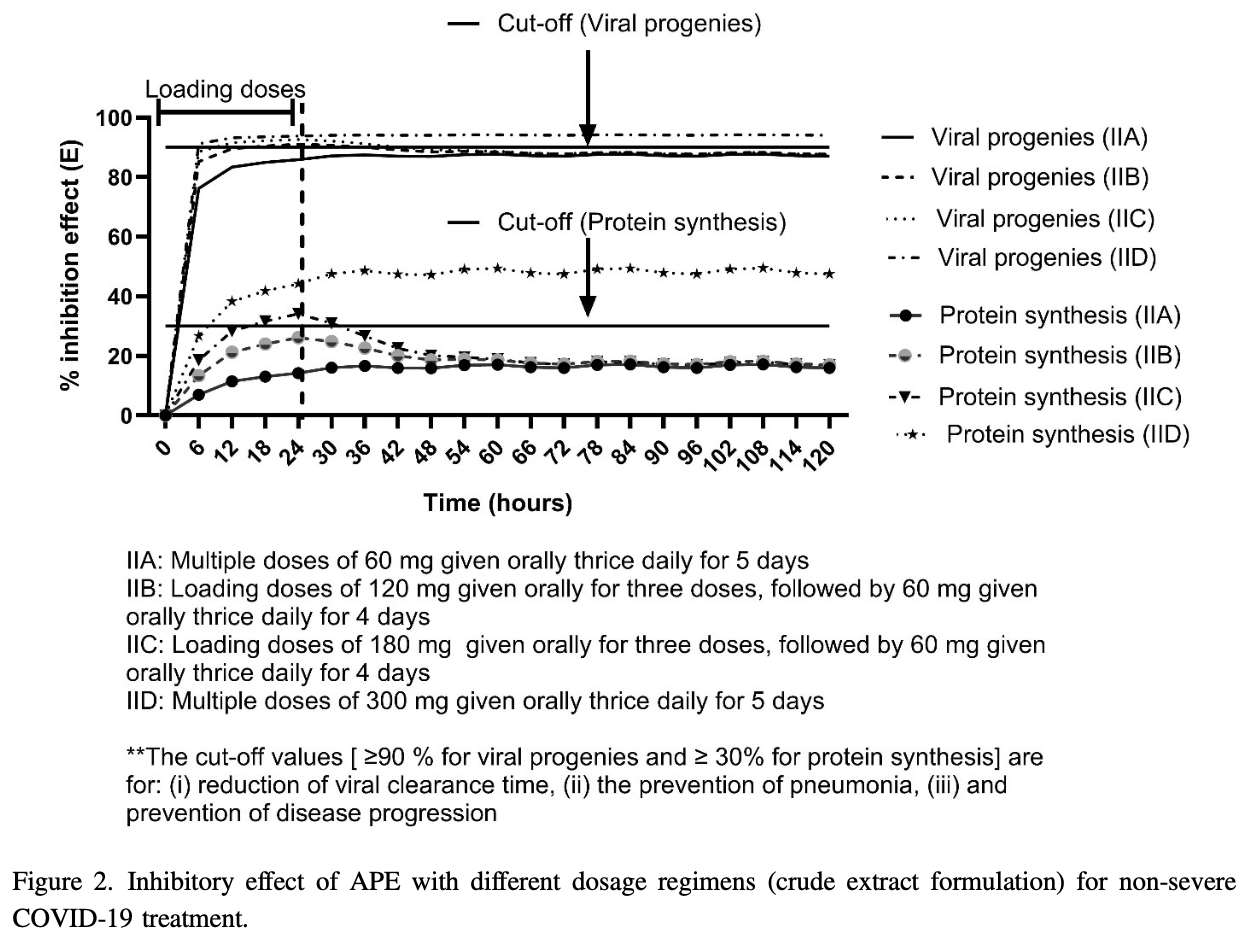
In Silico Prediction of Andrographolide Dosage Regimens for COVID-19 Treatment
et al., The American Journal of Chinese Medicine, doi:10.1142/S0192415X22500732, Jan 2022
In silico study showing potential benefits of andrographolide for COVID-19 treatment and optimal dosage regimens predicted by physiologically-based pharmacokinetic and pharmacodynamic (PBPK/PD) modeling. Authors find that oral crude extract formulation of andrographolide is recommended for patients with asymptomatic or mild COVID-19 at a loading dose of 120mg given for three doses, followed by 60mg every 8 hours for 4 consecutive days. Intravenous formulation is recommended for patients with mild-to-moderate COVID-19 at a dose of 500mg given by IV infusion at a rate of 25mg/h every 24 hours for 14 days. Authors suggest that andrographolide should be started within 10 days after infection.
25 preclinical studies support the efficacy of andrographolide for COVID-19:
In vitro studies demonstrate inhibition of the MproA,18 protein.
In vitro studies demonstrate efficacy in Calu-3B,18, A549C,14, and HUVECD,18 cells.
Animal studies demonstrate efficacy in Sprague Dawley miceE,18 and Golden Syrian hamstersF,14.
Andrographolide inhibits Mpro in a dose-dependent manner18, reduces ACE2 levels in the lung tissue of mice in combination with baicalein18, inhibits binding between the SARS-CoV-2 spike protein and ACE218, alleviates lung inflammation and cytokine storm in mice18, and improves survival and reduces lung inflammation via anti-inflammatory effects in Syrian hamsters14.
1.
Zhang et al., Effects and Mechanisms of Andrographolide for COVID-19: A Network Pharmacology-Based and Experimentally Validated Study, Natural Product Communications, doi:10.1177/1934578X241288428.
2.
Thomas et al., Cheminformatics approach to identify andrographolide derivatives as dual inhibitors of methyltransferases (nsp14 and nsp16) of SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-58532-7.
3.
Arifin et al., Computational exploration of Andrographis paniculata herb compounds as potential antiviral agents targeting NSP3 (6W02) and NSP5 (7AR6) of SARS-COV-2, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2023.25.2.0292.
4.
Bhattarai et al., Investigating the binding affinity of andrographolide against human SARS-CoV-2 spike receptor-binding domain through docking and molecular dynamics simulations, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2174596.
5.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
6.
Dassanayake et al., Molecular Docking and In-Silico Analysis of Natural Biomolecules against Dengue, Ebola, Zika, SARS-CoV-2 Variants of Concern and Monkeypox Virus, International Journal of Molecular Sciences, doi:10.3390/ijms231911131.
7.
Ningrum et al., Potency Of Andrographolide, L-Mimosine And Asiaticoside Compound As Antiviral For Covid-19 Based On In Silico Method, Proceedings Universitas Muhammadiyah Yogyakarta Undergraduate Conference, doi:10.18196/umygrace.v2i2.418.
8.
Ravichandran et al., Identification of Potential Semisynthetic Andrographolide Derivatives to Combat COVID-19 by Targeting the SARS-COV-2 Spike Protein and Human ACE2 Receptor– An In-silico Approach, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC132.155.
9.
Saeheng et al., In Silico Prediction of Andrographolide Dosage Regimens for COVID-19 Treatment, The American Journal of Chinese Medicine, doi:10.1142/S0192415X22500732.
10.
Khanal et al., Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19, RSC Advances, doi:10.1039/D0RA10529E.
11.
Rehan et al., A Computational Approach Identified Andrographolide as a Potential Drug for Suppressing COVID-19-Induced Cytokine Storm, Frontiers in Immunology, doi:10.3389/fimmu.2021.648250.
12.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
13.
Dey et al., The role of andrographolide and its derivative in COVID-19 associated proteins and immune system, Research Square, doi:10.21203/rs.3.rs-35800/v1.
14.
Kongsomros et al., In vivo evaluation of Andrographis paniculata and Boesenbergia rotunda extract activity against SARS-CoV-2 Delta variant in Golden Syrian hamsters: Potential herbal alternative for COVID-19 treatment, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2024.05.004.
15.
Chaopreecha et al., Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC), Phytomedicine, doi:10.1016/j.phymed.2024.156279.
16.
Li et al., Andrographolide suppresses SARS-CoV-2 infection by downregulating ACE2 expression: A mechanistic study, Antiviral Therapy, doi:10.1177/13596535241259952.
17.
Low et al., The wide spectrum anti-inflammatory activity of andrographolide in comparison to NSAIDs: a promising therapeutic compound against the cytokine storm, bioRxiv, doi:10.1101/2024.02.21.581396.
18.
Wan et al., Synergistic inhibition effects of andrographolide and baicalin on coronavirus mechanisms by downregulation of ACE2 protein level, Scientific Reports, doi:10.1038/s41598-024-54722-5.
19.
Siridechakorn et al., Inhibitory efficiency of Andrographis paniculata extract on viral multiplication and nitric oxide production, Scientific Reports, doi:10.1038/s41598-023-46249-y.
a.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
b.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
c.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
d.
HUVEC (Human Umbilical Vein Endothelial Cells) are primary endothelial cells derived from the vein of the umbilical cord. They are used to study vascular biology, including inflammation, angiogenesis, and viral interactions with endothelial cells.
e.
An outbred multipurpose breed of albino mouse used extensively in medical research.
f.
A rodent model widely used in infectious disease research due to their susceptibility to viral infections and similar disease progression to humans.
Saeheng et al., 31 Jan 2022, peer-reviewed, 3 authors.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
In Silico Prediction of Andrographolide Dosage Regimens for COVID-19 Treatment
The American Journal of Chinese Medicine, doi:10.1142/s0192415x22500732
Andrographolide (APE) has been used for COVID-19 treatment in various clinical settings in South-East Asia due to its benefits on reduction of viral clearance and prevention of disease progression. However, the limitation of APE clinical use is the high incidence of adverse events. The objective of this study was to find the optimal dosage regimens of APE for COVID-19 treatment. The whole-body physiologically-based pharmacokinetic (PBPK) models were constructed using data from the published articles and validated against clinical observations. The inhibitory effect of APE was determined for the potency of drug efficacy. For prevention of pneumonia, multiple oral doses such as 120 mg for three doses, followed by 60 mg three times daily for 4 consecutive days, or 200 mg intravenous infusion at the rate of 20 mg/h once daily is advised in patients with mild COVID-19. For prevention of pneumonia and reduction of viral clearance time, the recommended dosage regimen is 500 mg intravenous infusion at the rate of 25 mg/h once daily in patients with mild-tomoderate COVID-19. One hundred virtual populations (50 males and 50 females) were simulated for oral and intravenous infusion formulations of APE. The eligible PBPK/PD models successfully predicted optimal dosage regimens and formulations of APE for prevention of disease progression and/or reduction of viral clearance time. Additionally, APE should be co-administered with other antiviral drugs to enhance therapeutic efficacy for COVID-19 treatment.
Cholangiocarcinoma (No. 1/2556 , dated 12 October 2013 ), and the National Research Council of Thailand (No. 45/2561 , dated 10 September 2018) . K.N. was supported by the National Research Council of Thailand under the Research Team Promotion grant (grant number NRCT 820/2563, dated 12 November 2020).
Supplementary Information
References
Atzori, Villani, Regazzi, Cargnel, Detection of intrapulmonary concentration of lopinavir in an HIV-infected patient, AIDS
Baneyx, Parrott, Meille, Iliadis, Lavé, Physiologically-based pharmacokinetic modeling of CYP3A4 induction by rifampicin in human: Influence of time between substrate and inducer administration, Eur. J. Pharm. Sci
Benjapolpitak, Visitanon, Sawangthum, Thanirat, Vannarat, Short report on the use of Andrographis paniculata as treatment of COVID-19 patients, J. Thai. Trad. Alt. Med
Camon, Alonso, Munoz, Cardozo, Bernal-Maurandi et al., Hospital Clinic of Barcelona COVID-19 Research Group. C-reactive protein cut-off for early tocilizumab and dexamethasone prescription in hospitalized patients with COVID-19, Sci. Rep
Cui, Merritt, Assa, Mustehsan, Chung et al., Early and significant reduction in C-reactive protein levels after corticosteroid therapy is associated with reduced mortality in patients with COVID-19, J. Hosp. Med
Ernest, Hall, Jones, Mechanism-based inactivation of CYP3A by HIV protease inhibitors, J. Pharm. Exp. Ther
Eron, Feinberg, Kressler, Horowitz, Witt et al., Once-daily dose versus twice-daily lopinavir-ritonavir in antiretroviral-naïve HIV-positive patients: A 48week randomized clinical trial, J. Infect. Dis
Gao, Su, Wang, Xiong, Sun et al., Integrated computer-aided formulation design: A case study of andrographolide/cyclodextrin ternary formulation, Asian. J. Pharm
Hsu, Granneman, Witt, Locke, Denissen et al., Re-use and distribution is strictly not permitted, except for Open Access articles. pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects, Am. J. Chin. Med.
Kigen, Edwards, Drug-transporter mediated interactions between anthelminthic and antiretroviral drugs across the Caco-2 cell monolayers, BMC Pharmacol. Toxicol
Kirby, Collier, Kharasch, Whittington, Thummel et al., Complex drug interactions of HIV protease inhibitors 1: Inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir, Drug Metab. Dispos
Koudriakova, Iatsimirskaia, Utkin, Vouros, Storozhuk et al., Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P4503A4/3A5: mechanism-based inactivation of cytochrome P4503A by ritonavir, Drug Metab. Dispos
Li, He, Tang, Ding, Chu et al., Andrographolide inhibits inflammatory cytokines secretion in LPSstimulated RAW264.7 cells through suppression of NF-κB/MAPK signaling pathway, Evid.-Based Complement. Altern. Med
Lim, Chan, Tan, The, Razak et al., Andrographis paniculata (Burm.F.) Wall. Ex Nees, andrographolide, and andrographolide analogues as SARs-CoV-2 antivirals? A rapid review, Nat. Prod. Commun
Pandey, Rao, Andrographolide: Its pharmacology, natural bioavailability and current approaches to increase its content in Andrographis paniculata, Int. J. Complement. Alternat. Med
Panossian, Hovhannisyan, Mamikonyan, Abrahamian, Hambardzumyan et al., Pharmacokinetic and oral bioavailability of APE from Andrographis paniculata fixed combination Kan Jang in rats and human, Phytomedicine
Perazzolo, Zhu, Lin, Nguyen, Ho, Systems and clinical pharmacology of COVID-19 therapeutic candidates: A clinical and translational medicine perspective, J. Pharm. Sci
Pholphana, Panomvana, Rangkadilok, Suriyo, Puranajoti et al., Andrographis paniculata: Dissolution investigation and pharmacokinetic studies of four major active diterpenoids after multiple oral dose administration in healthy Thai volunteers, J. Ethnopharmacol
Rafiq, Iqbal, Jamil, Khan, Pharmacokinetic studies of rifampicin in healthy volunteers and tuberculosis patients, Int. J. Agric. Biol
Rasool, Khalid, Majeed, Saeed, Imran et al., Development and evaluation of physiologically-based pharmacokinetic drug-disease models for predicting rifampicin exposure in tuberculosis and cirrhosis populations, Pharmaceutics
Rattanaraksa, Poolwiwatchaikool, Nimitvilai, Loatrakul, Srimanee, The efficacy and safety of Andrographis paniculata Extract for treatment of COVID-19 patients with mild symptoms in Nakhonpathom hospital, Reg. 4-5 Med. J
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Thongsri, Kanjanasirirat et al., Anti-SARs-CoV-2 activity of Andrographolis paniculata Extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J. Nat. Prod
Saeheng, Karbwang, Na-Bangchang, None, Am. J. Chin. Med.
Saeheng, Na-Bangchang, Karbwang, Utility of physiologically-based pharmacokinetic (PBPK) modeling in oncology drug development and its accuracy: A systematic review, Eur. J. Clin. Pharmacol
Saeheng, Na-Bangchang, Siccardi, Rajoli, Karbwang, Physiologically-based pharmacokinetic modeling for optimal dosage prediction of quinine coadministered with ritonavir-boosted lopinavir, Clin. Pharmacol. Ther
Siccardi, Rajoli, Dickinson, Khoo, Owen et al., In silico simulation of interaction between rifampicin and boosted darunavir
Stigliani, Haghi, Russo, Young, Traini, Antibiotic transport across bronchial epithelial Effects of molecular weight, LogP and apparent permeability, Eur. J. Pharm. Sci
Stoeckle, Witting, Kapadia, An, Marks, Elevated inflammatory markers are associated with poor outcomes in COVID-19 patients treated with remdesivir, J. Med. Virol
Tanwettiyanont, Piriyachananusorn, Sangsoi, Boonsong, Sunpapoa et al., The efficacy of Andrographis paniculata (Burm. F.) Wall. Ex Nees crude extract in hospitalized mild COVID-19 patients: A retrospective cohort study, doi:10.1101/2022.01.01.22268609
Vannarat, Andrographis paniculata in COVID-19 patients
Varma, Lai, Feng, Litchfield, Goosen et al., Physiologically based modeling of pravastatin transporter-mediated hepatobiliary disposition and drug-drug interactions, Pharm. Res
Wagner, Zhao, Arya, Mullick, Struble et al., Physiologically based pharmacokinetic modeling for predicting the effect of intrinsic and extrinsic factors on darunavir or lopinavir exposure co-administered with ritonavir, Jr. Clin. Pharm
Wannaratna, Leethong, Inchai, Chueawiang, Sriraksa et al., Efficacy and safety of Andrographis paniculata extract in patients with mild COVID-19: A randomized controlled trial, medRXiv, doi:10.1101/2021.07.08.21259912
Ye, Wang, Tang, Liu, Yang et al., Poor oral bioavailability of a promising anticancer agent andrographolide is due to extensive metabolism and efflux by P-glycoprotein, J. Pharm. Sci
Zhang, Lv, Zhou, Xie, Xu et al., Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial, Phytother. Res
Zhang, Mcilleron, Ren, Van Der Walt, Karlsson et al., Population pharmacokinetics of lopinavir and ritonavir in combination with rifampicin-based antitubercular treatment in HIV-infected children, Antivir. Ther
Zhao, Hu, Wang, Comparative metabolism and stability of andrographolide in liver microsomes from humans, dogs and rats using ultra-performance liquid chromatography coupled with triple-quadrupole and Fourier transform ion cyclotron resonance mass spectrometry, Rapid Commun. Mass Spectrom
Ziglam, Baldwin, Daniels, Andrew, Finch, Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages, and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy, J. Antimicrob. Chemother
DOI record:
{
"DOI": "10.1142/s0192415x22500732",
"ISSN": [
"0192-415X",
"1793-6853"
],
"URL": "http://dx.doi.org/10.1142/S0192415X22500732",
"abstract": "<jats:p> Andrographolide (APE) has been used for COVID-19 treatment in various clinical settings in South-East Asia due to its benefits on reduction of viral clearance and prevention of disease progression. However, the limitation of APE clinical use is the high incidence of adverse events. The objective of this study was to find the optimal dosage regimens of APE for COVID-19 treatment. The whole-body physiologically-based pharmacokinetic (PBPK) models were constructed using data from the published articles and validated against clinical observations. The inhibitory effect of APE was determined for the potency of drug efficacy. For prevention of pneumonia, multiple oral doses such as 120[Formula: see text]mg for three doses, followed by 60[Formula: see text]mg three times daily for 4 consecutive days, or 200[Formula: see text]mg intravenous infusion at the rate of 20 mg/h once daily is advised in patients with mild COVID-19. For prevention of pneumonia and reduction of viral clearance time, the recommended dosage regimen is 500[Formula: see text]mg intravenous infusion at the rate of 25[Formula: see text]mg/h once daily in patients with mild-to-moderate COVID-19. One hundred virtual populations (50 males and 50 females) were simulated for oral and intravenous infusion formulations of APE. The eligible PBPK/PD models successfully predicted optimal dosage regimens and formulations of APE for prevention of disease progression and/or reduction of viral clearance time. Additionally, APE should be co-administered with other antiviral drugs to enhance therapeutic efficacy for COVID-19 treatment. </jats:p>",
"alternative-id": [
"10.1142/S0192415X22500732"
],
"author": [
{
"affiliation": [
{
"name": "Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Chulabhorn International College, Thailand"
}
],
"family": "Saeheng",
"given": "Teerachat",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Chulabhorn International College, Thailand"
}
],
"family": "Karbwang",
"given": "Juntra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Chulabhorn International College, Thailand"
},
{
"name": "Drug Discovery and Development Center, Office of Advanced Science and Technology, Thammasat University (Rangsit Campus), Klongneung, Pathumthani 12121, Thailand"
}
],
"family": "Na-Bangchang",
"given": "Kesara",
"sequence": "additional"
}
],
"container-title": "The American Journal of Chinese Medicine",
"container-title-short": "Am. J. Chin. Med.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
28
]
],
"date-time": "2022-08-28T04:55:47Z",
"timestamp": 1661662547000
},
"deposited": {
"date-parts": [
[
2022,
11,
12
]
],
"date-time": "2022-11-12T10:35:49Z",
"timestamp": 1668249349000
},
"funder": [
{
"award": [
"1/2556"
],
"name": "Thammasat Postdoctoral Fellowship, Thammasat University, Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma"
},
{
"DOI": "10.13039/501100004704",
"award": [
"45/2561"
],
"doi-asserted-by": "crossref",
"name": "the National Research Council of Thailand"
},
{
"award": [
"NRCT 820/2563"
],
"name": "the National Research Council of Thailand under the Research Team Promotion"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
21
]
],
"date-time": "2024-02-21T01:48:19Z",
"timestamp": 1708480099544
},
"is-referenced-by-count": 2,
"issue": "07",
"issued": {
"date-parts": [
[
2022,
1
]
]
},
"journal-issue": {
"issue": "07",
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.worldscientific.com/doi/pdf/10.1142/S0192415X22500732",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "219",
"original-title": [],
"page": "1719-1737",
"prefix": "10.1142",
"published": {
"date-parts": [
[
2022,
1
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "World Scientific Pub Co Pte Ltd",
"reference": [
{
"DOI": "10.1097/00002030-200307250-00022",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB001"
},
{
"DOI": "10.1016/j.ejps.2014.02.002",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB002"
},
{
"author": "Benjapolpitak A.",
"first-page": "229",
"journal-title": "J. Thai. Trad. Alt. Med.",
"key": "S0192415X22500732BIB003",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-08882-x",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB004"
},
{
"DOI": "10.12788/jhm.3560",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB005"
},
{
"DOI": "10.1124/jpet.104.075416",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB008"
},
{
"DOI": "10.1086/380799",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB009"
},
{
"author": "Gao H.",
"first-page": "494",
"journal-title": "Asian. J. Pharm.",
"key": "S0192415X22500732BIB010",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1128/AAC.41.5.898",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB011"
},
{
"DOI": "10.1186/s40360-017-0129-6",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB012"
},
{
"DOI": "10.1124/dmd.110.037523",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB013"
},
{
"author": "Koudriakova T.",
"first-page": "552",
"journal-title": "Drug Metab. Dispos.",
"key": "S0192415X22500732BIB014",
"volume": "26",
"year": "1998"
},
{
"author": "Li Y.",
"first-page": "8248142",
"journal-title": "Evid.-Based Complement. Altern. Med.",
"key": "S0192415X22500732BIB015",
"volume": "2017",
"year": "2017"
},
{
"author": "Lim X.Y.",
"first-page": "1",
"journal-title": "Nat. Prod. Commun.",
"key": "S0192415X22500732BIB016",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.15406/ijcam.2018.11.00425",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB017"
},
{
"DOI": "10.1016/S0944-7113(00)80054-9",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB018"
},
{
"author": "Perazzolo S.",
"first-page": "1002-1017",
"journal-title": "J. Pharm. Sci.",
"key": "S0192415X22500732BIB019",
"volume": "110",
"year": "2020"
},
{
"DOI": "10.1016/j.jep.2016.09.058",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB020"
},
{
"author": "Rafiq S.",
"first-page": "391",
"journal-title": "Int. J. Agric. Biol.",
"key": "S0192415X22500732BIB021",
"volume": "12",
"year": "2010"
},
{
"DOI": "10.3390/pharmaceutics11110578",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB022"
},
{
"author": "Rattanaraksa D.",
"first-page": "269",
"journal-title": "Reg. 4-5 Med. J.",
"key": "S0192415X22500732BIB023",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1021/acs.jnatprod.0c01324",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB024"
},
{
"DOI": "10.1007/s00228-018-2513-6",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB025"
},
{
"DOI": "10.1002/cpt.1721",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB026"
},
{
"DOI": "10.1016/j.ejps.2015.12.010",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB028a"
},
{
"DOI": "10.1002/jmv.27280",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB028"
},
{
"DOI": "10.1007/s11095-012-0792-7",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB028b"
},
{
"DOI": "10.1002/jcph.936",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB028d"
},
{
"DOI": "10.1002/jps.22693",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB028c"
},
{
"DOI": "10.3851/IMP1915",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB033"
},
{
"DOI": "10.1002/ptr.7141",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB034"
},
{
"DOI": "10.1002/rcm.6585",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB035"
},
{
"DOI": "10.1093/jac/dkf214",
"doi-asserted-by": "publisher",
"key": "S0192415X22500732BIB036"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.worldscientific.com/doi/10.1142/S0192415X22500732"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "<i>In Silico</i> Prediction of Andrographolide Dosage Regimens for COVID-19 Treatment",
"type": "journal-article",
"volume": "50"
}