Efficacy of Molnupiravir in Reducing the Risk of Severe Outcomes in Patients with SARS-CoV-2 Infection: A Real-Life Full-Matched Case–Control Study (SAVALO Study)
et al., Microorganisms, doi:10.3390/microorganisms13030669, SAVALO, Sep 2024 (preprint)
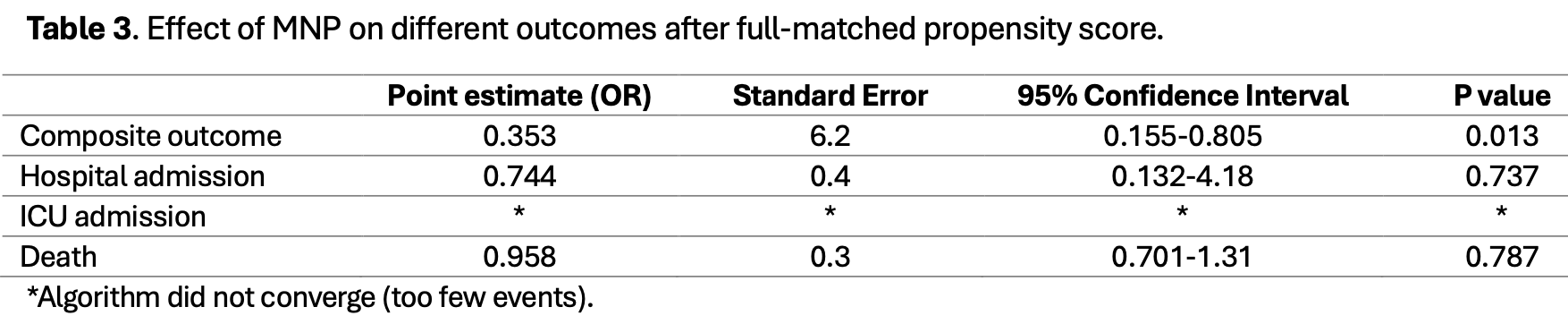
PSM retrospective case-control study with 1,382 SARS-CoV-2 positive outpatients in Italy, showing lower risk for a composite outcome of hospitalization, ICU admission, or death with molnupiravir, but no significant difference for mortality or hospitalization.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending molnupiravir also recommended them, or
because the patient seeking out molnupiravir is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity4-18. Multiple analyses have identified variants potentially created by molnupiravir19-23. Studies show significantly increased risk of acute kidney injury24, cardiovascular toxocity25, and neurological symptoms24. Treatment may increase viral rebound26,27.
|
risk of death, 4.2% lower, OR 0.96, p = 0.79, treatment 146, control 1,236, case control OR, propensity score matching.
|
|
risk of hospitalization, 25.6% lower, OR 0.74, p = 0.74, treatment 146, control 1,236, case control OR, propensity score matching.
|
|
hospitalization, ICU, mortality, 64.7% lower, OR 0.35, p = 0.01, treatment 146, control 1,236, case control OR, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
5.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
6.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
7.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
8.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
9.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
10.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
11.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
12.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
13.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
14.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
15.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
16.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
17.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
18.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
19.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
20.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
21.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
22.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
24.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
25.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Gentile et al., 9 Sep 2024, retrospective, Italy, peer-reviewed, 19 authors, study period 1 January, 2022 - 31 December, 2022, SAVALO trial.
Contact: riccardo.scotto@unina.it (corresponding author), ri.scotto@gmail.com, ivan.gentile@unina.it, mmichelascirocco@hotmail.com, francescodibrizzi@gmail.com, federicacuccurullo94@gmail.com, mariasilvitelli94@gmail.com, luigi.ametrano@outlook.com, francescoantimoalfe@gmail.com, pietroluongo.daria@gmail.com, irisirene@hotmail.it, chiariello.mr@outlook.it, noemidefelice@gmail.com, simoneseverino5f@gmail.com, giulio.viceconte@gmail.com, veghan@gmail.com, albertomaraolo@yahoo.com, antonioriccardobuonomo@gmail.com, agnesegiaccone94@gmail.com.
Efficacy of Molnupiravir in Reducing the Risk of Severe Outcomes in Patients with SARS-CoV-2 Infection: A Real-Life Full-Matched Case–Control Study (SAVALO Study)
Microorganisms, doi:10.3390/microorganisms13030669
We conducted a real-life case-control study among outpatients with Omicron SARS-CoV-2 infection to assess the effectiveness of molnupiravir (MNP) in reducing hospital admission, admission to the intensive care unit, and death at day 28. Cases were SARS-CoV-2-positive patients seeking medical care within five days of symptom onset from 1 January to 31 December 2022, who received MNP. Controls were selected from a regional database among positive subjects who did not receive antiviral treatment for SARS-CoV-2. A total of 1382 patients were included (146 cases, 1236 controls). Vaccinated patients had a lower risk of mortality and of the composite outcome (hospital admission, ICU admission, or all-cause death) than unvaccinated ones (0.6% vs. 7.8%, p < 0.001 and 2% vs. 7.8%, p = 0.001, respectively). After full-matching propensity score analysis, MNPtreated subjects had a lower incidence of the composite outcome, although no effect was observed on individual outcomes. In subgroup analyses by vaccination status, MNP was effective in preventing all outcomes among unvaccinated patients and reduced the risk of ICU admission in both vaccinated and unvaccinated patients. Molnupiravir treatment effectively reduced the composite outcome risk in outpatients with SARS-CoV-2 infection, with a more pronounced benefit in unvaccinated patients. These findings highlight MNP's potential to help prevent disease progression in high-risk patients, thereby supporting its role as an outpatient therapeutic option for COVID-19.
Supplementary Materials: The following supporting information can be downloaded at: https: //www.mdpi.com/article/10.3390/microorganisms13030669/s1 , Supplementary File S1: SAVALO Study telephone interview and case report form. Supplementary Table S1 : Number of patients stratified and outcome rates among included patients according to vaccination status. Supplementary Figure S1 Informed Consent Statement: Informed consent was obtained from all patients included in the study. For control patients, consent was obtained via telephone interview, a method that was rigorously reviewed and approved by the ethics committee to ensure it met all ethical standards. Informed consent from case patients was obtained in written form. Records of the telephone interviews with control participants, including their verbal consent, are securely stored by the study's data manager at the Department of Clinical Medicine and Surgery, University of Naples Federico II, Via Sergio Pansini 5, 80131, Naples, Italy. These records are available for review by any relevant authority upon reasonable request.
Data Availability Statement: The datasets generated and/or analyzed during the current study, along with the study's Case Report Forms (CRFs), the written informed consent from case participants, and Conflicts of Interest: Ivan Gentile reports personal fees from MSD, AbbVie, Gilead, Pfizer, GSK, SOBI, Nordic/Infecto Pharm, Angelini, and Abbott, as well as departmental grants from Gilead and support..
References
Baek, Park, Won, Park, Kim, Propensity score matching: A conceptual review for radiology researchers, Korean J. Radiol, doi:10.3348/kjr.2015.16.2.286
Bajema, Berry, Streja, Rajeevan, Li et al., Effectiveness of COVID-19 Treatment With Nirmatrelvir-Ritonavir or Molnupiravir Among U.S. Veterans: Target Trial Emulation Studies With One-Month and Six-Month Outcomes, Ann. Intern. Med, doi:10.7326/M22-3565
Bernal, Gomes Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N. Engl. J. Med, doi:10.1056/NEJMoa2116044
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Eikenboom, Le Cessie, Waernbaum, Groenwold, De Boer, Quality of Conduct and Reporting of Propensity Score Methods in Studies Investigating the Effectiveness of Antimicrobial Therapy, Open Forum Infect. Dis, doi:10.1093/ofid/ofac110
Gandhi, Hirsch, Treating Acute Covid-19-Final Chapters Still Unwritten, N. Engl. J. Med, doi:10.1056/NEJMe2402224
Green, Stuart, Examining moderation analyses in propensity score methods: Application to depression and substance use, J. Consult. Clin. Psychol, doi:10.1037/a0036515
Hansen, Klopfer, Optimal Full Matching and Related Designs via Network Flows, J. Comput. Graph. Stat, doi:10.1198/106186006X137047
Harris, Holmes, Gbinigie-Thompson, Rahman, Richards et al., Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): A randomised controlled trial, Lancet Infect. Dis, doi:10.1016/S1473-3099(24)00431-6
Hu, Jia, Zhao, Fu, Zhang et al., Clinical outcomes of the severe acute respiratory syndrome coronavirus 2 Omicron and Delta variant: Systematic review and meta-analysis of 33 studies covering 6 037 144 coronavirus disease 2019-positive patients, Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis, doi:10.1016/j.cmi.2023.03.017
Karniadakis, Mazonakis, Tsioutis, Papadakis, Markaki et al., Oral Molnupiravir and Nirmatrelvir/Ritonavir for the Treatment of COVID-19: A Literature Review with a Focus on Real-World Evidence, Infect. Dis. Rep, doi:10.3390/idr15060061
Khalifa, Al Ramahi, After the Hurricane: Anti-COVID-19 Drugs Development, Molecular Mechanisms of Action and Future Perspectives, Int. J. Mol. Sci, doi:10.3390/ijms25020739
Li, Wang, Chen, Lu, Song et al., Estimands in observational studies: Some considerations beyond ICH E9 (R1), Pharm Stat, doi:10.1002/pst.2196
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Molnupiravir in High-Risk Patients: A Propensity Score Matched Analysis, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am, doi:10.1093/cid/ciac781
Nguyen, Collins, Spence, Daurès, Devereaux et al., Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance, BMC Med. Res. Methodol, doi:10.1186/s12874-017-0338-0
Paraskevis, Gkova, Mellou, Gerolymatos, Psalida et al., Real-world Effectiveness of Molnupiravir and Nirmatrelvir/Ritonavir as Treatments for COVID-19 in Patients at High Risk, J. Infect. Dis, doi:10.1093/infdis/jiad324
Razonable, Ganesh, Bierle, Clinical Prioritization of Antispike Monoclonal Antibody Treatment of Mild to Moderate COVID-19, Mayo Clin. Proc, doi:10.1016/j.mayocp.2021.11.017
Richmond Dibello, Raziano, Liu, Puenpatom, Peebles et al., Molnupiravir Use Among Patients with COVID-19 in Real-World Settings: A Systematic Literature Review, Infect. Dis. Ther, doi:10.1007/s40121-024-00976-5
Snowden, Rose, Mortimer, Implementation of G-computation on a simulated data set: Demonstration of a causal inference technique, Am. J. Epidemiol, doi:10.1093/aje/kwq472
Thomas, Li, Pencina, Using Propensity Score Methods to Create Target Populations in Observational Clinical Research, JAMA, doi:10.1001/jama.2019.21558
Whitley, Molnupiravir-A Step toward Orally Bioavailable Therapies for Covid-19, N. Engl. J. Med
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: A retrospective cohort study, Lancet. Infect. Dis, doi:10.1016/S1473-3099(22)00507-2
Yip, Lui, Lai, Wong, Tse et al., Impact of the Use of Oral Antiviral Agents on the Risk of Hospitalization in Community Coronavirus Disease 2019 Patients (COVID-19), Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am, doi:10.1093/cid/ciac687
DOI record:
{
"DOI": "10.3390/microorganisms13030669",
"ISSN": [
"2076-2607"
],
"URL": "http://dx.doi.org/10.3390/microorganisms13030669",
"abstract": "<jats:p>We conducted a real-life case–control study among outpatients with Omicron SARS-CoV-2 infection to assess the effectiveness of molnupiravir (MNP) in reducing hospital admission, admission to the intensive care unit, and death at day 28. Cases were SARS-CoV-2-positive patients seeking medical care within five days of symptom onset from 1 January to 31 December 2022, who received MNP. Controls were selected from a regional database among positive subjects who did not receive antiviral treatment for SARS-CoV-2. A total of 1382 patients were included (146 cases, 1236 controls). Vaccinated patients had a lower risk of mortality and of the composite outcome (hospital admission, ICU admission, or all-cause death) than unvaccinated ones (0.6% vs. 7.8%, p < 0.001 and 2% vs. 7.8%, p = 0.001, respectively). After full-matching propensity score analysis, MNP-treated subjects had a lower incidence of the composite outcome, although no effect was observed on individual outcomes. In subgroup analyses by vaccination status, MNP was effective in preventing all outcomes among unvaccinated patients and reduced the risk of ICU admission in both vaccinated and unvaccinated patients. Molnupiravir treatment effectively reduced the composite outcome risk in outpatients with SARS-CoV-2 infection, with a more pronounced benefit in unvaccinated patients. These findings highlight MNP’s potential to help prevent disease progression in high-risk patients, thereby supporting its role as an outpatient therapeutic option for COVID-19.</jats:p>",
"alternative-id": [
"microorganisms13030669"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-5199-8451",
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Gentile",
"given": "Ivan",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-0178-2986",
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Scotto",
"given": "Riccardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Scirocco",
"given": "Maria Michela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Di Brizzi",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Cuccurullo",
"given": "Federica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Silvitelli",
"given": "Maria",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4181-4938",
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Ametrano",
"given": "Luigi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Alfè",
"given": "Francesco Antimo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Pietroluongo",
"given": "Daria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Irace",
"given": "Irene",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0002-9723-6767",
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Chiariello",
"given": "Mariarosaria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "De Felice",
"given": "Noemi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Severino",
"given": "Simone",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"family": "Viceconte",
"given": "Giulio",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8474-4776",
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Schiano Moriello",
"given": "Nicola",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7218-7762",
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Maraolo",
"given": "Alberto Enrico",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4378-8366",
"affiliation": [
{
"name": "Department of Clinical Medicine and Surgery—Section of Infectious Diseases, University of Naples Federico II, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Buonomo",
"given": "Antonio Riccardo",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4002-0930",
"affiliation": [
{
"name": "Department of Infectious Diseases, Unit of Geriatric Infectious Diseases, AORN Ospedali dei Colli, Cotugno Hospital, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Giaccone",
"given": "Agnese",
"sequence": "additional"
},
{
"affiliation": [],
"name": "on behalf of Federico II COVID Team",
"sequence": "additional"
}
],
"container-title": "Microorganisms",
"container-title-short": "Microorganisms",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
3,
17
]
],
"date-time": "2025-03-17T12:57:02Z",
"timestamp": 1742216222000
},
"deposited": {
"date-parts": [
[
2025,
3,
17
]
],
"date-time": "2025-03-17T13:44:39Z",
"timestamp": 1742219079000
},
"funder": [
{
"award": [
"CUPE63C22001830002"
],
"name": "Campania Region as part of the European Fund for Regional Development"
}
],
"indexed": {
"date-parts": [
[
2025,
3,
18
]
],
"date-time": "2025-03-18T04:11:33Z",
"timestamp": 1742271093730,
"version": "3.40.1"
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2025,
3,
15
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2025,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
15
]
],
"date-time": "2025-03-15T00:00:00Z",
"timestamp": 1741996800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-2607/13/3/669/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "669",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
3,
15
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
15
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref_1",
"unstructured": "(2024, August 14). WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c."
},
{
"DOI": "10.1093/infdis/jiad324",
"article-title": "Real-world Effectiveness of Molnupiravir and Nirmatrelvir/Ritonavir as Treatments for COVID-19 in Patients at High Risk",
"author": "Paraskevis",
"doi-asserted-by": "crossref",
"first-page": "1667",
"journal-title": "J. Infect. Dis.",
"key": "ref_2",
"volume": "228",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"article-title": "Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: A retrospective cohort study",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1681",
"journal-title": "Lancet. Infect. Dis.",
"key": "ref_3",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "Musungaie",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N. Engl. J. Med.",
"key": "ref_4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMe2117814",
"article-title": "Molnupiravir—A Step toward Orally Bioavailable Therapies for Covid-19",
"author": "Whitley",
"doi-asserted-by": "crossref",
"first-page": "592",
"journal-title": "N. Engl. J. Med.",
"key": "ref_5",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3390/ijms25020739",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Khalifa, H.O., and Al Ramahi, Y.M. (2024). After the Hurricane: Anti-COVID-19 Drugs Development, Molecular Mechanisms of Action and Future Perspectives. Int. J. Mol. Sci., 25."
},
{
"DOI": "10.3390/idr15060061",
"article-title": "Oral Molnupiravir and Nirmatrelvir/Ritonavir for the Treatment of COVID-19: A Literature Review with a Focus on Real-World Evidence",
"author": "Karniadakis",
"doi-asserted-by": "crossref",
"first-page": "662",
"journal-title": "Infect. Dis. Rep.",
"key": "ref_7",
"volume": "15",
"year": "2023"
},
{
"key": "ref_8",
"unstructured": "FDA (2024, August 14). Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults. 23 December 2021, Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain."
},
{
"key": "ref_9",
"unstructured": "AIFA Recommendations on Medicines to be Used in Home Management of COVID-19 Cases (2024, August 14). Vers. 7—Updated 09/02/2022, Available online: https://www.aifa.gov.it/documents/20142/1269602/EN_Raccomandazioni_AIFA_gestione_domiciliare_COVID-19_Vers7_09.02.2022.pdf."
},
{
"DOI": "10.1016/j.mayocp.2021.11.017",
"article-title": "Clinical Prioritization of Antispike Monoclonal Antibody Treatment of Mild to Moderate COVID-19",
"author": "Razonable",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "Mayo Clin. Proc.",
"key": "ref_10",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1001/jama.2019.21558",
"article-title": "Using Propensity Score Methods to Create Target Populations in Observational Clinical Research",
"author": "Thomas",
"doi-asserted-by": "crossref",
"first-page": "466",
"journal-title": "JAMA",
"key": "ref_11",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofac110",
"article-title": "Quality of Conduct and Reporting of Propensity Score Methods in Studies Investigating the Effectiveness of Antimicrobial Therapy",
"author": "Eikenboom",
"doi-asserted-by": "crossref",
"first-page": "ofac110",
"journal-title": "Open Forum Infect. Dis.",
"key": "ref_12",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.3348/kjr.2015.16.2.286",
"article-title": "Propensity score matching: A conceptual review for radiology researchers",
"author": "Baek",
"doi-asserted-by": "crossref",
"first-page": "286",
"journal-title": "Korean J. Radiol.",
"key": "ref_13",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1198/106186006X137047",
"article-title": "Optimal Full Matching and Related Designs via Network Flows",
"author": "Hansen",
"doi-asserted-by": "crossref",
"first-page": "609",
"journal-title": "J. Comput. Graph. Stat.",
"key": "ref_14",
"volume": "15",
"year": "2006"
},
{
"DOI": "10.1002/pst.2196",
"article-title": "Estimands in observational studies: Some considerations beyond ICH E9 (R1)",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "835",
"journal-title": "Pharm Stat",
"key": "ref_15",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1093/aje/kwq472",
"article-title": "Implementation of G-computation on a simulated data set: Demonstration of a causal inference technique",
"author": "Snowden",
"doi-asserted-by": "crossref",
"first-page": "731",
"journal-title": "Am. J. Epidemiol.",
"key": "ref_16",
"volume": "173",
"year": "2011"
},
{
"DOI": "10.1186/s12874-017-0338-0",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Nguyen, T.L., Collins, G.S., Spence, J., Daurès, J.P., Devereaux, P.J., Landais, P., and Le Manach, Y. (2017). Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Med. Res. Methodol., 17."
},
{
"DOI": "10.1037/a0036515",
"article-title": "Examining moderation analyses in propensity score methods: Application to depression and substance use",
"author": "Green",
"doi-asserted-by": "crossref",
"first-page": "773",
"journal-title": "J. Consult. Clin. Psychol.",
"key": "ref_18",
"volume": "82",
"year": "2014"
},
{
"article-title": "Clinical outcomes of the severe acute respiratory syndrome coronavirus 2 Omicron and Delta variant: Systematic review and meta-analysis of 33 studies covering 6 037 144 coronavirus disease 2019-positive patients",
"author": "Hu",
"first-page": "835",
"journal-title": "Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis.",
"key": "ref_19",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Lancet",
"key": "ref_20",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(24)00431-6",
"article-title": "Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): A randomised controlled trial",
"author": "Harris",
"doi-asserted-by": "crossref",
"first-page": "68",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_21",
"volume": "25",
"year": "2025"
},
{
"DOI": "10.7326/M22-3565",
"article-title": "Effectiveness of COVID-19 Treatment With Nirmatrelvir-Ritonavir or Molnupiravir Among U.S. Veterans: Target Trial Emulation Studies With One-Month and Six-Month Outcomes",
"author": "Bajema",
"doi-asserted-by": "crossref",
"first-page": "807",
"journal-title": "Ann. Intern. Med.",
"key": "ref_22",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac687",
"article-title": "Impact of the Use of Oral Antiviral Agents on the Risk of Hospitalization in Community Coronavirus Disease 2019 Patients (COVID-19)",
"author": "Yip",
"doi-asserted-by": "crossref",
"first-page": "e26",
"journal-title": "Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am.",
"key": "ref_23",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac781",
"article-title": "Effectiveness of Molnupiravir in High-Risk Patients: A Propensity Score Matched Analysis",
"author": "Gronich",
"doi-asserted-by": "crossref",
"first-page": "453",
"journal-title": "Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am.",
"key": "ref_24",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1007/s40121-024-00976-5",
"article-title": "Molnupiravir Use Among Patients with COVID-19 in Real-World Settings: A Systematic Literature Review",
"author": "Raziano",
"doi-asserted-by": "crossref",
"first-page": "1177",
"journal-title": "Infect. Dis. Ther.",
"key": "ref_25",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1056/NEJMe2402224",
"article-title": "Treating Acute Covid-19—Final Chapters Still Unwritten",
"author": "Gandhi",
"doi-asserted-by": "crossref",
"first-page": "1234",
"journal-title": "N. Engl. J. Med.",
"key": "ref_26",
"volume": "390",
"year": "2024"
},
{
"key": "ref_27",
"unstructured": "Italian Medicines Agency (AIFA) (2024, August 14). Sospensione di Utilizzo del Medicinale Lagevrio® (Molnupiravir), Available online: https://www.aifa.gov.it/-/sospensione_utilizzo_lagevrio_molnupiravir."
},
{
"key": "ref_28",
"unstructured": "European Medicines Agency (2024, August 14). Refusal of the Marketing Authorisation for Lagevrio (Molnupiravir). EMA/82948/2023 Rev.1 EMEA/H/C/005789. Available online: https://www.ema.europa.eu/en/documents/smop-initial/questions-and-answers-refusal-marketing-authorisation-lagevrio-molnupiravir_en.pdf."
},
{
"key": "ref_29",
"unstructured": "CDC—COVID-19 (2024, August 14). Clinical Course: Progression, Management, and Treatment. 13 June 2024, Available online: https://www.cdc.gov/covid/hcp/clinical-care/management-and-treatment.html."
},
{
"key": "ref_30",
"unstructured": "(2022). WHO Guidelines Approved by the Guidelines Review Committee. Therapeutics and COVID-19: Living Guideline, World Health Organization."
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-2607/13/3/669"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of Molnupiravir in Reducing the Risk of Severe Outcomes in Patients with SARS-CoV-2 Infection: A Real-Life Full-Matched Case–Control Study (SAVALO Study)",
"type": "journal-article",
"volume": "13"
}