Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC)
et al., Phytomedicine, doi:10.1016/j.phymed.2024.156279, Jul 2024 (preprint)
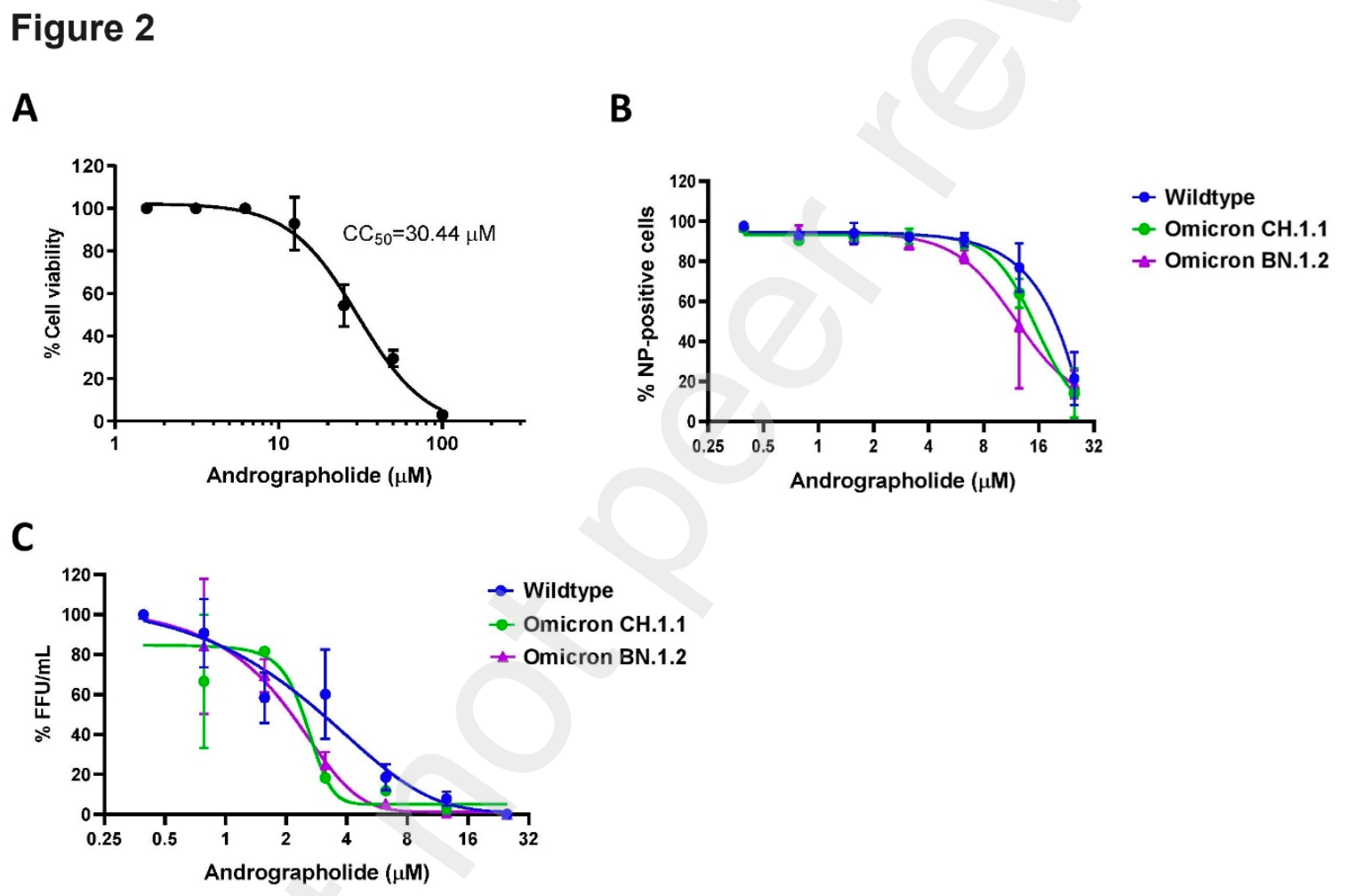
In vitro study showing andrographolide attenuates infection of SARS-CoV-2 wildtype and omicron variants in human lung epithelial cells and monkey kidney cells. Proteomic analysis revealed andrographolide induces expression of the glutathione synthesizing enzyme GCLC and the anti-oxidative stress response in infected cells. Ectopic over-expression of GCLC or treatment with the glutathione precursor N-acetylcysteine decreased SARS-CoV-2 infection, suggesting the glutathione pathway mediates the antiviral effects of andrographolide.
25 preclinical studies support the efficacy of andrographolide for COVID-19:
In vitro studies demonstrate inhibition of the MproA,18 protein.
In vitro studies demonstrate efficacy in Calu-3B,18, A549C,14, and HUVECD,18 cells.
Animal studies demonstrate efficacy in Sprague Dawley miceE,18 and Golden Syrian hamstersF,14.
Andrographolide inhibits Mpro in a dose-dependent manner18, reduces ACE2 levels in the lung tissue of mice in combination with baicalein18, inhibits binding between the SARS-CoV-2 spike protein and ACE218, alleviates lung inflammation and cytokine storm in mice18, and improves survival and reduces lung inflammation via anti-inflammatory effects in Syrian hamsters14.
Study covers andrographolide and N-acetylcysteine.
1.
Zhang et al., Effects and Mechanisms of Andrographolide for COVID-19: A Network Pharmacology-Based and Experimentally Validated Study, Natural Product Communications, doi:10.1177/1934578X241288428.
2.
Thomas et al., Cheminformatics approach to identify andrographolide derivatives as dual inhibitors of methyltransferases (nsp14 and nsp16) of SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-58532-7.
3.
Arifin et al., Computational exploration of Andrographis paniculata herb compounds as potential antiviral agents targeting NSP3 (6W02) and NSP5 (7AR6) of SARS-COV-2, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2023.25.2.0292.
4.
Bhattarai et al., Investigating the binding affinity of andrographolide against human SARS-CoV-2 spike receptor-binding domain through docking and molecular dynamics simulations, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2174596.
5.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
6.
Dassanayake et al., Molecular Docking and In-Silico Analysis of Natural Biomolecules against Dengue, Ebola, Zika, SARS-CoV-2 Variants of Concern and Monkeypox Virus, International Journal of Molecular Sciences, doi:10.3390/ijms231911131.
7.
Ningrum et al., Potency Of Andrographolide, L-Mimosine And Asiaticoside Compound As Antiviral For Covid-19 Based On In Silico Method, Proceedings Universitas Muhammadiyah Yogyakarta Undergraduate Conference, doi:10.18196/umygrace.v2i2.418.
8.
Ravichandran et al., Identification of Potential Semisynthetic Andrographolide Derivatives to Combat COVID-19 by Targeting the SARS-COV-2 Spike Protein and Human ACE2 Receptor– An In-silico Approach, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC132.155.
9.
Saeheng et al., In Silico Prediction of Andrographolide Dosage Regimens for COVID-19 Treatment, The American Journal of Chinese Medicine, doi:10.1142/S0192415X22500732.
10.
Khanal et al., Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19, RSC Advances, doi:10.1039/D0RA10529E.
11.
Rehan et al., A Computational Approach Identified Andrographolide as a Potential Drug for Suppressing COVID-19-Induced Cytokine Storm, Frontiers in Immunology, doi:10.3389/fimmu.2021.648250.
12.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
13.
Dey et al., The role of andrographolide and its derivative in COVID-19 associated proteins and immune system, Research Square, doi:10.21203/rs.3.rs-35800/v1.
14.
Kongsomros et al., In vivo evaluation of Andrographis paniculata and Boesenbergia rotunda extract activity against SARS-CoV-2 Delta variant in Golden Syrian hamsters: Potential herbal alternative for COVID-19 treatment, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2024.05.004.
15.
Chaopreecha et al., Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC), Phytomedicine, doi:10.1016/j.phymed.2024.156279.
16.
Li et al., Andrographolide suppresses SARS-CoV-2 infection by downregulating ACE2 expression: A mechanistic study, Antiviral Therapy, doi:10.1177/13596535241259952.
17.
Low et al., The wide spectrum anti-inflammatory activity of andrographolide in comparison to NSAIDs: a promising therapeutic compound against the cytokine storm, bioRxiv, doi:10.1101/2024.02.21.581396.
18.
Wan et al., Synergistic inhibition effects of andrographolide and baicalin on coronavirus mechanisms by downregulation of ACE2 protein level, Scientific Reports, doi:10.1038/s41598-024-54722-5.
19.
Siridechakorn et al., Inhibitory efficiency of Andrographis paniculata extract on viral multiplication and nitric oxide production, Scientific Reports, doi:10.1038/s41598-023-46249-y.
a.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
b.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
c.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
d.
HUVEC (Human Umbilical Vein Endothelial Cells) are primary endothelial cells derived from the vein of the umbilical cord. They are used to study vascular biology, including inflammation, angiogenesis, and viral interactions with endothelial cells.
e.
An outbred multipurpose breed of albino mouse used extensively in medical research.
f.
A rodent model widely used in infectious disease research due to their susceptibility to viral infections and similar disease progression to humans.
Chaopreecha et al., 1 Jul 2024, peer-reviewed, 9 authors.
Contact: arunee.thi@mahidol.edu (corresponding author), patompon.won@mahidol.ac.th.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Proteomic analysis identifies the glutathione synthesizing enzyme GCLC as an andrographolide target and a protective factor against SARS-CoV-2 infection
Even though advanced progresses have been made for COVID-19 vaccines, rapid and extensive mutations of the SARS-CoV-2 genomes have provided a selective advantage for the virus to escape the adaptive immunity leading to a critical challenge for current treatments and preventions of COVID-19. We report here the antiviral activity of andrographolide against SARS-CoV-2 wildtype and Omicron variants. Proteomic analysis was employed to identify cellular pathways and key proteins controlled by andrographolide in the human lung epithelial cells Calu-3 infected by SARS-CoV-2. Gene ontology analysis indicates that proteins involved in NRF2-regulated pathways are differentially expressed by andrographolide. Notably, andrographolide increases expression and nuclear localization of the transcription factor NRF2. In addition, transcriptional expression of GCLC and GCLM, which are NRF2 target genes, are induced by andrographolide. We further find that infection of SARS-CoV-2 results in a reduction of glutathione level in Calu-3; the effect that is rescued by andrographolide. Moreover, andrographolide also induces expression of the glutathione producing enzyme GCLC in SARS-CoV-2 infected lung epithelial cells. Importantly, an ectopic over-expression of GCLC or treatment of N-acetylcysteine in Calu-3 cells led to a decrease in SARS-CoV-2 infection. Collectively, our findings suggest the interplay between GCLC-mediated glutathione biogenesis induced by andrographolide and the anti-SARS-CoV-2 activity. The glutathione biogenesis and recycling pathways should be further exploited as a targeted therapy against SARS-CoV-2 infection.
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This preprint research paper has not been peer reviewed. Electronic copy available at: https://ssrn.com/abstract=4876852 P r e p r i n t n o t p e e r r e v i e w e d This preprint research paper has not been peer reviewed. Electronic copy available at: https://ssrn.com/abstract=4876852 P r e p r i n t n o t p e e r r e v i e w e d Supplementary Table 1 . Pathway analysis of up-regulated proteins upon andrographolide treatment in Calu-3 infected with SARS-CoV-2 versus to SARS-CoV-2 infection as shown in Figure 3D . The analysis was conducted using Reactome analysis.
Pathway
References
Bartolini, Stabile, Bastianelli, Giustarini, Pierucci et al., SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione, Redox Biol, doi:10.1016/j.redox.2021.102041
Burgos, Alarcon, Quiroga, Manosalva, Hancke, Andrographolide, an Anti-Inflammatory Multitarget Drug: All Roads Lead to Cellular Metabolism, Molecules, doi:10.3390/molecules26010005
Che, Zhou, Liu, Xie, Liu, Andrographolide exerts anti-respiratory syncytial virus activity by up-regulating heme oxygenase-1 independent of interferon responses in human airway epithelial cells, Mol Biol Rep, doi:10.1007/s11033-023-08346-z
Dai, Chen, Chai, Zhao, Wang et al., Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide, Crit Rev Food Sci Nutr, doi:10.1080/10408398.2018.1501657
Das, Das, Swain, Mukherjee, Bhattacharya, Andrographolide induces anti-SARS-CoV-2 response through host-directed mechanism: an in silico study, Future Virol, doi:10.2217/fvl-2021-0171
Ding, Li, Song, Xiao, Huang et al., Andrographolide prevents high-fat diet-induced obesity in C57BL/6 mice by suppressing the sterol regulatory element-binding protein pathway, J Pharmacol Exp Ther, doi:10.1124/jpet.114.217968
Elumalai, Suresh Kumar, Identification of neo-andrographolide compound targeting NS1 Lys14: an important residue in NS1 activity driving dengue pathogenesis, J Biomol Struct Dyn, doi:10.1080/07391102.2022.2068073
Enmozhi, Raja, Sebastine, Joseph, Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1760136
Hati, Bhattacharyya, Impact of Thiol-Disulfide Balance on the Binding of Covid-19 Spike Protein with Angiotensin-Converting Enzyme 2 Receptor, ACS Omega, doi:10.1021/acsomega.0c02125
Hillary, Ceasar, An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines, Heliyon, doi:10.1016/j.heliyon.2023.e13952
Hosakote, Liu, Castro, Garofalo, Casola, Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2008-0330OC
Hossain, Urbi, Karuniawati, Mohiuddin, Moh Qrimida et al., Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy, Life, doi:10.3390/life11040348
Ji, Shen, Jiang, Morahan, Wang, Critical roles of cellular glutathione homeostasis and jnk activation in andrographolide-mediated apoptotic cell death in human hepatoma cells, Mol Carcinog, doi:10.1002/mc.20741
Jiang, Sheng, Zhang, Ma, Gao et al., Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents, J Ethnopharmacol, doi:10.1016/j.jep.2021.113954
Jiaqi, Siqing, Qin, Di, Bei et al., Andrographolide promoted ferroptosis to repress the development of non-small cell lung cancer through activation of the mitochondrial dysfunction, Phytomedicine, doi:10.1016/j.phymed.2022.154601
Kanjanasirirat, Suksatu, Manopwisedjaroen, Munyoo, Tuchinda et al., High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents, Scientific reports
Kongsomros, Boonyarattanasoonthorn, Phongphaew, Kasorndorkbua, Sunyakumthorn et al., In vivo evaluation of Andrographis paniculata and Boesenbergia rotunda extract activity against SARS-CoV-2 Delta variant in Golden Syrian hamsters: Potential herbal alternative for COVID-19 treatment, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2024.05.004
Krobthong, Yingchutrakul, Samutrtai, Hitakarun, Siripattanapipong et al., Utilizing Quantitative Proteomics to Identify Species-Specific Protein Therapeutic Targets for the Treatment of Leishmaniasis, ACS Omega, doi:10.1021/acsomega.1c05792
Li, Dong, Liu, Chen, Shi et al., Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt, Molecular Vision
Li, Yuan, Wu, Zhen, Sun et al., Andrographolide, a natural antiinflammatory agent: An Update, Front Pharmacol, doi:10.3389/fphar.2022.920435
Liu, Wu, Bing, Qi, Zhu et al., H1N1 influenza virus infection through NRF2-KEAP1-GCLC pathway induces ferroptosis in nasal mucosal epithelial cells, Free Radic Biol Med, doi:10.1016/j.freeradbiomed.2023.05.004
Lu, Yang, Li, Liu, Lii et al., Andrographolide inhibits TNFαinduced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells, Biochemical Pharmacology, doi:10.1016/j.bcp.2014.06.024
Malat, Ekalaksananan, Heawchaiyaphum, Suebsasana, Roytrakul et al., Andrographolide Inhibits Epstein-Barr Virus Lytic Reactivation in EBV-Positive Cancer Cell Lines through the Modulation of Epigenetic-Related Proteins, Molecules, doi:10.3390/molecules27144666
Mir, Kapur, Singh, Sonpavde, Lillard et al., Andrographolide inhibits prostate cancer by targeting cell cycle regulators, CXCR3 and CXCR7 chemokine receptors, Cell Cycle, doi:10.1080/15384101.2016.1148836
Murae, Shimizu, Yamamoto, Kobayashi, Houri et al., The function of SARS-CoV-2 spike protein is impaired by disulfidebond disruption with mutation at cysteine-488 and by thiol-reactive N-acetyl-cysteine and glutathione, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2022.01.106
Nutho, Wilasluck, Deetanya, Wangkanont, Arsakhant et al., Discovery of C-12 dithiocarbamate andrographolide analogues as inhibitors of SARS-CoV-2 main protease: In vitro and in silico studies, Comput Struct Biotechnol J, doi:10.1016/j.csbj.2022.05.053
Panraksa, Ramphan, Khongwichit, Smith, Activity of andrographolide against dengue virus, Antiviral Res, doi:10.1016/j.antiviral.2016.12.014
Poe, Corn, N-Acetylcysteine: A potential therapeutic agent for SARS-CoV-2, Med Hypotheses, doi:10.1016/j.mehy.2020.109862
Rehan, Ahmed, Howladar, Refai, Baeissa et al., A Computational Approach Identified Andrographolide as a Potential Drug for Suppressing COVID-19-Induced Cytokine Storm, Front Immunol, doi:10.3389/fimmu.2021.648250
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Thongsri, Kanjanasirirat et al., Anti-SARS-CoV-2 Activity of Andrographis paniculata Extract and Its Major Component Andrographolide in Human Lung Epithelial Cells and Cytotoxicity Evaluation in Major Organ Cell Representatives, J Nat Prod, doi:10.1021/acs.jnatprod.0c01324
Saliu, Umar, Ogunsile, Okpara, Yanaka et al., Molecular docking and pharmacokinetic studies of phytocompounds from Nigerian Medicinal Plants as promising inhibitory agents against SARS-CoV-2 methyltransferase (nsp16), J Genet Eng Biotechnol, doi:10.1186/s43141-021-00273-5
Saravolatz, Depcinski, Sharma, Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs, Clinical Infectious Diseases, doi:10.1093/cid/ciac180
Schulte, Konig, Escher, Wittenburg, Proj et al., Andrographolide Derivatives Target the KEAP1/NRF2 Axis and Possess Potent Anti-SARS-CoV-2 Activity, ChemMedChem, doi:10.1002/cmdc.202100732
Shrestha, Foster, Rawlinson, Tedla, Bull, Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission, Rev Med Virol, doi:10.1002/rmv.2381
Soto, Manzano-Pech, Palacios-Chavarria, Valdez-Vazquez, Guarner-Lans et al., N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings, Pharmaceuticals, doi:10.3390/ph16040591
Srikanth, Sarma, Andrographolide binds to spike glycoprotein and RNA-dependent RNA polymerase (NSP12) of SARS-CoV-2 by in silico approach: a probable molecule in the development of anti-coronaviral drug, J Genet Eng Biotechnol, doi:10.1186/s43141-021-00201-7
Tavassolifar, Aghdaei, Sadatpour, Maleknia, Fayazzadeh et al., New insights into extracellular and intracellular redox status in COVID-19 patients, Redox Biol, doi:10.1016/j.redox.2022.102563
Tian, Chen, Feng, Nirmatrelvir-ritonavir compared with other antiviral drugs for the treatment of COVID-19 patients: A systematic review and meta-analysis, J Med Virol, doi:10.1002/jmv.28732
Valko, Leibfritz, Moncol, Cronin, Mazur et al., Free radicals and antioxidants in normal physiological functions and human disease, Int J Biochem Cell Biol, doi:10.1016/j.biocel.2006.07.001
Vetvicka, Vannucci, Biological properties of andrographolide, an active ingredient of Andrographis Paniculata: a narrative review, Ann Transl Med, doi:10.21037/atm-20-7830
Vitiello, Sars-Cov-2 and risk of antiviral drug resistance, Ir J Med Sci, doi:10.1007/s11845-021-02820-y
Wang, Li, Chen, Liu, Cui et al., Identification of PDCD2 as a Candidate Target of Andrographolide That Arrests the Tumor Cell Cycle by Human Proteome-Scale Screening, ACS Pharmacol Transl Sci, doi:10.1021/acsptsci.2c00092
Who, World health organization (WHO) coronavirus (COVID-19) dashboard
Wintachai, Kaur, Lee, Ramphan, Kuadkitkan et al., Activity of andrographolide against chikungunya virus infection, Sci Rep, doi:10.1038/srep14179
Wong, Ng, Leung, Boh, Lim et al., Regulation of the NRF2 transcription factor by andrographolide and organic extracts from plant endophytes, PLoS One, doi:10.1371/journal.pone.0204853
Woo, Waye, Tsui, Yeung, Cheng, Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury, J Pharmacol Exp Ther, doi:10.1124/jpet.107.133918
Wroblewska, Wroblewski, Holynska-Iwan, Modrzejewska, Nuszkiewicz et al., The Role of Glutathione in Selected Viral Diseases, Antioxidants, doi:10.3390/antiox12071325
Yu, Dai, Jiang, Li, Chen et al., Andrographolide as an anti-H1N1 drug and the mechanism related to retinoic acidinducible gene-I-like receptors signaling pathway, Chin J Integr Med, doi:10.1007/s11655-014-1860-0
Zhang, Ding, Cheng, Zhang, Xiang et al., Andrographolide attenuates oxidative stress injury in cigarette smoke extract exposed macrophages through inhibiting SIRT1/ERK signaling, Int Immunopharmacol, doi:10.1016/j.intimp.2020.106230
DOI record:
{
"DOI": "10.1016/j.phymed.2024.156279",
"ISSN": [
"0944-7113"
],
"URL": "http://dx.doi.org/10.1016/j.phymed.2024.156279",
"alternative-id": [
"S0944711324009358"
],
"article-number": "156279",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC)"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Phytomedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.phymed.2024.156279"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Elsevier GmbH. All rights are reserved, including those for text and data mining, AI training, and similar technologies."
}
],
"author": [
{
"affiliation": [],
"family": "Chaopreecha",
"given": "Jarinya",
"sequence": "first"
},
{
"affiliation": [],
"family": "Phueakphud",
"given": "Nut",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suksatu",
"given": "Ampa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krobthong",
"given": "Sucheewin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manopwisedjaroen",
"given": "Suwimon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Panyain",
"given": "Nattawadee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hongeng",
"given": "Suradej",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thitithanyanont",
"given": "Arunee",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6070-1843",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wongtrakoongate",
"given": "Patompon",
"sequence": "additional"
}
],
"container-title": "Phytomedicine",
"container-title-short": "Phytomedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
11,
30
]
],
"date-time": "2024-11-30T23:24:58Z",
"timestamp": 1733009098000
},
"deposited": {
"date-parts": [
[
2025,
1,
11
]
],
"date-time": "2025-01-11T19:27:06Z",
"timestamp": 1736623626000
},
"indexed": {
"date-parts": [
[
2025,
1,
12
]
],
"date-time": "2025-01-12T05:21:16Z",
"timestamp": 1736659276147,
"version": "3.32.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
1
]
],
"date-time": "2025-01-01T00:00:00Z",
"timestamp": 1735689600000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
1
]
],
"date-time": "2025-01-01T00:00:00Z",
"timestamp": 1735689600000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
1
]
],
"date-time": "2025-01-01T00:00:00Z",
"timestamp": 1735689600000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
1
]
],
"date-time": "2025-01-01T00:00:00Z",
"timestamp": 1735689600000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
1
]
],
"date-time": "2025-01-01T00:00:00Z",
"timestamp": 1735689600000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
1
]
],
"date-time": "2025-01-01T00:00:00Z",
"timestamp": 1735689600000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
1
]
],
"date-time": "2025-01-01T00:00:00Z",
"timestamp": 1735689600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0944711324009358?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0944711324009358?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "156279",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
1
]
]
},
"published-print": {
"date-parts": [
[
2025,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.redox.2021.102041",
"article-title": "SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione",
"author": "Bartolini",
"doi-asserted-by": "crossref",
"journal-title": "Redox. Biol.",
"key": "10.1016/j.phymed.2024.156279_bib0001",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26776",
"article-title": "In silico analyses on the comparative sensing of SARS-CoV-2 mRNA by the intracellular TLRs of humans",
"author": "Choudhury",
"doi-asserted-by": "crossref",
"first-page": "2476",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.phymed.2024.156279_bib0002",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.humimm.2021.05.001",
"article-title": "Chemotherapy vs. immunotherapy in combating nCOVID19: an update",
"author": "Choudhury",
"doi-asserted-by": "crossref",
"first-page": "649",
"journal-title": "Hum. Immunol.",
"key": "10.1016/j.phymed.2024.156279_bib0003",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.1002/jmv.25987",
"article-title": "In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs",
"author": "Choudhury",
"doi-asserted-by": "crossref",
"first-page": "2105",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.phymed.2024.156279_bib0004",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.2217/fvl-2021-0171",
"article-title": "Andrographolide induces anti-SARS-CoV-2 response through host-directed mechanism: an in silico study",
"author": "Das",
"doi-asserted-by": "crossref",
"first-page": "651",
"journal-title": "Future Virol.",
"key": "10.1016/j.phymed.2024.156279_bib0005",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1021/acsomega.0c02125",
"article-title": "Impact of Thiol-disulfide balance on the binding of Covid-19 spike protein with angiotensin-converting enzyme 2 receptor",
"author": "Hati",
"doi-asserted-by": "crossref",
"first-page": "16292",
"journal-title": "ACS Omega",
"key": "10.1016/j.phymed.2024.156279_bib0006",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1165/rcmb.2008-0330OC",
"article-title": "Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes",
"author": "Hosakote",
"doi-asserted-by": "crossref",
"first-page": "348",
"journal-title": "Am. J. Respir. Cell Mol. Biol.",
"key": "10.1016/j.phymed.2024.156279_bib0007",
"volume": "41",
"year": "2009"
},
{
"DOI": "10.1002/mc.20741",
"article-title": "Critical roles of cellular glutathione homeostasis and jnk activation in andrographolide-mediated apoptotic cell death in human hepatoma cells",
"author": "Ji",
"doi-asserted-by": "crossref",
"first-page": "580",
"journal-title": "Mol. Carcinog.",
"key": "10.1016/j.phymed.2024.156279_bib0008",
"volume": "50",
"year": "2011"
},
{
"DOI": "10.1016/j.phymed.2022.154601",
"article-title": "Andrographolide promoted ferroptosis to repress the development of non-small cell lung cancer through activation of the mitochondrial dysfunction",
"author": "Jiaqi",
"doi-asserted-by": "crossref",
"journal-title": "Phytomedicine",
"key": "10.1016/j.phymed.2024.156279_bib0009",
"volume": "109",
"year": "2023"
},
{
"DOI": "10.1038/s41598-020-77003-3",
"article-title": "High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents",
"author": "Kanjanasirirat",
"doi-asserted-by": "crossref",
"first-page": "19963",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.phymed.2024.156279_bib0010",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.jtcme.2024.05.004",
"article-title": "In vivo evaluation of Andrographis paniculata and Boesenbergia rotunda extract activity against SARS-CoV-2 Delta variant in Golden Syrian hamsters: Potential herbal alternative for COVID-19 treatment",
"author": "Kongsomros",
"doi-asserted-by": "crossref",
"first-page": "598",
"journal-title": "J Tradit Complement Med",
"key": "10.1016/j.phymed.2024.156279_bib0011",
"volume": "14",
"year": "2024"
},
{
"article-title": "Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt",
"author": "Li",
"first-page": "1656",
"journal-title": "Mol. Vis.",
"key": "10.1016/j.phymed.2024.156279_bib0012",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.1016/j.freeradbiomed.2023.05.004",
"article-title": "H1N1 influenza virus infection through NRF2-KEAP1-GCLC pathway induces ferroptosis in nasal mucosal epithelial cells",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "226",
"journal-title": "Free Radic Biol Med",
"key": "10.1016/j.phymed.2024.156279_bib0013",
"volume": "204",
"year": "2023"
},
{
"DOI": "10.1016/j.bcp.2014.06.024",
"article-title": "Andrographolide inhibits TNFα-induced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "40",
"journal-title": "Biochem. Pharmacol.",
"key": "10.1016/j.phymed.2024.156279_bib0014",
"volume": "91",
"year": "2014"
},
{
"DOI": "10.2217/fvl-2021-0249",
"article-title": "Toll-like receptor 4 in COVID-19: friend or foe?",
"author": "Mukherjee",
"doi-asserted-by": "crossref",
"first-page": "415",
"journal-title": "Future Virol.",
"key": "10.1016/j.phymed.2024.156279_bib0015",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.bbrc.2022.01.106",
"article-title": "The function of SARS-CoV-2 spike protein is impaired by disulfide-bond disruption with mutation at cysteine-488 and by thiol-reactive N-acetyl-cysteine and glutathione",
"author": "Murae",
"doi-asserted-by": "crossref",
"first-page": "30",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.phymed.2024.156279_bib0016",
"volume": "597",
"year": "2022"
},
{
"DOI": "10.1002/jmv.26387",
"article-title": "Targeting human TLRs to combat COVID-19: A solution?",
"author": "Patra",
"doi-asserted-by": "crossref",
"first-page": "615",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.phymed.2024.156279_bib0017",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.mehy.2020.109862",
"article-title": "N-Acetylcysteine: A potential therapeutic agent for SARS-CoV-2",
"author": "Poe",
"doi-asserted-by": "crossref",
"journal-title": "Med. Hypotheses",
"key": "10.1016/j.phymed.2024.156279_bib0018",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.648250",
"article-title": "A computational approach identified andrographolide as a potential drug for suppressing COVID-19-induced cytokine storm",
"author": "Rehan",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.phymed.2024.156279_bib0019",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1021/acs.jnatprod.0c01324",
"article-title": "Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives",
"author": "Sa-Ngiamsuntorn",
"doi-asserted-by": "crossref",
"first-page": "1261",
"journal-title": "J. Nat. Prod.",
"key": "10.1016/j.phymed.2024.156279_bib0020",
"volume": "84",
"year": "2021"
},
{
"article-title": "Andrographolide derivatives target the KEAP1/NRF2 axis and possess potent anti-SARS-CoV-2 activity",
"author": "Schulte",
"journal-title": "Chem Med Chem",
"key": "10.1016/j.phymed.2024.156279_bib0021",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.3390/ph16040591",
"article-title": "N-Acetyl Cysteine restores the diminished activity of the antioxidant enzymatic system caused by SARS-CoV-2 infection: preliminary findings",
"author": "Soto",
"doi-asserted-by": "crossref",
"first-page": "519",
"journal-title": "Pharmaceuticals",
"key": "10.1016/j.phymed.2024.156279_bib0022",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1016/j.redox.2022.102563",
"article-title": "New insights into extracellular and intracellular redox status in COVID-19 patients",
"author": "Tavassolifar",
"doi-asserted-by": "crossref",
"journal-title": "Redox. Biol.",
"key": "10.1016/j.phymed.2024.156279_bib0023",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1016/j.biocel.2006.07.001",
"article-title": "Free radicals and antioxidants in normal physiological functions and human disease",
"author": "Valko",
"doi-asserted-by": "crossref",
"first-page": "44",
"journal-title": "Int. J. Biochem. Cell Biol.",
"key": "10.1016/j.phymed.2024.156279_bib0024",
"volume": "39",
"year": "2007"
},
{
"DOI": "10.1007/s11845-021-02820-y",
"article-title": "Sars-Cov-2 and risk of antiviral drug resistance",
"author": "Vitiello",
"doi-asserted-by": "crossref",
"first-page": "2367",
"journal-title": "Ir. J. Med. Sci.",
"key": "10.1016/j.phymed.2024.156279_bib0025",
"volume": "191",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0204853",
"article-title": "Regulation of the NRF2 transcription factor by andrographolide and organic extracts from plant endophytes",
"author": "Wong",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.phymed.2024.156279_bib0026",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1124/jpet.107.133918",
"article-title": "Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury",
"author": "Woo",
"doi-asserted-by": "crossref",
"first-page": "226",
"journal-title": "J. Pharmacol. Exp. Ther.",
"key": "10.1016/j.phymed.2024.156279_bib0027",
"volume": "325",
"year": "2008"
},
{
"DOI": "10.3390/antiox12071325",
"article-title": "The role of glutathione in selected viral diseases",
"author": "Wroblewska",
"doi-asserted-by": "crossref",
"first-page": "1325",
"journal-title": "Antioxidants",
"key": "10.1016/j.phymed.2024.156279_bib0028",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1016/j.intimp.2020.106230",
"article-title": "Andrographolide attenuates oxidative stress injury in cigarette smoke extract exposed macrophages through inhibiting SIRT1/ERK signaling",
"author": "Zhang",
"doi-asserted-by": "crossref",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.phymed.2024.156279_bib0029",
"volume": "81",
"year": "2020"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0944711324009358"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC)",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "136"
}