-01914-8.",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2102446",
"article-title": "\"Platform Trials - Beware the Noncomparable Control Group,\"",
"author": "Dodd LE",
"doi-asserted-by": "publisher",
"first-page": "1572",
"journal-title": "N Engl J Med",
"key": "ref32",
"unstructured": "L. E. Dodd, B. Freidlin, and E. L. Korn, \"Platform Trials - Beware the Noncomparable Control Group,\" N Engl J Med, vol. 384, no. 16, pp. 1572–1573, Apr 22 2021, doi: 10.1056/NEJMc2102446.",
"volume": "384",
"year": "2021"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-3876022/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Oral antivirals for COVID-19 among patients with cancer",
"type": "posted-content"
}